Hesperis matronalis L. is an herbaceous species used in traditional medicine for stimulatory, antiscorbutic, diuretic, diaphoretic and expectorant properties. The antioxidant effect of a 20% hydroalcoholic extract from the flowering aerial part of H. matronalis (HMHE) was evaluated in mice with chronic experimental streptozotocin-induced diabetes (STZ-ID). Four groups of mice included one control group treated with saline and three test groups with STZ-ID. The second group remained with untreated diabetes and was evaluated for 35 days. The third and fourth groups were treated daily for 35 days with 20% hydroalcoholic extract from the leaves of Vaccinium myrtillus (VMHE, positive control group) and HMHE (experimental group), respectively. At the end of experiment, the levels of some oxidative stress markers were determined in blood samples, including malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), and the total antioxidant capacity (TAC) was assessed. In addition to the hypoglycemic action, HMHE exhibited a decrease in the MDApro-oxidant activity and increase in the antioxidant activity of SOD, GPx and TAC. The anti-oxidative stress effect was correlated with decreased blood sugar in chronic STZ-ID mice. Based on these results, HMHE can be recommended as a source of natural polyphenols with adjunctive role in the prophylaxis and treatment of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Diabetes mellitus, a chronic metabolic disorder, is characterized by hyperglycemia and impaired metabolism of glucose, lipids, and proteins. In addition to the hyperglycemia, some authors consider oxidative stress as the main factor in the pathophysiology of diabetes and its complications [1].
Oxidative stress, an imbalance between the oxidative and antioxidant systems of cells and tissues, is the result of the overproduction of oxidative free radicals and associated reactive oxygen species (ROS) and a depression of antioxidant systems [2]. These hyperactive elements have unmatched electrons in their outer layer of molecules and therefore can bind to other biomolecules and modify them (they can oxidize proteins, lipids, and nucleic acids) and produce toxic bioproducts that cause tissue dysfunction to cell death [3]. The pathophysiological changes of diabetes are characterized by (i) β-cell pancreatic dysfunction in the periphery (e.g., the liver, skeletal muscle, and adipose tissue) and (ii) inflammation-dependent ROS generated locally by tissue or due to immune cells interacting with the insulin receptor, as well as their downstream signaling pathways, resulting in a failure to respond adequately to insulin levels [4]. All of these factors form a basis for insulin resistance and chronic inflammation, which progressively hamper blood glucose control, leading to the development of micro- and macrovascular complications [4].
Oxidative stress is positively correlated with metabolic syndromes such as hyperglycemia. Chronic hyperglycemia leads to overactivation of the polyol pathway (which reduces antioxidant mechanisms), activation of protein kinase C (PKC) isoforms and increased hexosamine flow [5], generates ROS, non-enzymatic glycation of proteins involved in glycation, with the formation of advanced glycation end products (AGEs), which interacts with specific receptors [AGE-R1, AGE-R2, AGE-R3, receptor for AGE (RAGE)] [1] and causes oxidative stress. ROS modify the structures of biological molecules and even break them, as in the case of deoxyribonucleic acid (DNA) affecting many genes and cell death [3, 6]. Free radicals also can indirectly damage cells by activating a variety of stress-sensitive intracellular signaling pathways, such as nuclear factor-kappa B (NF-κB), p38 mitogen-activated protein kinases (MAPKs), c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK), hexosamine pathways, PKC, AGE/RAGE interactions, and sorbitol synthesis [3]. Chronic hyperglycemia and consequent overproduction of ROS in β-pancreatic cells decreases insulin production by involving cytosolic calcium and activating PKC by promoting diacylglycerol synthesis [1, 5]. They also interact abnormally with extracellular matrix components to promote ROS generation and invariably promote oxidative stress [1]. In addition, cells may also be chronically exposed to glucose overload in the diabetic milieu, leading to elevated glycolytic flux and glucose oxidation, elevated tricarboxylic acid cycle activity, and increased Ca2+ oscillations [6]. Together, these metabolic reactions increase the probability of further electron leak, \( {\textrm{O}}_2^{\bullet -} \) generation, and the potential pathological consequences that may affect cell function in terms of reduced insulin secretion or promote cell dysfunction and death, reducing overall pancreatic insulin output [6].
In case of diabetes, the cells are more susceptible to lipid peroxidation [7]. Lipid peroxidation is a complex process involving an increase in free fatty acids (FFAs) that can also lead to the formation of ROS, contribution to mitochondrial DNA damage, and impaired pancreatic β-cell function [8]. ROS can inhibit complex enzymes in the electron transport chain of mitochondria, resulting in blocked mitochondrial respiration [8]. Recent studies provided evidence that transient receptor potential cation channel subfamily M member 2 (TRPM2), a non-selective Ca2+-permeable cation channel with a wide distribution throughout the body, plays an important role in mediating cell death induced by miscellaneous oxidative stress-inducing pathological factors [9]. Abnormally high levels of free radicals and the simultaneous decline of antioxidant defense mechanisms can lead to damage of cellular organelles and enzymes, increased lipid peroxidation, and development of insulin resistance [9]. Cell damage in myocardial tissue, vessels, striated muscles, and adipose tissue eventually leads to secondary complications of diabetes [9]: cardiovascular disorders, nephropathy, retinopathy, neuropathy [–, 3, 10, 15]. Oxidative stress also plays a pivotal role in the pathophysiology of various complications of diabetes through lipid peroxidation, DNA damage, and mitochondrial dysfunction [3].
In diabetes, oxidative stress is caused by auto-oxidation of glucose, oxidation of proteins, and non-enzymatic glycated proteins, which decrease the capacity of the antioxidant defense system and generate the production of free radicals [16]. Endogenous oxidants (free radicals) are superoxide anion (\( {\textrm{O}}_2^{\bullet -} \)), hydroxyl radical (•OH), hydrogen peroxide (H2O2), hypochlorous acid, peroxyl radicals, hydroperoxyl radical [17]. Lipid hydroperoxides are very unstable and easily decompose to secondary products such as aldehydes [e.g., 4-hydroxy-2,3-nonenal and malondialdehyde (MDA)] [17]. Nonradical oxidant species including peroxides, aldehydes, quinones, and epoxides, are generated enzymatically from both endogenous and exogenous precursors and do not require free radicals as intermediates to oxidize or modify these thiols [18]. Nonradical thiol-reactive agents include conjugated aldehydes (e.g., acrolein, 4-hydroxy-2,3-nonenal, MDA), quinones, epoxides, Zn2+, Hg2+, and other cations [19].
