A biologically active complex of polypeptides was obtained from fetal bone to stimulate chondrogenesis. Its effectiveness was studied on the model of cartilage defect healing in rats. The biologically active complex was isolated from the intercellular matrix of long tubular bones of dog fetuses (57 – 63 d of fetal development). The resulting complex (0.1 mLvolume) was introduced into a cartilage defect in 10 rats (experimental group). Normal saline (0.1 mL) was injected into the defect in 10 animals of the control group. It was found that the sizes of the defects in experimental animals on the 30th day of the experiment were statistically significantly less than in the control group. The defect region was completely filled in animals of both groups on the 180th day of the experiment. However, the cartilage flexibility was statistically significantly lower in the control than in the experimental group. It is believed that the intercellular substance of fetal bone tissue can be a source of biologically active components that can stimulate reparative chondrogenesis (growth factors, procollagen and/or its derivatives).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intra-articular injections of various drugs are currently widely used methods for treating chronic inflammatory joint diseases and their post-traumatic damage [1,2, – 3]. However, the effectiveness of using them remains questionable [4]. Therefore, research on improving the effectiveness of drugs by seeking new chondrogenesis stimulators, new combinations of various active substances, and variation of administration regimes of registered drugs is continuing [5,6,7, – 8].
Complex protein-containing preparations can be used effectively to treat osteoarthrosis [9,10,11, – 12]. However, few studies have focused on the use of intra-articular injected preparations to treat traumatic cartilage damage [13,14, – 15]. Fetal bone tissue possessing the potential for both osteo- and chondrogenesis could be a rather promising material for seeking such preparations [16].
Therefore, the goal of the present study was to prepare a biologically active complex of polypeptides from fetal bone for stimulation of chondrogenesis and to study its effectiveness on a model of cartilage defect healing in rats.
Experimental Part
The material was obtained from long tubular bones of mongrel dog fetuses that perished as a result of asphyxiation during birth (57 – 63 d of fetal development). In all instances, the pregnancy proceeded without complications. All fetuses had no visible signs of pathology.
The isolated bone was cleaned of traces of muscle tissue, marrow, cartilage, and tendons, rinsed in distilled H2O heated to 30°C, and ground to particle size ≤0.5 and ≥ 0.1 cm3. Ground and rinsed bone tissue was placed into a bottle made of material resistant to the action of inorganic acids and treated with HCl solution (0.1 N, seven times the volume of the material). The mixture at pH 1.5 – 2.5 was stirred on a magnetic stirrer at 4 – 8°C. The extractant was corrected with dilute HCl as necessary. The extraction continued for 3 d.
Next, the liquid from the bottle was separated from the remaining bone tissue and filtered through filter paper. The obtained solution was dialyzed against distilled H2O using a CelluSep cellophane film for dialysis (USA) of pore diameter <5 kDa. The dialysis continued until Ca2+ in the counter-dialysate disappeared as confirmed by the reaction with barium sulfate. Fragments of coagulated protein were removed from the obtained dialysate. For this, the dialysate was first centrifuged at 300 g. Then, the supernatant liquid was filtered through filter paper and purified through a cellulose acetate filter of pore diameter 0.22 _m. The purified solution was placed into a heat resistant vessel and frozen at –70°C.
Next, the obtained fraction was concentrated by lyophilization in a HETO LyoLab 3000 low-temperature vacuum dryer (Denmark). The lyophilized protein was hermetically packed into glass vials and sterilized by a beam of fast electrons at a dose of (18 ± 5) kGy on an LUE-8-5M accelerator (Russia) before administration to experimental animals.
The effectiveness of intra-articular injection of the dissolved lyophilizate into the knee joint of Wistar rats was assessed. A conical bone-cartilage defect in the patellar region of the femur was created in 20 animals. For this, a 1-mm diameter dental drill was used. The drill was inserted through the whole lateral cartilage to a depth of 1 mm into the subchondral bone. The animals were aged 10 months. The body mass was from 287 to 295 g. Experimental animals (n = 10) were administered the prepared drug (0.1 mL) into the joint cavity 7 d after modeling the defect. The lyophilizate (1 mg per kg of animal body mass) was dissolved in normal saline (1.5 mL). The injected solution was standardized for total protein content as determined by the Lowry method. The protein concentration in the aqueous solution was 25 g/L. Control animals (n = 10) were injected with normal saline (0.1 mL) into the joint cavity 7 d after modeling the defect.
Animals of both groups were withdrawn from the experiment on the 30th and 180th days after modeling the defect (five animals at each time). In addition, the test parameters were studied in five intact animals (intact group). All animals received during the post-operative period a standard balanced food ration with free access to water. The general condition of the animals, their activity, and demand for food were visually assessed daily. The rectal temperature was measured.
The study was performed in compliance with the principles of humanitarian handling of laboratory animals according to the requirements of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and European Parliament and European Union Committee Directive 2010/63/EU dated Sept. 22, 2010 on the protection of animals used for scientific purposes. The experimental study was approved by the Ethics Committee of G. A. Ilizarov NMRC, Ministry of Health of the RF.
Histological, biomechanical, and biochemical research methods were used to assess the effectiveness of the studied preparation.
Histomorphological studies used epoxide semi-thin sections of large area (6 – 8 mm2) perpendicular to the joint surface that were stained with Methylene Blue and Methylene Blue-Basic Fuchsin. Images of micropreparations were digitized on a DiaMorf apparatus-program complex (Moscow) and analyzed using the VideoTest-Master-Morphology program to calculate the defect size.
The biomechanical flexibility of the damaged cartilage was also assessed [17]. The concentration of glucuronic acids in blood serum was determined from the reaction with carbazole [18].
