Paracetamol (PARA) and Pseudoephedrine (PSE) are co-formulated drugs that and widely used over the world for the treatment of symptoms associated with common cold. In the present study a specific, rapid and simple UV spectrophotometric method with good sensitivity was developed and validated for the simultaneous quantification of PARA and PSE in bulk and tablet dosage form using methanol as diluent. This method is based on the direct measurement of absorbance at 248 nm wavelength for PARA solution, whereas dual-wavelength absorption measurement is applied for PSE solution (i.e. subtraction of absorbance at 217 nm from that at 201 nm, in which PARA absorbance subtraction is equal to zero). The calibration curves were linear (R2 = 0.9988 and 0.9976 for PARA and PSE, respectively) in a concentrations range of 2 – 10 μg/mL for both drugs. Limit of detection is LOD = 0.4159 μg/mL and 0.29679 μg/mL and the limit of quantification is LOQ = 1.26 μg/mL and 0.8994 μg/mL for PARA and PSE, respectively. The RSD% values for all levels of precision (repeatability, intra-day and inter-day) are within the specified limit for both drugs. The accuracy of PARA determination was ascertained by standard addition method, and the percentage recovery for 50, 100, and 150 % of standard were 97.93, 100, and 101.39%, respectively (RSD% = 1.7). The accuracy of PSE determination was studied by percentage recovery (n = 3), and the results were 100.7, 101.16, and 99.57% (RSD% = 0.6648). The proposed spectrophotometric method was successfully applied to the analysis of commercial tablets and the obtained results were satisfactory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
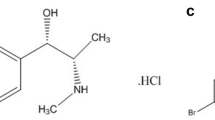
Paracetamol (Fig. 1) is over-the-counter analgesic widely used for the relief of headache and other minor aches and pains, also being a major ingredient in numerous cold and flu remedies [1]. Pseudoephedrine (PSE), a sympathomimetic amine (Fig. 1), is also found in many over-the- counter preparations as a single component or more commonly in combination with other drugs used in cold and flu [2]. Previous studies proved superiority of the combined therapy over mono-therapy for the treatment of cold and flu [3].
Both PARA and PSE are official drugs in the United States Pharmacopoeia (USP) [4] and British Pharmacopoeia (BP) [5]. A variety of methods previously reported in the literature for the simultaneous determination of PARA and PSE with other components included HPLC [6,7,8,9], capillary electrophoresis [10, 11], chemometric methods [12], and LC-MS [13]. Several multicomponent UV spectrophotometric methods have been disclosed in the literature. These methods can be used for the simultaneous determination of multidrug dosage forms in mixtures without separation [14]. Dual-wavelength spectrophotometry is one of the multicomponent UV methods based on calculations of the concentration of analyte of interest in the presence of interfering components. In this method, calculation of the absorbance difference between two points in the spectrum of mixture is directly proportional to the concentration of the analyte of interest. On the other hand, the interfering component exhibits the same absorbance at the two selected wavelengths while significant absorance difference is noticed for the component of interest [15].
To the best of our knowledge from extensive literature survey, there is neither official nor published UV spectrophotometric method for simultaneous determination of PARA and PSE in their co-formulated tablets. The present study was aimed to develop accurate, reliable and selective spectrophotometric method for simultaneous determination of PARA and PSE in their combined tablet dosage form. The validation characteristics were evaluated as per ICH guidelines. These characteristics include selectivity, intraday and interday precision, linearity, accuracy, limit of detection (LOD) and limit of quantification (LOQ).
2. Experimental
2.1. Instrument and Materials
UV-VIS spectrophotometer equipped with 1 cm matched quartz cells, PG Instruments Ltd. (South Korea). Pseudoephedrine (PSE) working standard (100.6% w/w) was obtained as a gift from Azal Pharmaceuticals, Sudan. Paracetamol (PARA) working standard (101% w/w) was obtained as a gift from Wafra Pharmaceutical, Sudan. Panadol sinus tablets claimed containing 500 mg PARA and 30 mg PSE hydrochloride manufactured in GlaxoSmithKline (Australia) was collected from local market in Khartoum State, Sudan. Methanol of analytical grade manufactured in SDFCL, India, was used without further purification.
