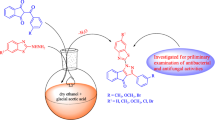
A series of new 2-(4/3-substitutedbenzyl)thieno[2,3-d]pyrimidin-4(3H)-ones (2a-f) and 2-(4/3-substituted benzyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one derivatives (3a-f) were synthesized for the first time by the reaction of corresponding 2-aminothiophene-3-carboxamide derivatives and appropriate iminoester hydrochlorides under suitable conditions. Using microdilution method, the antibacterial and antifungal activities of the synthesized compounds were tested against selected strains of Gram positive and Gram negative bacteria (Staphylococcus aureus, Enterecoccus faecalis, Escherichia coli and Pseudomonas aeruginosa) and yeast-like fungi (Candida albicans, C. krusei and C. parapsilosis). In these tests, compounds 3a-f showed higher antifungal activity than fluconazole against Candida fungus species. 2-Substituted thieno-[2,3-d]pyrimidin-4(3H)-ones (2a-f) showed better antibacterial activity than 2-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one derivatives (3a-f).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
The synthesis of new heterocyclic compounds and elucidation of their structures have attracted growing scholarly attention. For this purpose, the pursuit of alternative methods for the synthesis of new compounds has captured considerable interest [1]. Pyrimidine derivatives in bicyclic and tricyclic form presented increasing focus for many years due to their spectrum of biological properties [2,3,4, – 5] including antibacterial, antioxidant and anti-inflammatory activities [6]. In addition, research results suggested that thienopyrimidines exhibited antimicrobial activity [7]. In another study, biological properties of thieno[2,3-d] pyrimidin-4-amine derivatives have been reported [8]. Recently, new substituted 6-alkenylamides of 4-anilinothieno[2,3-d]pyrimidines have been synthesized, and their irreversible epidermal growth factor receptor inhibitor activity was observed [9]. Furthermore, tricyclic thienopyrimidines were reported as new inhibitors of Mycobacterium tuberculosis DNA GyrB domain [10, 11]. There were studies showing the effect of these systems on neurodegenerative disorders [12]. Palladium-catalyzed synthesis of these systems was reported [13] and it was established that revealed that such systems were formed via cleavage of the C-C bond of ketoalkyne [14]. In another study, effective method for the synthesis of thienopyrimidines from acid anhydrides or benzoyl chloride was used [15]. Microwave heating has been used for the synthesis of 3H-thieno[2,3-d]pyrimidin-4-ones as alternative to conventional heating [16].
In addition to these studies, there is a growing body of literature that suggests significant biological activity results about this heterocyclic system. In one of these studies, Goudar, et al. synthesized a new series of thienopyrimidine derivatives and evaluated their anti-inflammatory activities. As a result, the thiadiazole-substituted thienopyrimidine derivatives were found to be the most potent [17]. Ouyang, et al. [18] reported some compounds containing this skeleton to be significantly active against breast cancer. In another study [19], a series of new N-(sugar pyranosyl)thienopyrimidine 4-amine derivatives were synthesized and screened for antimicrobial and anti-inflammatory properties, showing significant to moderate activities. Abd El-All, et al. [20] synthesized new thienopyrimidine derivatives, screened them for antiviral activity, and established that all of the tested compounds produced remarkable inhibition of neuraminidase of influenza A virus (H3N2).
Fascinated by above descriptions, herein we report the synthesis and antimicrobial activity of 2-substituted thieno-[2,3d]pyrimidin-4(3H)-one derivatives.
2. RESULTS AND DISCUSSION
2.1. Chemistry
The target compounds (2a-f, 3a-f) were synthesized using reactions of 2-aminothiophene-3-carboxamide derivatives with iminoester hydrochlorides (1a-f). For this purpose, iminoester hydrochlorides 1a-f were prepared according to the related literature [21, 22]. and reacted with 2-aminothiophene-3-carboxamide in dioxane to obtain 2-substituted thieno[2,3-d]pyrimidin-4(3H)-one derivatives (2a-f). In addition, compounds 1a-f were treated with 2-amino-4,5,6,7-tetrahydrobenzo(b)thiophene-3-carboxamide in dioxane to obtain 2-subtituted-5,6,7,8-tetrahydrobenzo[4, 5]thieno-[2,3-d]pyrimidin-4(3H)-one derivatives (3a-f). The synthetic path to the target compounds (2a-f, 3a-f) is shown in Scheme 1. The structures of new compounds were studied by 1H NMR, 13C NMR spectroscopy and mass spectrometry. The spectroscopic characteristics of newly synthesized compounds are in accordance with the proposed structures.