The statistical significance of differences between two sets was assessed using the Wilcoxon W-criterion for independent sets [19].
Results and Discussion
The rate of healing of the defect (decrease of width and depth of defect zone) at the experimental times was found to be statistically significantly faster in experimental animals than in control animals (Table 1, Fig. 1). The concentration of cartilage degradation products, i.e., glucuronic acids, was significantly less in blood serum of control animals than in intact animals.
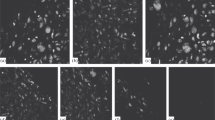
Histograms of damage zone in experimental and control rats: Damage zone, 30th day of experiment. Paraffin section, Mason stain. Control (a), experimental (b); damage zone, 30th day of experiment. Paraffin section, Mason stain. Oc. 12.5, ob. 6.3. Control (c), experimental (d); damage zone, 180th day of experiment. Paraffin section, Mason stain. Control (e), experimental (f).
It is noteworthy that cartilage was also more flexible in experimental animals at both observation times although this parameter by the 180th day was statistically significantly greater than that of the control group but less than that of intact animals. Also, the volume of newly formed cartilage in experimental rats was 15% greater than the defect volume. The outer edge of the newly formed tissue had small protuberances above the level of the joint surface (Fig. 1f).
It is noteworthy that newly formed tissue in rats of both groups was histologically fibrous connective tissue and had the same source, i.e., marrow and osteogenic cells (Fig. 1c and 1d).
The assessment of the general condition of experimental animals during the experiment found an increase relative to the pre-operative level of 1°C in the rectal temperature of five experimental rats for 2 d after administration of the studied lyophilizate solution. Lethargy and reduced demand for food were also noted at these times for all animals of this group. Instances of unplanned deaths of animals did not occur in both groups.
Thus, the study showed that already on the 30th day of the experiment (which corresponded to the 23rd day after injection of the lyophilizate solution) statistically significant differences in the condition of the damaged cartilage were noted between animals of the experimental and control groups. The sizes of the defects decreased more significantly in the experimental group at this time. The area of the defect was practically completely filled in animals of both groups as the observation time increased (to 180 d). However, the flexibility of the cartilage in the control was less than that of the experimental and intact animals. Furthermore, not only was the defect in experimental rats fully replaced but also cartilage hypertrophy in the defect zone was present.
Based on the research results, it could be supposed that the intercellular substance of fetal bone tissue contained a complex of biologically active components capable of stimulating reparative chondrogenesis after traumatic damage of joint cartilage. These potential stimulators in fetal bone could be low-molecular-mass polypeptides such as growth factors and immature collagen molecules (procollagen and/or its derivatives).
References
Yu. A. Olyunin, Sovrem. Revmatol., No. 1, 78 – 83 (2015).
M. I. Udovika, Terapiya, No. 7 – 8, 89 – 95 (2018).
E. A. Strebkova and L. I. Alekseeva, Sovrem. Revmatol., No. 2, 96 – 104 (2019).
A. E. Karateev, E. Yu. Pogozheva, V. N. Amirdzhanova, and E. S. Filatova, Sovrem. Revmatol., No. 3, 40 – 52 (2018).
V. V. Badokin, M. A. Strakhov, I. F. Akhtyamov, et al., Eff. Farmakoter., No. 19, 6 – 17 (2018).
E. A. Belyaeva and V. N. Razin, Klin. Med. Farmakol., 4(2), 31 – 35 (2018).
S. V. Popovich, Ortop., Travmatol. Protez., No. 4, 81 – 84 (2015).
O. A. Shavlovskaya, Med. Sovet., No. 1, 76 – 83 (2019).
A. V. Garkavi, V. A. Meshcheryakov, and V. S. Kaikov, Kafedra Travmatol. Ortop., No. 3, 23 – 30 (2018).
A. I. Gorbatenko and N. O. Kostyanaya, Vestn. Travmatol. Ortop. im. N. I. Priorova, No. 2, 40 – 45 (2016).
R. N. Kil’debekova, R. K. Urazbakhtin, V. T. Kaibyshev, and R. Sh. Mirkhaidarov, Klin. Gerontol., 25, No. 7 – 8, 48 – 53 (2019).
M. A. Gerasimenko, E. V. Zhuk, A. S. Lenkovets, and S. I. Tret’yak, Med. Zh., No. 3, 13 – 16 (2018).
K. S. Kazanin, E. I. Ardasheva, and V. I. Rudaev, Doktor. Ru, No. 4, 38 – 43 (2018).
A. V. Karpenko, V. N. Obolenskii, P. D. Prilutskii, et al., Khirurg, No. 5 – 6, 60 – 72 (2019).
T. A. Silant’eva, V. V. Krasnov, and N. V. Kubrak, Usp. Sovrem. Estestvozn., No. 9, No. 3, 471 – 475 (2015).
N. A. Kononovich, N. V. Petrovskaya, E. N. Gorbach, et al., Genii Ortop., No. 4, 20 – 25 (2011).
L. I. Gracheva and A. A. Uten’kin, Method for Determination of Visco-Elastic Properties of Cartilage Tissue [in Russian], Zaural’e, Kurgan (1992).
T. Bitter and H. M. Muir, Anal. Biochem., 4(4), 330 – 334 (1962).
O. I. Mokhov, Kach. Klin. Prakt., No. 4, 24 – 33 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 10, pp. 56 – 59, October, 2020.
Rights and permissions
About this article
Cite this article
Stogov, M.V., Kovin’ka, M.A., Kononovich, N.A. et al. Preparation and Effectiveness of Biologically Active Polypeptides from Fetal Bones for Stimulation of Chondrogenesis. Pharm Chem J 54, 1071–1074 (2021). https://doi.org/10.1007/s11094-021-02322-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02322-2