2.2. Preparation of Standard Drug Solutions
PARAand PSE (100 mg/mL) standard stock solutions . Standard PARA or standard PSE 10 mg each was weighed and transferred to separate 100 mL volumetric flask, dissolved in 10 mL methanol, and completed to the mark.
PARA – PSE mixture standard stock solution (100 mg/mL). Standard PARA (10 mg) and standard PSE (10 mg) each were weighed and transferred to 100 mL volumetric flask, dissolved with 10 mL methanol and then completed to the mark to get solution mixture of PSE and PARA, each of 100 μg/mL.
Estimation of optimum λ max . From the prepared stock solutions (100 μg/mL each), test concentrations of 10 μg/mL were prepared from each PARA and PSE stock solution and were scanned over the wavelength range of 200 – 400 nm against their reagent blanks.
Construction of calibration curves. From the mixture stock solution, five concentration levels (2, 4, 6, 8 and 10 μg/mL) were prepared and then the absorbance (ABS) of each concentration was measured at 248 nm for PARA and at both wavelengths, 201 and 217 nm, for PSE. Then, the concentrations were plotted versus absorbance for PARA and versus ABS difference for PSE, and finally the calibration curves were constructed.
2.3. Verification of Method
Limit of detection (LOD) and limit of quantification (LOQ). The limits of detection and quantification were calculated from the linear regression equations using the following formulas for both drugs:
Precision. Precision of the proposed method was studied by measuring repeatability, intra-day and inter-day variations of results. A standard solution mixture containing PSE and PARA at concentrations of 3 and 6 μg/mL, respectively, was used to check for various precision levels. For the repeatability study, six solutions were analyzed. In case of in intraday variation, the absorbance of prepared solutions was measured three times on the same day at specific time interval. On the other hand, inter-day variation was performed via taking the absorbance of standard solution on three consecutive days.
Accuracy. Accuracy of the method was determined using standard addition technique for PARA at three levels, so that standard amounts of the drug equivalent to 50, 100 and 150% were added to pre-analyzed sample solutions. On the other hand, recovery technique was applied to assess the accuracy for PSE, and the percentage recovery values were calculated.
Robustness. This characteristic was determined for PARA by measuring the absorbance of the mixture solution at 246, 248 and 250 nm (±2 nm from the wavelength of maximum absorbance). Assay deviations at 246 nm and 250 nm from the value at 248 nm were calculated.
Assay of PSE/PARA in brand tablets. Ten tablets of brand product, each containing 30 mg of PSE and 500 mg of PARA were weighed and ground, weight equivalent of one tablet was taken and transferred to 100 mL volumetric flask, dissolved in methanol, and the volume was completed to the mark. This solution was further diluted to afford final PSE concentration of 3 μg/mL and PARA concentration of 5 μg/mL. Absorbances of these solutions were measured at 248 nm for PARA and at 217 and 201 nm for PSE. The percentage content of PARA and PSE in the brand was calculated from the linear regression equations.
3. Results and Discussion
Extensive literature analysis revealed no single multicomponent UV spectrophotometric method for simultaneous determination of PARA and PSE in bulk or in their combined dosage form and the reported methods were either expensive or tedious. This scene was further complicated by the unavailability of an official method for simultaneous determination of these drugs in their combined dosage form. Accordingly, there was a challenge to develop UV spectrophotometric method for the simultaneous estimation of these drugs in their combined dosage form and this could possibly be attributed to two reasons. The first is a significant overlap seen in the overlaid UV spectra of these drugs, and the second reason is the existence of drugs in a far different ratio in the dosage form, almost at 1 : 15 PSE/PARA, respectively. In view of what have been mentioned, the aim of the present study was to develop and validate a new and affordable UV spectrophotometric method for the simultaneous assay of PARA and PSE in bulk and in their combined tablet dosage form.
