The major bioactive constituents in extracts from roots of Eleutherococcus senticosus (Rupr. & Maxim) (Araliaceae) and in a multi-adaptogen herbal formulation (Multiphytoadaptogen) were analyzed using high-performance liquid chromatography (HPLC) in combination with tandem mass spectrometry. Chromatography was performed on an ACQUITY UPLC BEH C18 column in gradient mode. A TSQ Vantage triple quadrupole mass spectrometer with electrospray ionization was used for the analysis. Eleutherosides B (syringin, a phenylpropanoid) and E (syringaresinol diglucoside, a lignan) were identified in both the multi-adaptogen herbal formulation and E. senticosus root extract. The results could be used for standardization and quality testing of herbal formulations including eleutherosides B and E and for justification of the biological action of Multiphytoadaptogen and studies of its new properties considering the identified bioactive constituents. The probable mechanisms of the antitumor and additive/synergistic effects of eleutherosides B and E were established by in silico analysis of their biological activity profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The term adaptogens was introduced into the scientific literature in the 1950s by the Leningrad pharmacologist Prof. N. V. Lazarev. It characterizes the physiological mechanisms of action of natural compounds and medicinal plants such as phytoadaptogens that increase the nonspecific resistance of an organism to various types of stressors, including antitumor resistance. Classical phytoadaptogens include genuine ginseng Panax ginseng, Rhodiola rosea, Aralia mandshurica, Eleutherococcus senticosus, Oplopanax elatus, Schizandra chinensis, etc. However, the use of individual phytoadaptogens is often limited by tolerance to the drug that develops over time. Therefore, research on phytocomplexes based on the principle of rational combination of biologically active substances and the creation of unique synergistic effects that cannot be produced using separate phytoadaptogens is scientifically justified and relevant. Moreover, an organism can be affected without causing tolerance if several adaptogens are used in a single pharmaceutical formulation. In addition, both standardization and validation of the pharmacological activity of multicomponent phytoadaptogens considering their chemical composition are pressing problems [1,2,3,4,5, – 6].
The pharmaceutical formulation Multiphytoadaptogen (MPA) for preventive oncology and gerontology was developed at N. N. Blokhin National Medical Research Center of Oncology [7]. MPA contains constituents of extracts from 40 official plants, including the adaptogens ginseng, Aralia, Eleutherococcus, Rhodiola rosea, Oplopanax, and Schizandra. MPA was shown to be effective in preventive oncology. Preventive oncology comprises primary (prevention of the generation or chemoprevention); secondary (prevention of recidivism and tumor metastases); and tertiary (prevention of side effects of chemotherapy and radiation therapy) prevention of oncological diseases [3]. The antitumor and protective effects are certainly the main properties that drugs for preventive oncology should possess. Experimental and clinical research on MPA found antimutagenic (which is important for primary prevention of cancer), radioprotective, hormone- modulating, antioxidant, neuroprotective, and immunomodulating, including adhesiogenic effects (which are important for tertiary prevention of cancer) [7,8,9,10,11,12,13,14,15,16,17, – 18]. Undoubtedly, the efficacy of MPA is due to a complex of biologically active compounds (BACs) included in its composition.
Research on the BACs of the components of MPA is being conducted to evaluate the potential for quality control and standardization of the formulation. For example, polyphenolic compounds, essential oils, amino acids, and vitamins were detected in MPA using reversed-phase high-performance liquid chromatography (HPLC) with UV detection, GC-MS, and NMR spectroscopy [19,20, – 21]. In addition, HPLC in combination with tandem mass spectrometry (HPLC-MS/MS) identified the major BACs among the MPA components, particularly ginseng and aralia, as triterpene saponins, e.g., ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Rg2, Re, Rf, and Ro and aralosides A, B, and C [22, 23]. The phenylethanol glycoside salidroside, phenylpropanoid glycosides rosavin and rosarin, monoterpene glycoside rosiridin, and the flavonoid rhodionin were also determined as constituent BACs of Rhodiola rosea [24].