The body’s antioxidant defense system protects cells from excess ROS and is composed of endogenous component [non-enzymatic-reduced glutathione and antioxidant enzymes – superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), etc.] and an exogenous component (ascorbate, bioflavonoids, vitamin A, carotenoids, tocopherols, dietary minerals, etc.) [20]. Other antioxidants include lipoic acid, coenzyme Q10, several minerals (copper, zinc, manganese, and selenium), and the cofactors (folic acid, vitamins B1, B2, B6, B12) [9]. Both types of antioxidants can help preventing the formation of free radicals by scavenging or promoting their decomposition [4]. Recently, considerable research interest was devoted to natural antioxidants from plants capable of replacing synthetic antioxidants. In the literature, many herbal medicines have been recommended for treating diabetes mellitus. Hypoglycemic effects have been reported for some plants containing terpenoids, glycoside iridoids, flavonoids, and other phenolic compounds [21]. In addition, several metabolites such as flavonoids, phenolic acids, phenylpropanoids and terpenoids exhibited important antioxidant properties [21].
Hesperis genus of flowering plants (Brassicaceae family) comprises almost 60 species well represented with many taxa at the junctions of the Irano-Turanian, Mediterranean, and Euro-Siberian phytogeographic regions [22]. Hesperis matronalis L. has many common names: Evening violet, Night violet, Damask-violet, Dame’s rocket, Mother-of-theevening, Summer lilac, Sweet rocket, Winter gillyflower. It is a biannual or perennial herbaceous species, originating from Southern Europe (Mediterranean region) and southwest Asia, more precisely from Greece, Italy, and Turkey, and cultivated all over the world for its ornamental appearance. In some countries, it is treated as an invasive plant [23,24,25, – 26]. The whole plant is edible, the leaves being used for salads, in combination with other vegetables. Some extracts from the flowering aerial parts of H. matronalis are recommended for the treatment or prophylaxis of certain skin disorders. The infusion of leaves has stimulatory, antiscorbutic (high content of vitamin C) [24], diuretic, diaphoretic, and expectorant properties [23,24,25,26,27, – 28]. A high percentage of seed oil has the potential to be used as edible oil [24, 28].
Although H. matronalis has a very wide spreading area and is known since antiquity, we did not encounter any special literature regarding researches in the therapeutic properties of this plant and its use in traditional medicine. That is why we investigated the possible antidiabetic action of H. matronalis hydroalcoholic extract (HMHE) and tried to find if a decrease in the oxidative stress can be one of the mechanisms of HMHE action. The aim of this work was to assess the antioxidant capacity of HMHE from the flowering aerial parts (flowering tops of H. matronalis) after five-week administration of the preparation in mice. For this purpose, we investigated the serum levels of MDA, SOD, GPx and total antioxidant capacity (TAC) in the experimental model of oxidative stress of chronic streptozotocin-induced diabetes (STZ-ID) mice, compared with the levels of these markers in the group of untreated STZ-ID mice, control (saline) group, and the group treated with the hydroalcoholic extract from the leaves of Vaccinium myrtillus L. (Bilberry, European blueberry, Wild bilberry of the Ericaceae family), a plant known for its antidiabetic effect and anti-oxidative stress action (positive control group). The choice of hydroalcoholic extract from V. myrtillus leaves (VMHE) as a positive control was based on the data of special papers indicating that this natural product was successfully used as antidiabetic and antioxidant medicine also in Romanian ethnopharmacology [29,30,31,32,33, – 34]. The HMHE and VMHE exhibited low acute and subacute toxicity in mice, being assigned to Category 5 of the acute oral toxicity with a median lethal dose (LD50) > 2000 mg/kg body weight (b.w.), according to the Organization for Economic Co-operation and Development (OECD) Guidelines [35], as we have seen at the end of our experiments on the histopathological analysis of vital organs (unpublished data).
RESULTS AND DISCUSSION
Standardization of Hydroalcoholic Extracts
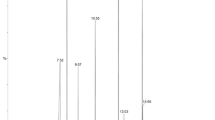
The 20% hydroalcoholic extracts were standardized according to the content of caffeic acid as determined using ultra-high-performance liquid chromatography–ultraviolet–mass spectrometry (UHPLC–UV–MS) analysis, as follows: 7.44 ± 0.16 μg/mL for HMHE and 116.92 ± 2.35 μg/mL for VMHE (Table 1, Fig. 1).
Oral Glucose Tolerance Test
The oral glucose tolerance test in mice was performed for the two hydroalcoholic extracts to observe their influence on the body’s ability to process glucose and to determine their effective dose. Hydroalcoholic extracts significantly decrease (p < 0.05) blood glucose in mice with glucose-induced hyperglycemia by oral gavage, at the following doses: 200 mg/kg b.w. for HMHE and 100 mg/kg b.w. for VMHE (Table 2).
Changes in Blood Glucose after 35 Days of Experiment
In the untreated STZ-ID group, blood glucose increased from 92.40 ± 10.36 mg/dL, before the administration of the substance that causes diabetes, to 358.60 ± 27.44 mg/dL (p < 0.01), after 35 days of experiment. In the control (saline) group, after 35 days, glycemia varied insignificantly (from 92.20 ± 6.76 mg/dL to 99.80 ± 8.35 mg/dL; p > 0.068). For positive (VMHE-treated STZ-ID) control group, blood glucose ranged from 92.20 ± 12.52 mg/dL to 174.80 ± 12.64 mg/dL (p < 0.009). For experimental (HMHE-treated STZ-ID), glycemia ranged from 102.20 ± 8.11 mg/dL to 194.80 ± 12.91 mg/dL (p < 0.009 at 28 days, but p < 0.117 at 35 days). Compared with the untreated STZ-ID group, after 35 days of experiment, the decrease of blood glucose for positive (VMHE-treated STZ-ID) control group and for experimental (HMHE-treated STZ-ID) group was statistically significant (p < 0.009 and p < 0.028, respectively). On day 35, compared to untreated STZ-ID group, blood glucose decreased for positive (VMHE-treated STZ-ID) control group and for experimental (HMHE-treated STZ-ID) group by 48.75% and 54.72%, respectively (Fig. 2).
Status of Oxidative Stress Markers after 35 Days of Experiment
Comparison of the data obtained for oxidative stress markers between the untreated STZ-ID group and control (saline) group showed different statistically significant values for GPx and MDA, and statistically insignificant values for SOD and TAC, with an increase of MDA values in the untreated STZ-ID group and an increase of TAC, SOD and GPx values in the control (saline) group (Tables 3 and 4).
VMHE exhibited antioxidant activity against oxidative stress of STZ-ID in the measured values of MDA, SOD, GPx and TAC, but it did not show statistically significant effects (cf. Tables 3, 5 and 6). The antioxidant effect of VMHE in STZ-ID was not correlated with a large amount of polyphenols such as flavonoids and polyphenolic acids (caffeic acid, chlorogenic acid) as was highlighted by the phytochemical analysis.
From analysis of the values of oxidative stress markers, HMHE against STZ-ID showed some antioxidant effect, but it was not statistically significant. Thus, the value of GPx activity was higher for experimental (HMHE-treated STZ-ID) group compared to the untreated STZ-ID group and even higher than in the control (saline) group (p = 0.053, close to statistically significant) (cf. Tables 3, 7 and 8).