3.1. Selection of Suitable Wavelength
In the spectra of PSE and PARA recorded in a UV spectrophotometer (200 – 400 nm), PARA showed a significant absorption at 248 nm wavelength (Fig. 2) where no absorption was detected for PSE (Fig. 3). The absorption of PARA at longer wavelength relative to PSE can be ascribed to the extended chromophoric feature inherent in PARA, represented by existence of the phenolic hydroxyl group.
At the same time, the UV spectrum of PSE (20 μg/mL) standard solution showed no UV absorption at PARA absorbance maxima, and its wavelength corresponding to maximum absorbance was 208 nm as shown in Fig. 3.
From the overlaid spectrum (Fig. 4), the wavelength selected for estimating PARA was 248 nm, at which PSE exhibited no absorbance. On the other hand, the absorbance difference at 201 and 217 nm was found significant for PSE while being zero for PARA.
3.2. Linearity
Linearity of the proposed method for standard PARA solutions was checked over a concentration range of 2 – 10 μg/mL. The zero-order absorbance of serial diluted standard solutions at 248 nm was measured, the calibration curve of absorbance was constructed versus the corresponding concentrations, and the regression equation was computed. The constructed curve was found linear for PARA within the tested concentration range as reflected from the highly significant correlation coefficient (R2 = 0.9988). Equation of the regression analysis was found to be Y = 0.0912x + 0.0287. Linearity data are shown in Table 1 and Fig. 5.
Linearity of the proposed method for PSE was verified within a concentration range of 2 – 10 μg/mL. The calibration curve was constructed as the absorbance difference at wavelengths 201 and 217 nm versus the variable concentration, and the regression equation was computed as Y = 0.0313x + 0.005. This method was found linear for PSE within the tested range as confirmed by the highly significant correlation coefficient (R2 = 0.9976). PARA shows an absorbance difference equal to zero at these two wavelengths and hence will be suitable for single determination of PSE in the presence of the interfering component. The data are presented in Table 2 and Fig. 6.
3.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
Limit of detection (LOD) and limit of quantification (LOQ) are two important performance characteristics in method validation. LOD is taken as the lowest concentration of an analyte in a sample that can be detected, but not necessarily quantified, under stated conditions of the test. The LOQ is the lowest concentration of an analyte in a sample that can be determined with acceptable precision and accuracy under stated conditions of the test [16]. In the present method, LOD and LOQ for both analytes were calculated from regression equations of the corresponding calibration lines, which yielded the following values:
The obtained low LOD values for both drugs are indicative of the precision of UV response obtained for the given method when the drug concentration is zero. Moreover, the low LOQ values for both drugs in this method reflect the sensitivity of the developed method and its capability to detect and quantify very low concentration levels for both drugs.
3.4. Precision
To check for precision of the method, suitable statistical evaluation via the percentage relative standard deviations (%RSD) of the predicted concentrations from the regression equations were employed as the measure of precision. A standard solution mixture (3 μg/mL and 6 μg/mL of PSE and PARA, respectively) was measured six times at once (repeatability), three times on the same day at 3 hours interval and on three consecutive days for intra-day and inter-day precision, respectively. The standard deviation (SD) and relative standard deviation (RSD) were calculated and results are given in Tables 3 and all confirmed the precision of the method (RSD% < 2% for all levels of precision). The developed method exhibits an equal precision compared to the reported chromatographic methods [6,7,8,9].