The next stage of the analysis of the constituent composition of MPA was HPLC-MS/MS determination of BACs of the extract from rhizomes and roots of E. senticosus included in the MPA formulation. This method is highly specific and accurate and enables the determination of minimal quantities of compounds.
E. senticosus (Rupr. & Maxim) Maxim (Araliaceae, wild pepper) is a bush with fruit clustered into large black balls. Its medicinal properties are close to those of ginseng. Therefore, it is sometimes called Siberian ginseng. It is distributed in Japan, northern China, and Manchuria. It grows in Russia in Primorsky and Khabarovsk Krais, Amur Region, and southern Sakhalin. Total BACs from rhizomes and roots of Eleutherococcus include eleutherosides belonging to various classes of chemical compounds, e.g. sterols (eleutheroside A), phenylpropanoids (eleutheroside B), coumarins (eleutheroside B1), lignans (eleutherosides D and E), and triterpene saponins (eleutherosides K, L, and M). Furthermore, rhizomes and roots of this plant contain essential oils, anthocyans, chromones, flavonoids, resins, lipids, pectinic substances, free sugars and polysaccharides, and the alkaloid aralin. The contents of eleutherosides in rhizomes and roots were greatest in late autumn after fruiting, decreased in spring, and fell sharply in July and during flowering. The effects of Eleutherococcus preparations are largely like those of ginseng but milder. The extract exhibits antioxidant, immunomodulating, cardiotonic, hypoglycemic, tonic, gonadotropic, antistressor, and general strengthening properties. Eleutherococcus preparations in experiments inhibited the growth of malignant tumors. They were recommended for serious physical stresses and radiation illness and for reduction of the toxicity of x-ray and radiation exposure during rehabilitation after serious diseases and operations. They increase the acuity of hearing and vision and improve memory. Muscular activity under the influence of Eleutherococcus strengthened because of lower losses of carbohydrate energy sources due to earlier inclusion in lipid metabolism [25,26, – 27].
The aim of the present study was to identify BACs of Eleutherococcus in MPA using HPLC-MS/MS and to evaluate the biological activity profile of the identified phytoconstituents.
EXPERIMENTAL PART
Two MPA samples and extracts from rhizomes and roots of Eleutherococcus included in the MPA formulation were studied in the work. Samples of the extracts were obtained using certified raw material and the same technology (raw material specific weight, extraction temperature and time regimes, extractant composition, raw-material:extractant ratio).
An MPA sample was mixed with MeOH in a 1:2 ratio and centrifuged for 5 min at 13,000 rpm. The supernatant liquid was passed through a 0.22-μm filter and centrifuged for 1 min at 13,000 rpm.
An aliquot (1 mL) of the Eleutherococcus extract was evaporated in a Concentrator 5301 rotary evaporator (Eppendorf, Germany) to dryness at 30°C. The residue was dissolved in MeOH (100 μL) and centrifuged for 1 min at 13,000 rpm.
Samples were analyzed using a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific) connected to an Accela HPLC chromatograph.
The chromatographic analytical conditions were ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters); mobile phase composition: phase A, 100% H2O and formic acid (FA, 0.1%); phase B, MeCN (95%), H2O (5%), and FA (0.1%).
Extracts were analyzed using gradient elution by mobile phase (%B) 0 – 68 min (0 – 60%), 68 – 70 min (60 – 100%), 70 – 75 min (100%), 75 – 80 min (0%). Samples (5 μL) were injected into a 25-μL injector loop (mobile phase, 20 μL). The flow rate was 450 μL/min.
Electrospray ionization was used in negative-ion mode; spray capillary potential, 4 kV; gas (spray), 60 psi; bypass gas, 15 rel. units; capillary temperature, 270°C. Spectra of total ion scans and selected ion monitoring (SIM) were taken in the range 150 – 1500 Da with scan time 0.1 sec.