The value of SOD activity was higher for experimental (HMHE-treated STZ-ID) group as compared to the untreated STZ-ID group, but lower than that in the control (saline) group. TAC was slightly increased for experimental (HMHE-treated STZ-ID) group compared to the untreated STZ-ID group, but smaller than in the control (saline) group. At the same time, the value of MDAactivity was much lower for experimental (HMHE-treated STZ-ID) compared to the untreated STZ-ID group and to the control (saline) group. Thus, HMHE has good antioxidant effect against oxidative stress in experimental STZ-ID, inducing both a decrease in the pro-oxidative effect of MDAand an increase in the activity of antioxidant enzymes. It is worth to mention that, comparing to VMHE after 35 days of experiment, HMHE decreases more significantly the pro-oxidant activity of MDA and increases more significantly the GPx activity and TAC (Table 9, Fig. 3). No animals died by the end of the experiment.
In the present study, we analyzed the activity of HMHE against oxidative stress in experimental STZ-ID mice. In animals, STZ induces experimental insulin-dependent diabetes mellitus (type 1) through its cytotoxic effects on pancreatic β-cells, via a mechanism associated with the generation of ROS [36]. It leads to a deficiency of insulin, acting as a diabetogenic agent [36]. STZ is selectively accumulated in pancreatic β-cells via the low-affinity GLUT2 glucose transporter in the plasma membrane [37]. STZ generated lipid peroxidation and DNA breaks in pancreatic islets of (Langerhans) cells [37].
Yaribeygi, et al. [3] cited a series of experimental animal studies that demonstrate the involvement of oxidative stress in diabetes and the improvement in insulin signal transduction and glucose homeostasis by using antioxidative agents. The antioxidative effect of green tea on insulin sensitivity markedly improved insulin resistance in diabetic rats [38], antioxidative properties of resveratrol reversed oxidative stress-dependent insulin resistance in diabetic rats [39], myricitrin improved insulin sensitivity and glucose homeostasis by attenuating the inflammatory responses and oxidative damage in diabetic mice [40], allyl isothiocyanate significantly increased insulin sensitivity by lowering oxidative stress and inflammatory responses in diabetic rats [41], and selenium promoted insulin sensitivity by ameliorating inflammation and oxidative stress in diabetic rats [42]. Based on the obtained results, we noted the presence of diabetes in the STZ-treated group and the lowering of blood glucose (antidiabetic effect) at the end of HMHE daily administration for 35 days. The effect of blood glucose lowering was slightly higher for HMHE as compared to that obtained upon VMHE administration.
In investigations of the pathogenesis of diabetes, emphasis is placed on studying the influence of oxidative stress and inflammation. Metabolic disorders in diabetes can lead to oxidative stress, which adversely affects insulin activity by interacting with different pathways and generating ROS as hydrogen peroxide and superoxide anion [43]. ROS can damage the β-cells of Langerhans islands in the pancreas, resulting in reduced insulin release. ROS can also activate some signaling pathways in cells, such as NF-κB and PKC [43, 44]. This activation may lead to interference with insulin signaling pathways, developing insulin resistance [43]. Hyperglycemia can increase oxidative stress through several mechanisms, including auto-oxidation of glucose, non-enzymatic glycation of proteins, and activation of the polyol pathway [45]. Increased activity of free radicals plays an important role in lipid peroxidation and protein oxidation of cellular structures, resulting in cellular alterations involved in the pathogenesis of vascular disease, which is the leading cause of diabetes related morbidity and mortality [45].
Hydrogen peroxide, superoxide radical, oxidized glutathione (GSSG), MDA, isoprostanes, carbonyls, and nitrotyrosine can be easily measured in blood plasma as biomarkers of oxidation by standardized assays [17]. From these biomarkers, we determined MDA. Increased MDAlevels in diabetics suggest that peroxidation injury may be involved in the development of diabetic complications, and it is also an indicator of the decline in the defense mechanisms of enzymatic and non-enzymatic antioxidants [46]. Oxidized lipids can produce MDA as a decomposition product and the mechanism is involved in the formation of prostaglandins, similar to polyunsaturated fatty acid endoperoxides with two or more linkages [46]. Lipid peroxidation was measured indirectly by MDA formed after lipid oxidation; thus, MDA was recognized as a good biomarker for lipid oxidation and is highly reactive.
To counteract the damaging effect of free radicals, the body has antioxidant defense mechanisms, such as SOD and CAT, copper and iron transport proteins, as well as water and lipid soluble antioxidants [47, 48]. The oxidative stress leads to inactivation of protein or enzymes such as SOD, GPx, CAT, and to reduced glutathione, and again the reduction in these proteins promotes oxidative stress [49]. SOD is a copper-dependent cytosol enzyme, while mitochondrial SOD is a manganese-dependent enzyme. SOD and CAT play an important role in cellular stress. SOD stabilizes the superoxide molecule, removing the superoxide radical by converting it into hydrogen peroxide and molecular oxygen [50, 51]. In untreated STZ-ID group, there is a decrease in SOD relative to the control (saline) group without diabetes. Such decrease in SOD activity in hyperglycemic state could be due to oxidative stress-induced inactivation. Increased hydrogen peroxide concentration for example is known to inactivate SOD, while glycosylation of SOD and/or loss of Cu2+, a cofactor required for the enzyme activity, can decrease its activity [49]. GPx is a selenium-dependent enzyme that stabilizes the peroxide molecule [52]. Selenium is a cofactor for GPx, the major detoxification enzyme for hydrogen peroxide. Plasma selenium reduction decreases serum GPx activity. GPx1 is more abundant and highly effective as a scavenger for hydrogen peroxide, and GPx4 is more active on lipid hydroperoxides. GPx does not act on fatty acids in membranes or on low-density lipoprotein (LDL) lipids [53]. In this process, glutathione disulfide (GSSG) is obtained by oxidation of reduced glutathione (GSH). The peroxisome CAT enzyme converts hydrogen peroxide into water and molecular oxygen [53]. GPx is present in all tissues, in variable amounts; it is enzymatically induced and is found in the cytosol (70%) but also in the mitochondria (30%). It is irreplaceable in the antioxidant arsenal, especially in the mitochondria, because they do not contain CAT for the metabolism of peroxide. GPx provides protection against organic hydroperoxides and helps regenerate the reduced form of vitamin C. Vitamin C is a reducing agent that can reduce and thereby neutralize ROS such as hydrogen peroxide [44].
For a compound to act as an antioxidant, it must perform two steps: (i) when present in low concentrations compared to the oxidizable substrate, it should delay or prevent the oxidation of the substrate significantly, and (ii) the resulting radical must be sufficiently stable to prevent its action as a lipid peroxidation chain propagating radical [47]. Typically, this stabilization is achieved by electron delocalization, intramolecular hydrogen bonding or by subsequent oxidation by reaction with another lipid radical [47].
In our experiments, we compared the mean values of MDA, SOD, GPx and TAC obtained in experimental (HMHE-treated STZ-ID) group with those established for positive (VMHE-treated STZ-ID) control, untreated STZ-ID, and control (saline) groups. At the end of the study after 35 days, the control (saline) group had MDA activity value of 30.98% that was lower than in the untreated STZ-ID group, which showed that the oxidative stress in diabetes mellitus intervenes in the pathogenesis of the disease. HMHE demonstrated the highest inhibitory activity of MDA as a pro-oxidant enzyme, compared with the untreated STZ-ID and control (saline) groups, by 45.89% and 21.60%, respectively.