3.5. Accuracy
Accuracy of the proposed method was assessed via recovery experiments. The recovery studies were carried out at spiking levels of 50, 100 and 150 % of the tested sample concentration for PARA. Results of recovery were presented in Table 4. The obtained accuracy values were 97.93, 100, and 101.39%, respectively, assuring accuracy of the method in determination of PARA in the combined dosage form in addition to the absence of interference from the excipients. On the other hand, recovery percent method was adopted to ensure the accuracy of the developed method in determination of PSE. To this end, a standard drug concentration of 3 μg/mL was used and the obtained recovery results are 99.57, 100.7 and 101.16%, respectively (Table 4). These results are within the acceptable range as per ICH guidelines which indicate the accuracy of this method in determination of PSE in its combined dosage form with PARA.
3.5. Robustness
Robustness of the proposed method was checked by making a deliberate change in PARA assay conditions using a standard solution at a concentration of 6 μg/mL. To this end, a slight variation in the detector wavelength within ±2 nm for PARA relative to the wavelength of the maximum absorbance showed no effect and further proves reliability of the developed method, RSD% being 1.15% to fall within the acceptable limit.
3.6. Assay of PARA/PSE Commercial Brand
Finally, the validated UV spectrophotometric method was applied for the determination of PSE/PARA in tablet dosage form and the percentage content of each drug was calculated by applying equations of the standard calibration curves. The content of PARA and PSE in the commercial brand was satisfactory, 100.5% and 100%, respectively. Since all statistical data proved validity of the methods, this method can successfully be utilized for routine analysis of PSE/PARA in their combined pharmaceutical dosage forms.
References
S. Behera, S. Ghanty, F. Ahmad, et al., Int. J. Chem. Anal. Sci., 3(2), 1 – 12 (2012).
H. Raviteja, M. Nasare, V. V. L. N. Prasad, and P. V. Diwan, Brit. J. Pharm. Res., 4(4), 418–428 (2014).
The United States Pharmacopoeia 35 – National Formulary 30, United States Pharmacopoeial Convention, Rockville, MD (2012), pp. 2029, 2242, 4476.
British Pharmacopoeia, British Pharmacopoeial Commission, Her Majesty Stationery Office, London (2013), Electronic Version.
G. M. Hadad, S. Emara, and W. M. M. Mahmoud, Talanta, 79(5), 1360 – 1367 (2009).
F. Al-Rimawi, Saudi Pharm. J., 18(2), 103–106 (2010).
L. Sivasubramanian and K. S. Lakshmi, Pharma Chemica, 1(1), 238 (2015).
M. J. Akhtar, S. Khan and M. Hafiz, J. Pharm. Biomed. Anal., 12(3), 379 – 382 (1994).
S. Di Berardino and R. Jasionowska, Am. J. Anal. Chem., 5(9), 613 – 619 (2014).
X. Liu, L. Liu, H. Chen, and X. Chen, J. Pharm. Biomed. Anal., 43(7), 1700 – 1705 (2007).
S. H. Youssef, M. A. M. Hegazy, D. Mohamed, and A. M. Badawey, World J. Pharm. Sci., 6 (6), 1644 – 1659 (2017).
H. Li, C. Zhang, J. Wang, Y. Jiang, et al., J. Pharm. Biomed. Anal., 51(3), 716 – 722 (2010).
Q. Liao, Z. Xie, B. Pan, et al., Chromatographia, 67, 687 – 694 (2008).
R. Hajian and A. Soltaninezhad, J. Spectrosc., 2013, 405210 (2013).
J. Jain, R. Patadia, D. Vanparia, et al., Int. J. Pharm. Pharm. Sci., 2(4), 76 – 79 (2010).
Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls, Federal Register (Notices), 65, 776 – 777 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, M.S., Elsaman, T. Development and Validation of the UV Spectrophotometric Method for Simultaneous Determination of Paracetamol and Pseudoephedrine in Bulk and Combined Tablet Dosage Form. Pharm Chem J 54, 1306–1310 (2021). https://doi.org/10.1007/s11094-021-02360-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02360-w