Mass spectra were obtained by direct sample introduction through a syringe at 5 μL/min. The gas pressure in the collision chamber was 0.9 Torr. The potential in the collision chamber was selected separately for each compound.
Probable spectra of BACs were calculated using the Prediction of Activity Spectra for Substances (PASS) computer program. PASS Refined 2020 allowed 1945 types of biological activity to be predicted with an average accuracy of 97% [28]. The PASS algorithm was based on the naïve Bayesian classifier and represented the structures of chemical compounds as multilevel neighborhoods of atoms (MNA) descriptors [28]. The calculations yielded a list of activities for each compound with the corresponding probable evaluations: Pa, probability of activity and Pi, probability of inactivity. All activities for which the calculated Pa values exceeded the Pi values were considered probable [29].
It is noteworthy that natural compounds were considered in the present work. This group of compounds had several structural features that differentiated them from synthetic compounds [30]. Therefore, the question arises whether this group falls within the application area of PASS. The last is determined by the MNA set, i.e., the training set descriptors. The accuracy of the prediction was shown to decrease if more than four new descriptors are observed [31]. New descriptors were not observed for the chemical compounds examined in the present work. Therefore, the studied compounds correspond to the PASS application area. PASS was already successfully used earlier to assess the biological activity of individual natural compounds and to analyze the additive/ synergistic action of pharmaceutical formulations [24, 32,33,34,35].
The additive/synergistic action of the studied chemical compounds was analyzed using the PharmaExpert computer program, which is based on a knowledge base containing information on greater than 15,000 known interactions between mechanisms of action and pharmacological effects [32].
RESULTS AND DISCUSSION
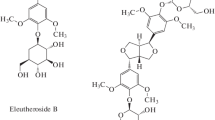
An analysis of literature data on the chemical composition of Eleutherococcus roots allowed the most important BACs (standard markers) of this plant to be identified as eleutherosides B and E. Table 1 presents their molecular weights and structural formulas.
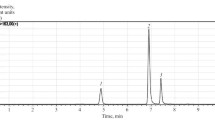
The major BACs in the extract of Eleutherococcus included in the MPA formulation were studied using a chromatogram of the extract taken in total ion scanning mode (Fig. 1).
TABLE 2 presents the results from tandem mass spectrometry of the Eleutherococcus extract (Rt, m/z for the main molecular ion and it fragments) and the molecular weight of the compound according to literature data.
TABLE 2 shows that the studied Eleutherococcus compounds formed ion adducts [M + FA – H]+ with FA included in the mobile phase. Eleutheroside B (syringin) corresponded to one of the peaks with m/z 417 and retention time Rt = 11.2 min. Eleutheroside E (syringaresinol diglucoside) corresponded to one of the peaks with m/z 787 and retention time Rt = 19.4 min.
Thus, the major BACs of Eleutherococcus, primarily eleutherosides B and E, were identified in its extract.
The chromatogram of MPA with respect to contents of eleutherosides was analyzed by knowing the major molecular ion and the retention time and m/z value corresponding to it (Fig. 2).
The MPA chromatogram obtained under the same conditions as for the chromatogram of the Eleutherococcus extract was analyzed in selected ion monitoring mode corresponding to the major molecular ions of the eleutherosides (based on data given in Table 2). The sought peaks were identified by analyzing in the MPA chromatogram. Also, the presence of eleutherosides B and E in the formulation was confirmed.
Figure 3 illustrates the quantitative characteristics of the biological activity profiles calculated using the PASS computer program for eleutherosides B and E.
An analysis of the biological activity profiles of eleutherosides B and E established that 306 biological activities were predicted with positive (Pa – Pi) values for eleutheroside B. Of these, 70 were predicted with threshold (Pa – Pi) > 0.5. A total of 294 biological activities with positive (Pa – Pi) values were predicted for eleutheroside E, 69 of which had (Pa – Pi) > 0.5.