VMHE produced much lower decrease in MDA value compared to that in the untreated STZ-ID group (7.68%).
From the group of antioxidant markers, we tested the effect of HMHE at the end of experiment (after 35 days) on the values of SOD, GPx and TAC. Cellular defense against ROS includes the activation of antioxidant enzymes, such as SOD, CAT, GPx. Our experiment evaluated the degree of activation of these enzymes, except CAT, by treatment with hydroalcoholic extracts (HMHE, VMHE) applied against oxidative stress in experimental STZ-ID mice. Hyperglycemia not only stimulates ROS production, but also attenuates the mechanisms of antioxidant enzymes by glycosylation [54, 55]. Hyperglycemia can directly increase the production of hydrogen peroxide and increase lipid peroxidation in glomeruli. Furthermore, it can produce AGEs by glycosylation of proteins in blood and tissues [56]. In our experiment, high blood glucose levels in untreated STZ-ID group were maintained at 35 days (358.60 ± 27.44 mg/dL). After 35 days in the untreated STZ-ID group, the antioxidant enzymes remained low compared to the control (saline) group at decreased values with 30.14% for SOD, 24.56% for GPx and 17.87% for TAC, which indicated the presence of chronic diabetes in test mice. Compared to untreated STZ-ID group, HMHE produced a limited increase in SOD activity (by 4.88%), much less than the value in the control (saline) group (43.41%) and that induced by VMHE (46.25% increase). In contrast, as compared to untreated STZ-ID group, the increase in GPx activity was 48.59% for experimental (HMHE-treated STZ-ID) group, 27.61% for the positive (VMHE-treated STZ-ID) control group, and 32.56% for the control (saline) group.
To evaluate the heterogeneous appearance of various classes of antioxidants, we also performed a non-specific integral analysis (TAC), which allowed complete dosing of all components with antioxidant potential in plasma. Paradoxically, TAC tests do not measure total antioxidant capacity. In general, they predominantly measure the activity of low molecular weight antioxidants, with chain breaking effect, excluding the contribution of antioxidant enzymes and metal binding proteins [57, 58]. Biological fluids contain numerous compounds with antioxidant chain breaking activity, including urate, ascorbate, bilirubin and thiols in the aqueous phase and á-tocopherol, carotenoids, and flavonoids in the lipid phase [59]. A comprehensive assessment of oxidative stress would include measuring all these antioxidants, although this would be time consuming, costly, and in some cases technically difficult [59]. In addition, many other compounds that are not normally measured as antioxidants have antioxidant activity that breaks the chain. The combined activity of all of them is evaluated in a TAC test, which could also consider some of the complex interactions that occur between antioxidants and chain breaking [59]. Overall, TAC is reduced under conditions associated with oxidative stress and administration of antioxidant chains increases antioxidant capacity. Antioxidants work at different levels, creating the possibility to regulate and limit excess free radicals or reactive species (peroxides) [57,58, – 59]. Concerning TAC activity, compared to untreated STZ-ID group, the highest increase (31.22%) was recorded for experimental (HMHE-treated STZ-ID) group, followed by 21.75% for control (saline) group and 18.16% for the positive (VMHE-treated STZ-ID) control group.
The serum contains many different antioxidants important for the maintenance of general health. These include ascorbic acid, α-tocopherol, β-carotene, uric acid, bilirubin, and albumin. In addition, antioxidant enzymes, such as GPx and SOD, are found in serum to a lesser extent [60,61, – 62]. In diabetes, increased markers of oxidative stress have been shown, while the level of vitamin C is low. Some studies have shown that antioxidant treatment, especially with vitamin C, can reduce both oxidative stress and protein glycation and can help reduce the risk of diabetic complications. The number of different antioxidant components in serum and tissues makes it relatively difficult to measure each component of separate antioxidant. In addition, because there is a cooperation between different antioxidants, evaluating one isolated from the rest, their combined action cannot be accurately reflected. The measurement of serum TAC appears to be an appropriate biochemical parameter for the assessment of the general antioxidant status resulting from the intake or production of antioxidants and their consumption by increasing the levels of oxidative stress [60, 62,63,64,65]. The antioxidant defense system has many components. A deficiency of either component can reduce the individual total antioxidant status [66]. The decreased TAC and SOD activity may be a response to increased production of hydrogen peroxide and oxygen by the autoxidation of glucose [48]. These enzymes play an important role in maintaining physiological levels of oxygen and hydrogen peroxide by hastening the dismutation of oxygen radicals and eliminating organic peroxides and hydroperoxides generated from inadvertent exposure to STZ [67].
Low molecular weight antioxidants such as minerals, vitamins, carotenoids, cofactors, glutathione, and polyphenols are crucial for antioxidative defense mechanisms of cells and organisms. Ascorbic acid (vitamin C) and tocopherol (vitamin E) are the most important low molecular weight antioxidants that cannot be synthesized by a human [68]. Plant polyphenols are secondary metabolites with strong antioxidant capabilities. Although primarily synthesized for self-defense of plants against oxidative stress [62], these compounds retain the ability to act as antioxidants outside the plant and, therefore, greatly contribute to the obtaining of drugs and the dietary properties of derived foods from plants [67, 69]. Consequently, the characterization of polyphenols as antioxidants is essential for both plant biology and human nutrition. Flavonoids, as well as many other plant polyphenols, have an ideal chemical structure for cleaning free radicals [68]. Their antioxidant properties include reactivity to a variety of ROS [67], as well as metal chelation [67,70]. Due to the high diversity of these compounds, the antioxidant properties are generally characterized by TAC. TAC methods evaluate antiradical activities using either synthetic free radicals or metal ions, such as Fe3+ or Cu2+ complexes. There are two mechanisms for phenolic antioxidant radical scavenging reactions: a transfer of hydrogen atoms from the OH phenolic group; and an electron transfer followed by a proton transfer [67].
Natural polyphenols become noticeable as potential agents for prevention and treatment of cancer, cardiovascular diseases, diabetes mellitus, aging, and neurodegenerative diseases. Polyphenols have been found to possess a variety of pharmacological effects on oxidative stress, lipid metabolism, insulin resistance, and inflammation, the most important pathological processes in the diabetes [71]. In the V. myrtillus leaves, Ștefãnescu, et al. [72] identified 21 phenolic compounds originating from four phenolic groups including hydroxycinnamic acids, flavonols (mainly quercetin derivatives), flavanols, and anthocyanins. Starting from results of the above-mentioned study [72], the phytochemical composition found by us for VMHE was highly correlated with the antioxidative stress effects of VMHE against experimental diabetes in mice.