The potential additive/synergistic activity of eleutherosides E and B was evaluated in silico by us because the identified compounds were proposed for use in the extract of E. senticosus rhizomes and roots. The analysis of the additive/synergistic activity considered the types of activity predicted for both compounds with (Pa – Pi) values exceeding 0.5. Figure 4 shows the probably additive/synergistic effects.
It was found that the above criteria corresponded to 56 types of biological activity. Of these, eight were predicted with threshold (Pa – Pi) > 0.8 (Table 3).
TABLE 3 shows that additivity/synergism of NF-êB transcription factor inhibition was predicted with the greatest probability. This is the mechanism of the antitumor effect for lung and breast cancer.
The in silico analysis allowed the most probable additive/ synergistic mechanisms of the antitumor activity of eleutherosides E and B to be identified. Table 4 presents the results.
TABLE 4 shows that the most probable mechanisms of antitumor activity were stimulation of apoptosis, inhibition of NF-κB transcription factor, and stimulation of caspase 3, which were predicted with (Pa – Pi) > 0.7. Based on the analytical data, use of PharmaExpert established that these mechanisms were involved in the development of antitumor effects against kidney cancer, melanoma, non-Hodgkin’s lymphoma, colorectal cancer, osteosarcoma, and pancreatic cancer in addition to antitumor activity against lung, breast, and liver cancer.
An analysis of literature studies on the biological activity of the identified compounds gave the following results.
Eleutherosides B (syringin) and E (syringaresinol diglucoside) exhibited neuroprotective, immunomodulating, and anti-inflammatory activity, promoting correction of adhesive intercellular interactions [36,37,38, – 39]. Radioprotector properties were found for eleutheroside E [40].
Studies in silico, in vitro, and in vivo established antitumor activity for eleutherosides B and E against glioblastoma and liver, lung, and breast cancer [41, 42].
The literature data also suggested that eleutheroside B possessed antiangiogenic activity and inhibited isoenzymes hCA IX and hCA XII, which led to progression and metastasis of malignant tumors [43, 44].
Eleutheroside B was found to exhibit antioxidant activity upon activation of the Nrf2 signaling pathway and to suppress hyperproduction of pro-inflammatory cytokines (interleukins IL-1β, IL-6, and tumor necrosis factor TNF-α) and pro-inflammatory factors (induced NO-synthase iNOS, cyclooxygenase COX-2), exhibiting anti-inflammatory activity with colitis [45]. In addition, antioxidant properties of eleutheroside B were found with increased activity of liver superoxide dismutase, catalase, and glutathione peroxidase together with neutralizing action on free radicals [46]. Eleutherosides B and F could relieve tiredness and improve memory and cognitive functions. These effects may have been due to inhibition of cholinesterase or increased acetylcholine synthesis in hippocampal neurons. It was also demonstrated that eleutheroside E could relieve tiredness with reduced activity of NK cells and delay an increased corticosterone level caused by stress during swimming [47, 48].
It is noteworthy that the MPA pharmaceutical formulation also showed the above properties characteristic of eleutherosides B and E, i.e., immunomodulating (including adhesiogenic and diminished increase of IL-6 level), antioxidant, radioprotective, neuroprotective, antistress, chemopreventive (against squamous cell skin carcinoma), and antitumor against hepatocarcinoma and lung and stomach cancer [8,9,10,11,12,13,14,15,16,17, 49,50,51,52].
Furthermore, the following should be noted. MPA could be assumed to have high neuroprotective activity in experiments and the clinic against dopaminergic neurons owing to the identification in the MPA formulation of eleutheroside B (syringin) in particular. The latter facilitated increased effectiveness of pathogenetic therapy with Parkinson’s disease and enabled MPA to be characterized as a geroprotector among others [11, 53, 54]. The similarity of the molecular structures of eleutheroside B (syringin) and the levo-dopamine isomer L-DOPA (Fig. 3) suggested that syringin itself included in the MPA formulation helped significantly to replenish and protect cerebral dopaminergic neurons from damage. This could also explain the fact that MPA, affecting the level of central dopamine (produced in the brain), passed the blood–brain barrier because this property is characteristic only of the levo-isomer of dopamine.