Shikimic acid present in the leaves of H. matronalis has antioxidant effect [24]. Rimpapa, et al. [73] studied the antioxidant action of various plants in Bosnia by photolysis method and showed that H. matronalis flowers have an average antioxidant capacity of 40%. The high vitamin C content of H. matronalis may partly explain the good antioxidant action manifested in our experiment. Ascorbic acid (vitamin C) serves as a reducing agent, reducing agent, scavenge free radicals, and quench \( {\textrm{O}}_2^{\bullet -} \) [65]. At high levels of ascorbic acid (1000 mg/kg), it tends to shift the balance between ferric (Fe3+) and ferrous (Fe2+) ions and thereby scavenge the oxygen and inhibit oxidation [65]. Vitamin C can reduce both oxidative stress and protein glycation and can help reduce the risk of diabetic complications.We did not determine the amount of vitamin C in the H. matronalis leaves, but we indirectly deduced its presence in the leaves after their use in the treatment of scurvy (a disease caused by C avitaminosis), as cited in the literature [74,75,76, – 77]. The in vivo antioxidant activity of HMHE was correlated with the quantified phytochemical composition of hydroalcoholic extract, namely total polyphenols amount of 237.72 ± 4.71 mg/L gallic acid equivalents (GAE), total flavonoids content of 129.34 ± 2.58 mg/L quercetin equivalents (QE) and 7.44 ± 0.16 μg/mL caffeic acid; the values of three above-mentioned phytochemical parameters for HMHE are smaller than for VMHE (433.89 ± 8.67 mg/L GAE, 154.38 ± 3.08 mg/L QE, and 116.92 ± 2.35 μg/mL caffeic acid, respectively). The in vitro antioxidant capacity of HMHE and VMHE as highlighted by analysis of the total polyphenols and total flavonoids contents showed that VMHE had higher total polyphenols content than HMHE. The hypoglycemic effect of HMHE was in positive correlation with the antioxidant action and phytochemical composition of H. matronalis flowering aerial parts.
Caffeic acid is a naturally occurring phenolic compound commonly found in many herbs, fruits, vegetables [78]. It exhibits different pharmacological activities, such as antidiabetic, anti-inflammatory, antioxidant [78]. Caffeic acid has been shown to lower blood sugar and glycosylated hemoglobin. Also, plasma insulin, C-peptide, and leptin levels in the caffeic acid group were significantly higher than those of the control group, whereas the plasma glucagon level was lower on the experimental model in db/db mice [78, 79]. Caffeic acid also markedly increased glucokinase activity and glycogen content and simultaneously lowered glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities, accompanied by a reduction in the GLUT2 expression in the liver [78]. Caffeic acid acts through different targets regulating the glucose metabolism. It elevates the adenosine monophosphate-activated protein kinase (AMPK) level in skeletal muscles, liver, adipocytes and GLUT4 translocation and expression in skeletal muscles thus stimulating glucose utilization [78]. It also suppresses the glucose output from liver. Caffeic acid inhibits the differentiation and adipogenesis. In addition, it also helps to increase the exogenous antioxidants, i.e., SOD, CAT, thiobarbituric acid (TBA), glutathione reductase (GRx) and thus helps in reducing oxidative stress [78]. Matboli, et al. [80] postulated that caffeic acid modulates autophagy pathway through inhibition of autophagy regulatory micro-ribonucleic acids (miRNAs), that could explain its curative properties against diabetic kidney disease. In our study, we found that both HMHE and VMHE contain caffeic acid in an amount of 7.44 ± 0.16 μg/mL and 116.92 ± 2.35 μg/mL, respectively, which is a natural compound responsible for other mechanisms that can support antidiabetic and antioxidative action.
Thus, our results regarding the antioxidative stress action of H. matronalis are quite conclusive due to significant differences in GPx and TAC between untreated STZ-ID and experimental (HMHE-treated STZ-ID) groups. It is also worth noting the insignificant difference in the values of MDA, SOD, GPx activity and TAC between experimental (HMHE-treated STZ-ID) and control (saline) groups. This fact demonstrates that the activity of enzymes involved in oxidative stress approaches normal in diabetic animals treated with HMHE.
MATERIALS AND METHODS
Plant Materials
The plant materials (aerial parts of H. matronalis L. and leaves of V. myrtillus L.) were collected during the flowering period, in June 2019, from the Alexandru Buia Botanical Garden, University of Craiova, Romania. Our research did not involve endangered or protected species. Voucher specimens (HM-1032019 and VM-1052019, respectively) were also deposited in the Herbarium of the Department of Pharmacognosy and Phytotherapy, Faculty of Pharmacy, University of Medicine and Pharmacy of Craiova.
Preparation of Hydroalcoholic Extracts
Samples of accurately weighed, air-dried, and powdered plant materials have been macerated for 14 days with diluted alcohol (70% ethanol), at room temperature, according to the Romanian Pharmacopoeia Xth Edition [81]. HMHE and VMHE (20% hydroalcoholic extracts) were filtered and then stored in dark bottles, in the refrigerator, until use. We used hydroalcoholic extracts prepared according to the official standards considering that, for the leaves of V. myrtillus species, such extracts are studied by many specialty articles [29, 30, 34, 46, 72].
UHPLC–UV–MS Analysis of Hydroalcoholic Extracts
3.3.1. Solvents and standard compounds. Solvents (acetonitrile, formic acid, methanol, water) of chromatographic purity (LiChrosolv®, Merck Millipore, Darmstadt, Germany) were used for analysis. Standard polyphenolic compounds were purchased from Merck Millipore, Sigma-Aldrich (Taufkirchen, Germany) and Alfa Aesar (Kandel, Germany).
3.3.2. Preparation of standard solutions. The standard stock solutions were obtained by dissolving 8 mg of each reference compound (caffeic acid, chlorogenic acid, epicatechin, ferulic acid, p-coumaric acid, protocatechuic acid, and quercetin) in 100 mL methanol. The stock solutions were kept refrigerated at 4°C until use. The calibration curve concentrations were obtained by diluting the previous mentioned stock solutions.
3.3.3. Preparation of sample solutions. HMHE and VMHE (20% hydroalcoholic extracts) were diluted five times, then filtered using 0.2 μm syringe filters and transferred to autosampler vials to be used as such, prior the UHPLC–UV–MS analysis with the first line of the mobile phase gradient.
3.3.4. UHPLC–UV–MS analysis. The separation of polyphenols was carried out on Waters (Milford, Massachusetts, USA) Arc System coupled with Waters 2998 photodiode array (PDA) detector and Waters QDa mass detector equipped with an electrospray ionization (ESI) source. The equipment was controlled using EmPower 3 (Waters) software package. The system used Waters CORTECS C18 (4.6 × 50 mm, 2.7 μm) column eluted with solvent A (0.1% formic acid in water), solvent B (0.1% formic acid in methanol), and solvent C (0.1% formic acid in acetonitrile). Solvent B was set at 1% during the entire separation. The gradient was as follows: 0 – 4 min, 3% to 14% C; 4 – 9 min, 14% to 39%C; 9 – 11 min, 29% to 3% C. The flow rate of the mobile phase was set at 1.0 mL/min. The column temperature was equilibrated to 35°C. Five μL of each sample was injected into the column. All samples were kept at 20°C during the entire analysis [82].