All above activities characteristic of eleutherosides B and E were analyzed in silico and using the scientific literature. They were fully consistent with a formulation designed for preventive oncology, as mentioned above.
In addition, properties unstudied for the MPA pharmaceutical formulation were demonstrated experimentally for eleutherosides B and E. In particular, eleutherosides B and E were shown to possess cardioprotective activity. For example, eleutheroside B in experiments on rabbit heart reduced the number of episodes of atrial and ventricular fibrillation and death from heart failure. Also, it had anti-inflammatory activity as a sodium channel inhibitor. It was also shown that eleutheroside B prevented hypertrophy of myocardium, suppressing oxidative stress with myocardial damage [55,56, – 57]. Eleutheroside B exhibited antidepressant properties. The mechanism of action in this instance could consist of reduction of the level of pro-inflammatory cytokines such as TNF-α and IL-1â and inhibition of neuro-inflammatory reactions [58]. Eleutherosides B and E could reduce the metabolic activity of several drugs by suppressing the activity of cytochrome genes CYP2C9 and CYP2E. Thus, they could increase the activity of cytostatics against tumor cells [59].
All these properties could be the subject of future research on the MPA pharmaceutical formulation and development of complex adaptogenic geroprotector formulations for preventive oncology.
New properties of the MPA could be predicted considering the characteristics of eleutherosides E and B obtained using in silico analysis and literature data.
The potential for quality control and standardization of complex phytopreparations containing phenylpropanoid compounds (eleutheroside B) and lignans (eleutheroside E) were demonstrated by the completed research.
References
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Ross. Bioterapevticheskii Zh., 19(2), 13 (2020).
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Ross. Bioterapevticheskii Zh., 19(3), 12 (2020).
O. A. Bocharova, M. I. Davydov, A. Yu. Baryshnikov, et al., Vestn. Ross. Akad. Med. Nauk, No. 8, 21 – 25 (2009).
E. L. Al’perina, E. V. Bocharov, O. A. Bocharova, et al., Current Problems in Neuroimmunopathology [in Russian], Moscow (2012), p. 131.
A. Panossian, G. Wikman, and J. Sarris, Phytomedicine, 17, No. 7, 481 (2010).
A. N. Shikov, O. N. Pozharitskaya, V. G. Makarov, et al., J. Ethnopharmacol., 154(3), 481 (2014).
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Ross. Bioterapevticheskii Zh., 19(4), 35 (2020).