The chromatograms were obtained using the PDA detector at 280 nm. Eluted compounds were analyzed using the QDa mass detector with ESI source. Capillary voltage was maintained at 0.8 kV, cone voltage was kept at 20 V, and the mass spectra were recorded in negative ion mode in the range 100 – 800 m/z. Quantification was established in the selective ion recording (SIR) mode for each compound (Table 1, Figs. 4 and 5) using external calibration curves prepared for each reference compound.
Animals and Experimental Protocol
Swiss albino breed mice, mean 6 weeks old males, were used for experimental purposes. The animals were housed in plastic cages in a climate-controlled environment (19 – 23°C), well ventilated, with a 12 h light/dark cycle (light from 8 a.m. to 8 p.m.) and had an acclimatization period of seven days. Test mice had distinct marking and were numbered separately in each batch. The animals were fed with standard diet (granulated combination feed, complete feed for mice, rats or hamsters used for scientific research, from the Cantacuzino National Institute for Medical Research, Bãneasa Station, Bucharest, Romania) and water ad libitum.
Experiments were carried out in accordance with the rules of the Commission for Animal Welfare and with the rules and approval of the Ethics Committee of the University of Medicine and Pharmacy of Craiova (Approval No. 9/March 28, 2018). European Council Directive No. 86/609/EEC on the protection of laboratory animals used for experimental or other scientific purposes and Directive 2010/63/EU (revising Directive 86/609/EEC on animal protection) were also respected. The Directives are firmly based on the ‘three R’ principles to replace, reduce and refine the use of animals for scientific purposes.
Determination of the effective dose of 20% hydroalcoholic extracts was performed using the oral glucose tolerance test in mice with normal pancreatic function, according to a recent (2020) validated protocol [83]. The mice were divided into three groups: three mice for control group, and nine mice for each of the HMHE and VMHE groups. In turn, HMHE and VMHE groups were subsequently divided in three subgroups, each of three animals. By oral gavage, the control group was treated with saline and received 2 g/kg b.w. glucose. Also, by oral gavage, the hydroalcoholic extracts were administered to mice without anesthesia, 30 minutes before administration of glucose (2 g/kg b.w.). The animals from the two experimental groups (HMHE and VMHE) were pretreated by oral gavage with the corresponding hydroalcoholic extract, but in three different doses for each of the three subgroups: 100 mg/kg b.w., 150 mg/kg b.w., and 200 mg/kg b.w. The effective dose of 200 mg/kg b.w. for HMHE and 100 mg/kg b.w. for VMHE, respectively, represents 1/10 of the LD50 for the hydroalcoholic extracts.
To investigate the effect of HMHE against oxidative stress in experimental STZ-ID, mice were divided into four groups, each of five animals, of which one group was taken as a control and was treated with 0.9% saline. For the other three groups, STZ-induced type 1 diabetes was achieved by intraperitoneally administration of a single dose of 180 mg/kg b.w. STZ, without exceeding 1 mL/kg b.w. solution. The second group, with STZ-ID, remained untreated. The third group, positive control, was treated daily by oral gavage, for 35 days, with effective dose of 100 mg/kg b.w. VMHE. The fourth group was treated daily by oral gavage, with effective dose of 200 mg/kg b.w. HMHE. Hyperglycemia was confirmed in all three groups at day 3 and day 7 after STZ administration. The experiment lasted 35 days (five weeks).
To determine the action of HMHE against oxidative stress, in experimental model of STZ-ID, at the end of the study (after 35 days), the levels of oxidative stress markers (MDA, SOD, GPx and TAC) for experimental (HMHE-treated STZ-ID) group were compared to those obtained for control (saline), untreated STZ-ID and positive (VMHE-treated STZ-ID) control groups.
Body weight, blood sugar, cholesterol, triglycerides, food, and water intake were monitored during the experiment, the results of which are not covered by this report.
Obtaining and Preparing Blood Samples
For the oral glucose tolerance test, blood was obtained at 30, 60, 90, and 120 min. To determine the HMHE effect against oxidative stress in experimental STZ-ID, blood was collected at the end of the treatment (day 35). Mice were placed under anesthesia with 60 mg/kg i.p. pentobarbital sodium and intracardiac blood samples were rapidly collected in syringes containing ethylenediaminetetraacetic acid (EDTA). To obtain serum from a portion of the blood samples, they were placed in gel tubes; the remaining portion was placed in heparin tubes to remove plasma. Blood samples in gel tubes were immediately placed in a cooled centrifuge at +4°C and processed at 3000 rpm for 10 min to obtain serum samples. Blood samples in heparin tubes were centrifuged at +4°C, with 1000 rpm for 30 minutes, to separate plasma. The as-prepared serum and plasma samples were stored in a freezer at –20°C until analysis. Blood glucose level [mg/dL] was quantified using a glucometer (Accu-Check, Roche Diagnostics, Basel, Switzerland).
Blood samples were taken in EDTA treated tubes for MDA test, hemolyzed blood was obtained for SOD analysis, heparinized blood for GPx assay, serum was used for TAC determination. The animals were sacrificed for histopathological analysis of organs. Determination of the oxidative stress parameters from the collected biological samples were performed in the Department of Biochemistry, Faculty of Pharmacy, University of Medicine and Pharmacy of Craiova, using commecially available kits according to the manufacturers’ instructions.
MDA values [ng/mL] were determined using enzyme-linked immunosorbent assay (ELISA). The blood was collected in EDTA tubes. The samples were centrifuged for 15 min at 1000 g, +4°C, within 30 min of harvesting, and the separated plasma was stored at -20°C until the analysis for MDA.
Determination of SOD activity [U/mL] was performed with a reagent kit produced by Randox Laboratories for in vitro assessment of SOD activity in whole blood. The measurements were made with a Beckman DU-65 UV-VIS spectrophotometer equipped with a thermostat system. SOD activity was determined by measuring the degree of inhibition of the reaction resulting from formazan dye. A SOD unit is the one that causes a 50% inhibition of the rate of reduction of iodonitrotetrazolium (INT) under the reaction conditions. To obtain the hemolysate, 0.5 mL of whole blood was centrifuged at 3000 rpm for 10 min and the plasma was aspirated. Erythrocytes were washed three times with saline and centrifuged at 3000 rpm for 10 min after each wash. Finally, erythrocytes were resuspended in cold bidistilled water and then kept for 15 min at +4°C to produce hemolysis. SOD enzymatic activity was expressed as U/mg protein (one unit of enzyme is defined as the enzyme activity that inhibits autoxidation of pyrogallol by 50%).
Quantification of GPx activity [U/mL] was performed with the usage of a kit produced by Randox Laboratories for in vitro determination of GPx activity in whole blood. The measurements were performed with the Beckman DU-65 UV-VIS spectrophotometer. GPx enzymatic activity was calculated as nanomoles of nicotinamide adenine dinucleotide phosphate reduced form (NADPH) oxidized/min/mg protein, using molar extinction coefficient of 6.22 × 103/M/cm.
TAC was determined using a kit produced by Randox Laboratories (NX2332) for in vitro analysis of serum, plasma, and other fluids. The measurements were performed with the same spectrophotometer. TAC was expressed in mmol/mL [34]. All methods were those used in this laboratory for any oxidative stress studies. All reagents and solvents were purchased from Merck (Darmstadt, Germany).
Statistical Data Analysis
Statistical analysis was performed using a dedicated software: IBM Statistical Package for Social Sciences (SPSS) ver. 23. For the descriptive analysis of the groups, the mean, the minimum and maximum value, and the standard deviation (SD) were used. For data comparison, the Z-score with 95% specificity threshold (p < 0.05) was used, and this was calculated by the following nonparametric tests: Mann–Whitney U-test, when independent data series were compared and Wilcoxon W-test, when comparisons were made between measurements from the same subjects.
CONCLUSION
We did not find, in the scientific literature, any study regarding some pharmacological properties of H. matronalis, although in some countries it is a known decorative plant, and in folk medicine it was used for some therapeutic qualities, such as scurvy. That is why our original study represent the first pharmacological research concerning the hydroalcoholic extract of H. matronalis aerial parts.
HMHE showed antioxidative-stress effects, decreasing MDA activity, as a pro-oxidant enzyme, and increasing the activity of the enzymatic and non-enzymatic antioxidant enzymes (SOD, GPx and TAC). The antioxidative stress effect was correlated with decreased blood sugar in chronic STZ-ID. The small number of determinations (due to the small number of animals in the groups) did not allow us to find statistically significant values for experimental (HMHE-treated STZ-ID) group, but also for the control (saline) group and for the positive (VMHE-treated STZ-ID) control group.
Considering the content of flavonoids and phenolic acids, which are responsible for hypoglycemic and antioxidant properties of H. matronalis flowering aerial parts, HMHE could be recommended as a source of natural polyphenols with an adjunctive role in the prophylaxis and treatment of diseases caused by the presence of ROS, such as diabetes. We consider that this study is preliminary, research on animals larger than mice being necessary for an adequate statistical analysis to highlight even more clearly the antioxidant action of H. matronalis species.
LIMITATIONS OF THE STUDY
The blood samples were too few for adequate statistical processing. The smaller number of TAC samples was due to insufficient blood, although the number of animals was equal until the end of experiment. That is why we consider this study prior to a more in-depth study with a larger number of greater animals (rats) that would allow us to draw more accurate conclusions. Because of the same inconveniences (animals too small for the required blood amount), we did not perform the CAT analysis either.
References
O. M. Ighodaro, Biomed. Pharmacother., 108, 656 – 662 (2018).
P. Newsholme, V. F. Cruzat, K. N. Keane, et al., Biochem. J., 473, 4527 – 4550 (2016).
H. Yaribeygi, T. Sathyapalan, S. L. Atkin, and A. Sahebkar, Oxid. Med. Cell. Longev., 2020, 8609213 (2020).
V. R. Pasupuleti, C. S. Arigela, S. H. Gan, et al., Oxid. Med. Cell. Longev., 2020, 8878172 (2020).
V. Saio, D. Syiem, and R. Sharma, J. Basic Clin. Pharm., 3, 249 – 254 (2012).
P. Newsholme, K. N. Keane, R. Carlessi, and V. Cruzat, Am. J. Physiol. Cell Physiol., 317, C420 – C433 (2019).
U. Asmat, K. Abad, and K. Ismail, Saudi Pharm. J., 24, 547 – 553 (2016).
H. Yang, X. Jin, C. W. Kei Lam, and S. K. Yan, Clin. Chem. Lab. Med., 49, 1773 – 1782 (2011).
P. Malko and L. H. Jiang, Redox Biol., 37, 101755 (2020).
A. C. Maritim, R. A. Sanders, and J. B. Watkins, J. Biochem. Mol. Toxicol., 17, 24 – 38 (2003).
P. Wang, Y. C. Lu, Y. F. Li, et al., J. Diabetes Res., 2018, 3274084 (2018).
J. M. Forbes and M. E. Cooper, Physiol. Rev., 93, 137–188 (2013).
N. C. Chilelli, S. Burlina, and A. Lapolla, Nutr. Metab. Cardiovasc. Dis., 23, 913–919 (2013).
L. Zhang, B. Chen, and L. Tang, Diabetes Res. Clin. Pract., 96, 283–293 (2012).
A. Hosseini and M. Abdollahi, Oxid. Med. Cell. Longev., 2013, 168039 (2013).
S. Singh, V. Garg, and D. Yadav, Int. J. Pharm. Pharmaceut. Sci., 5, 297–302 (2013).
E. Birben, U. M. Sahiner, C. Sackesen, et al., World Allergy Organ J., 5, 9 – 19 (2012).
K. K. Dennis, Y. M. Go, and D. P. Jones, J. Nutr., 149, 553 – 565 (2019).
D. P. Jones, Am. J. Physiol. Cell Physiol., 295, C849 – C868 (2008).
J. Bouayed and T. Bohn, Oxid. Med. Cell. Longev., 3, 228 – 237 (2010).
M. Ben Salah, A. Hafedh, and A. Manef, J. Mater. Environ. Sci., 8, 1359 – 1364 (2017).
T. Mandáková, P. Hloušková, D. A. German, and M. A. Lysak, Plant Physiol., 174, 2062 – 2071 (2017).
C. Guarino, L. De Simone, and S. Santoro, Ethnobot. Res. Appl., 6, 255 – 317 (2008).
Natural Medicine Facts. Available online: https://www.naturalmedicinefacts.info/plant/hesperis-matronalis.html (accessed on 14 March 2021).
P. W. Ball, in: Flora Europaea: Psilotaceae to Platanaceae, 2nd edn, T. G. Tutin, N. A. Burges, A. O. Chater, et al. (eds.), Cambridge University Press: Cambridge, UK (1993), Vol. 1, pp. 336 – 337.
Atlas Florae Europaea. Distribution of vascular plants in Europe (Cruciferae: Sisymbrium to Aubrieta), J. Jalas and J. Suominen (eds.), Helsinki University Printing House: Helsinki (1994), Vol. 10, pp. 115 – 148.
C. J. Majetic, R. A. Raguso, and T. L. Ashman, Ann. Bot., 102, 911 – 922 (2008).
A. Francis, P. B. Cavers, and S. I. Warwick, Can. J. Plant Sci., 89, 191 – 206 (2009).
S. Brasanac-Vukanovic, J. Mutic, D. M. Stankovic, et al., Molecules, 23, 1864 (2018).
K. Bljajiæ, R. Petlevski, L. Vujiæ, et al., Molecules, 22, 703 (2017).
B. Salehi, A. Ata, N. V. Anil Kumar, et al., Biomolecules, 9, 551 (2019).
A. Hameed, M. Galli, E. Adamska-Patruno, et al., Nutrients, 12, 2538 (2020).
B. E. Ștefãnescu, K. Szabo, A. Mocan, and G. Crişan, Molecules, 24, 2046 (2019).
C. Ancillotti, L. Ciofi, D. Pucci, et al., Food Chem., 204, 176 – 184 (2016).
Organization for Economic Co-operation and Development (OECD). Available online: https://www.oecd.org/env/ehs/risk-management/37182285.pdf (accessed on 12 January 2020).