O. Bocharova, R. Serebriakova, T. Philippova, et al., Farm. Vestn., 48 (Special Issue), 414 (1997).
O. A. Bocharova, M. M. Pozharitskaya, T. L. Chekalina, et al., Bull. Exp. Biol. Med., 138(6), 578 (2004).
O. A. Bocharova and M. M. Pozharitskaya, Byull. Eksp. Biol. Med., 138(12), 652 – 657 (2004).
E. V. Bocharov, V. G. Kucheryanu, G. N. Kryzhanovsky, et al., Bull. Exp. Biol. Med., 141(5), 560 (2006).
O. A. Bocharova, M. I. Davydov, A. A. Klimenkov, et al., Byull. Eksp. Biol. Med, 148(7), 96 (2009).
O. A. Bocharova, M. A. Lyzhenkova, M. V. Mezentseva, et al., Byull. Eksp. Biol. Med., 136(12), 670 (2003).
N. P. Bochkov, O. A. Bocharova, A. A. Aksenov, et al., Med. Genet. (Moscow, Russ. Fed.), 4(1), 15 (2005).
O. A. Bocharova, V. B. Matveev, R. V. Karpova, et al., Bull. Exp. Biol. Med., 141(5), 616, (2006).
O. N. Kurennaya, R. V Karpova, O. A. Bocharova, et al., Russ. J. Genet., 49(12), 1190 (2013).
E. V. Bocharov, R. V. Karpova, I. V. Kazeev, et al., Patol. Fiziol. Eksp. Ter., No. 3, 55 (2013).
R. V. Karpova, E. V. Bocharov, I. V. Kazeev, et al., Patol. Fiziol. Eksp. Ter., No. 4, 51 (2013).
V. I. Sheichenko, O. A. Bocharova, O. P. Sheichenko, et al., Zavod. Lab., Diagn. Mater., 72(8), 15 (2006).
O. P. Sheichenko, O. A. Bocharova, V. I. Sheichenko, et al., Vopr. Biol., Med. Farm. Khim., 5(2), 20 (2007).
O. P. Sheichenko, O. A. Bocharova, B. A. Krapivkin, et al., Vopr. Biol., Med. Farm. Khim., 10, 52 (2012).
I. V. Kazeev, O. A. Bocharova, V. E. Shevchenko, et al., Teor. Osn. Khim. Tekhnol., 55(6), 780 (2021).
I. V. Kazeev, O. A. Bocharova, V. E. Shevchenko, et al., Teor. Osn. Khim. Tekhnol., 54(6), 733 (2020).
O. A. Bocharova, I. V. Kazeev, V. E. Shevchenko, et al., Khim.-farm. Zh., 56(1), 32 – 38 (2022); Pharm. Chem. J., 56(1) 78 – 84 (2022).
S.-P. Liu, R. Wang, Q. Li, et al., Molecules, 17(7), 7903 (2012).
T. Li, K. Ferns, Z. Q. Yan, et al., Am. J. Chin. Med., 44(8), 1543 (2016).
A. Jia, Y. Zhang, H. Gao, et al., J. Ethnopharmacol., 268, 113586 (2021).
V. V. Poroikov, Biochemistry (Moscow), Suppl. Ser.: Biomed. Chem., 14(3), 216 – 227 (2020).
D. A. Filimonov, D. S. Druzhilovskiy, A. A. Lagunin, et al., Biomed. Chem.: Res. Methods, 1(1), e00004-e00004 (2018).
S. Wang, G. Dong, and C. Sheng, Chem. Rev., 119(6), 4180 – 4220 (2019).
P. V. Pogodin, A. A. Lagunin, A. V. Rudik, et al., Front. Chem., 6, 133 (2018).
A. A. Lagunin, R. K. Goel, D. Y. Gawande, et al., Nat. Prod. Rep., 31(11), 1585 – 1611 (2014).
D. Y. Gawande, D. S. Druzhilovsky, R. C. Gupta, et al., J. Ethnopharmacol., 202, 97 – 102 (2017).
N. S. Ionov, M. A. Baryshnikova, E. V. Bocharov, et al., Biochemistry (Moscow), Suppl. Ser.: Biomed. Chem., 15(4), 290 – 300 (2021).
R. K. Goel, D. Y. Gawande, A. A. Lagunin, et al., SAR QSAR Environ. Res., 29(1), 69 – 81 (2018).
J. Tan, J. Luo, C. Meng, et al., Int. Immunopharmacol., 90, 107268 (2021); https://doi.org/10.1016/j.intimp.2020.107268.
D. Che, B. Zhao, Y. Fan, et al., J. Anim. Physiol. Anim. Nutr. (Berlin), 103(4), 1174 (2019); https://doi.org/10.1111/jpn.13087.
K.-M. Lau, G. G.-L. Yue, Y.-Y. Chan, et al., Chin. Med., 14, 25 (2019); https://doi.org/10.1186/s13020-019-0250-0.
A. Panossian, T. Davtyan, N. Gukassyan, et al., Phytomedicine, 9(7), 598 (2002); https://doi.org/10.1078/094471102321616409.
M. Liu, Y. Xiong, S. Shan, et al., Nat. Prod., 83(11), 3315 (2020); https://doi.org/10.1021/acs.jnatprod.0c00650.
S. Ahmed, D. A. Moni, K. D. Sonawane, et al., Biomol. Struct. Dyn., 39(17), 6553 (2021); https://doi.org/10.1080/07391102.2020.1803135.