D. A. T. Almeida, C. P. Braga, E. L. B. Novelli, and A. A. H. Fernandes, Braz. Arch. Biol. Technol., 55, 527 – 536 (2012).
S. Lenzen, Diabetologia, 51, 216 – 226 (2008).
I. Hininger-Favier, R. Benaraba, S. Coves, et al., J. Am. Coll. Nutr., 28, 355 – 361 (2009).
P. K. Bagul, H. Middela, S. Matapally, et al., Pharmacol. Res., 66, 260 – 268 (2012).
B. Zhang, Q. Shen, Y. Chen, et al., Sci. Rep., 7, 44239 (2017).
N. Sahin, C. Orhan, F. Erten, et al., J. Biochem. Mol. Toxicol., 33, e22328 (2019).
S. A. Abdulmalekand M. Balbaa, PLoS One, 14, e0220779 (2019).
X. Ma, Z. Chen, L. Wang, et al., Front. Pharmacol., 9, 782 (2018).
E. B. Kurutas, Nutr. J., 15, 71 (2016).
J. E. Ghoul, M. Smiri, S. Ghrab, et al., Pathophysiology, 19, 35–42 (2012).
L. T. Rotaru, R. M. Varut, M. Banicioiu Covei, et al., Rev. Chim. (Bucharest), 69, 1860 – 1865 (2018).
A. K. Verma and R. Pratap, Nat. Prod. Rep., 27, 1571 – 1593 (2010).
B. Halliwell, R. Aeschbach, J. Löliger, and O. I. Aruoma, Food Chem. Toxicol., 33, 601 – 617 (1995).
O. O. Oguntibeju, Int. J. Physiol. Pathophysiol. Pharmacol., 11, 45 – 63 (2019).
Manisha, W. Hasan, R. Rajak, and D. Jat, Int. J. Adv. Res. Rev., 2, 110 – 119 (2017).
V. N. Shilpa, N. Rajasekaran, V. K. Gopalakrishnan, and K. Devaki, J. Appl. Pharmaceut. Sci., 2, 60 – 65 (2012).
E. Lubos, J. Loscalzo, and D. E. Handy, Antioxid. Redox Signal., 15, 1957 – 1997 (2011).
V. Lobo, A. Patil, A. Phatak, and N. Chandra, Pharmacogn. Rev., 4, 118 – 126 (2010).
G. Paolisso, A. D’Amore, D. Galzerano, et al., Diabetes Care, 16, 1433 – 1437 (1993).
M. Mandal, A. Varghese, V. K. Gaviraju, et al., Clin. Diabetol., 8, 215 – 222 (2019).
G. Vessal, M. Akmali, P. Najafi, et al., Ren. Fail., 32, 733 – 739 (2010).
C. I. Sajeeth, P. K. Manna, and R. Manavalan, Pharm. Sin., 2, 220 – 226 (2011).
S. S. Chung, M. Kim, B. S. Youn, et al., Mol. Cell. Biol., 29, 20 – 30 (2009).
I. S. Young, J. Clin. Pathol., 54, 339 (2001).
G. Cao, H. M. Alessio, and R. G. Cutler, Free Radic. Biol. Med., 14, 303 – 311 (1993).
I. L. C. Chapple, J. Clin. Periodontol., 24, 287 – 296 (1997).
I. L. C. Chapple, G. I. Mason, I. Garner, et al., Ann. Clin. Biochem., 34, 412 – 421 (1997).
G. Cao and R. L. Prior, Clin. Chem., 44, 1309 – 1315 (1998).
P. Pinzani, E. Petruzzi, C. Orlando, et al., J. Biolumin. Chemilumin., 13, 321 – 325 (1998).
A. Nemec, M. Drobniè-Košorok, M. Skitek, et al., Acta Vet. Brno, 69, 297 – 303 (2000).
E. Bobescu, Rev. Rom. Med. Lab., 8, 13 – 19 (2007).
K. Csepregi, S. Neugart, M. Schreiner, and É. Hideg, Molecules, 21, 208 (2016).
B. L. Tan, M. E. Norhaizan, W. P. Liew, and H. Sulaiman Rahman, Front. Pharmacol., 9, 1162 (2018).
B. H. Havsteen, Pharmacol. Ther., 96, 67 – 202 (2002).
C. Rice-Evans, N. Miller, and G. Paganga, Trends Plant Sci., 2, 152 – 159 (1997).
S. Li, H. Y. Tan, N. Wang, et al., Oxid. Med. Cell. Longev., 2018, 8394818 (2018).
B. E. Ștefãnescu, L. F. Cãlinoiu, F. Ranga, et al., Antioxidants (Basel), 9, 495 (2020).
Z. Rimpapa, F. Korac, J. Toromanovic, et al., Planta Med., 74, PC61 (2008).
University of Ioanina, School of Health Sciences, Faculty of Medicine, Department of Pharmacology. Available online: http: //mediplantepirus.med.uoi.gr/pharmacology en/plant details.php?id=222 (accessed on 26 February 2021).
Wildflowers West, Available online: https://wildflowerswest.org/hesperis matronalis.htm (accessed on 26 February 2021).
Herbs-Treat and Taste. Available online: https://herbs-treatandtaste.blogspot.com/2012/06/dames-rocket-or-sweetrocket-symbol-of.html (accessed on 26 February 2021).
Mother Earth Living. Available online: https://www.motherearthliving.com/plant-profile/an-herb-to-know-dames-rocket (accessed on 26 February 2021).
M. Yusuf, M. Nasiruddin, N. Sultana, et al., Res. J. Pharm. Technol., 12, 4735 – 4740 (2019).
U. J. Jung, M. K. Lee, Y. B. Park, et al., J. Pharmacol. Exp. Ther., 318, 476 – 483 (2006).
M. Matboli, S. Eissa, D. Ibrahim, et al., Sci. Rep., 7, 2263 (2017).
Romanian Pharmacopoeia Xth Edition, National Agency for Medicines and Medical Devices of Romania, Medical Publishing House, Bucharest (1993), pp. 335, 921, 922.
J. Hokkanen, S. Mattila, L. Jaakola, et al., J. Agric. Food Chem., 57, 9437 – 9447 (2009).
R. Benedé-Ubieto, O. Estévez-Vázquez, P. Ramadori, et al., Diabetes Metab. Syndr. Obes., 13, 439 – 450 (2020).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kostici, R., Pisoschi, C.G., Popescu, F. et al. Antioxidant Action of Hesperis matronalis L. in Chronic Experimental Diabetes. Pharm Chem J 56, 1092–1106 (2022). https://doi.org/10.1007/s11094-022-02759-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02759-z