C.-H. Lee, C.-W. Huang, P.-C. Chang, et al., Phytomedicine, 61, 152844 (2019); https://doi.org/10.1016/j.phymed.2019.152844.
S. A. Aventurado, D. B. Billones, R. D. Vasquez, et al., Drug Des., Dev. Ther., 4, 5189 (2020); https://doi.org/10.2147/DDDT.S271952.
G. Costa, A. Maruca, R. Rocca, et al., Antioxidants (Basel), 9(9), 775 (2020); https://doi.org/10.3390/antiox9090775.
H. Zhang, H. Gu, Q. Jia, et al., Arch. Biochem. Biophys., 680, 108242 (2020); https://doi.org/10.1016/j.abb.2019.108242.
S. Lee, D. Son, J. Ryu, et al., Arch. Pharm. Res., 27(1), 106 (2004); https://doi.org/10.1007/BF02980055.
D. Huang, Z. Hu, and Z. Yu, Neural. Regen. Res., 8(12), 1103 (2013); https://doi.org/10.3969/j.issn.1673-5374.2013.12.005.
Y. Kimura and M. Sumiyoshi, J. Ethnopharmacol., 95(2–3), 447 (2004); https://doi.org/10.1016/.jep.2004.08.027.
O. A. Bocharova, E. V. Bocharov, R. V. Karpova, et al., Bull. Exp. Biol. Med., 157(2), 258 (2014); https://doi.org/10.1007/s10517-014-2539-4.
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Bull. Exp. Biol. Med., 159(5), 655 (2015).
E. V. Bocharov, R. V. Karpova, O. A. Bocharova, et al., Bull. Exp. Biol. Med., 163(6), 789 (2017).
E. V. Bocharov, O. A. Bocharova, R. V. Karpova, et al., Ross. Bioterapevticheskii Zh., 17(2), 69 – 78 (2018).
E. V. Bocharov, I. F. Ivanova-Smolenskaya, V. V. Poleshchuk, et al., Bull. Exp. Bio. Med., 149(6), 682 (2010).
O. A. Bocharova, A. V. Revishchin, E. V. Bocharov, et al., Lab. Zhivotn. Nauchn. Issled., No. 2, 47 (2021); https://doi.org/10.29296/2618723X-2021-02-06.
P.-P. Zhang, Z.-F. Guo, P.-H. Zhang, et al., Acta Pharmacol. Sin., 42(2), 209 (2020); https://doi.org/10.1038/s41401-020-0453-z.
F. Li, N. Zhang, Q. Wu, et al., Int. J. Mol. Med., 39(1), 199 (2017); https://doi.org/10.3892/ijmm.2016.2824.
S. Wang and X. Yang, Int. Immunopharmacol., 84, 106513 (2020); https://doi.org/10.1016/j.intimp.2020.106513.
B. Zhang, H.-S. Chang, K.-L. Hu, et al., Chin. J. Integr. Med., 27(7), 534 (2021); https://doi.org/10.1007/s11655-019-3051-5.
S. Guo, Y. Liu, Z. Lin, et al., BMC Complement. Altern. Med., 14, 1 (2014); https://doi.org/10.1186/1472-6882-14-1.
Acknowledgments
The work was performed with partial support by grants from the Commission on Biomedical Innovations and Technologies, Ministry of Science of the Russian Federation and in the framework of the Basic Research Program in the Russian Federation for the Long Term (2021 – 2030) (No. 122030100170-5).
CONFLICT OF INTEREST
We confirm no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 6, pp. 29 – 37, June, 2022.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bocharova, O.A., Shevchenko, V.E., Kazeev, I.V. et al. Analysis of Eleutherosides by Tandem Mass Spectrometry: Possibilities of Standardizing a Multi-Phytoadaptogen Formulation for Preventive Oncology. Pharm Chem J 56, 806–814 (2022). https://doi.org/10.1007/s11094-022-02712-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02712-0