2.2. Antimicrobial Activity
The antimicrobial activities of synthesized compounds were studied by microdilution method as proposed by the National Committee for Clinical Laboratory Standards (NCCLS) [23, 24], and the minimum inhibitory concentration (MIC) values of all compounds were determined based on the results of this examination. The microdilution method has been used because of its precise and rapid results, ease of operation and economic efficiency in examining the antibacterial and antifungal properties of compounds. Ciprofloxacin was employed as the reference compound for bacteria, fluconazole was selected for fungi, and the NCCLS guidelines were followed. The results of antibacterial and antifungal activity testing are shown in Tables 1 and 2.
2.3. Statistical Analysis
Statistical data analysis was performed and the corresponding activity bar plots were constructed using Statistical Program for Social Sciences (SPSS, version 23). The results are shown in Figs. 1 and 2, where the height of bars encodes the mean and 95% confidence interval for each compound.
All the obtained compounds, namely 2-(4/3-substitutedbenzyl) thieno[2,3-d]pyrimidin-4(3H)-one (2a-f) and 2-(4/3-substitutedbenzyl)-5,6,7,8-tetrahydrobenzo[4, 5]thieno-[2,3-d]pyrimidin-4(3H)-one (3a-f) derivatives, were synthe-sized by the proposed method. Ten of these compounds are new. Compound 3c was reported previously in the literature [25, 26] and compound 3d has a CAS number. The reason for including these compounds in this study was that they were obtained by different synthesis pathways, and same activities were under investigation.
According to the statistical results of antibacterial activity testing shown in Fig. 1, compounds 2b, 2e and 3c (MIC = 64 μg/mL; piperacillin–tazobactam MIC = 1 μg/mL) are the most active against S. aureus; compounds 2b-f and 3b, 3c (MIC = 128 μg/mL; piperacillin–tazobactam MIC = 1 μg/mL) are the most active against P. aeruginosa; compounds 2b, 2c and 2e (MIC = 64 μg/mL; piperacillin– tazobactam MIC = 2 μg/mL) are the most active against E. faecalis; and compounds 2d (MIC = 256 μg/mL; piperacillin–tazobactam MIC = 1 μg/mL) are the most active against E. coli.
According to the statistical results of antifungal activity testing shown in Fig. 2, compounds 3a-d (MIC = 47 μg/mL) and 3f (MIC = 8 μg/mL; fluconazole MIC = 0.5 μg/mL) are the most active against C. albicans; compounds 3a-d (MIC = 4 μg/mL; fluconazole MIC = 1 μg/mL) are the most active against C. parapsilosis; and compounds 3a, 3c (MIC = 4 μg/mL), 3b (MIC= 8 μg/mL) and 3d-f (MIC = 16 μg/mL; fluconazole MIC = 32 μg/mL) are the most active against C. krusei.
In this study, the new thienopyrimidine derivatives did not show stronger antibacterial activity than the compounds used as standards (piperacillin-tazobactam). However, our compounds showed particularly high activity in comparison with fluconazole used as a standard against yeast-like fungi C. krusei. Based on these antifungal activities data, further studies with new derivatives can be expedient.
3. EXPERIMENTAL
3.1. General Chemistry
Materials and methods. The chemicals were supplied from Merck, Aldrich and Fluka. The melting points were taken in capillary tubes on a Stuart SMP30 melting point apparatus and remained uncorrected. The reactions were monitored by thin-layer chromatography (TLC) using precoated aluminum sheets (silica gel 60 F 2.54 0.2 mm thickness). The mobile phase was ethyl acetate and hexane (2:1 or 3:1), and the detection was made using UV light. The 1H and 13C NMR spectra were recorded on Varian-Mercury 400 (1H, 400 MHz; 13C, 100 MHz) spectrometer using DMSO-d6 as solvent and TMS as the internal standard. All chemical shifts are expressed in ppm. The mass spectra of synthesized compounds were obtained on Thermo Scientific Quantum Access max LC-MS spectrometer.
3.2. Chemical Synthesis
Initial iminoester hydrochloride derivatives (compounds 1a-f) were synthesized according to the Pinner method as described in [21, 22].
General procedure for the synthesis of compounds 2a-f. A mixture of 2-aminothiophene-3-carboxamide (0.010 mol) and corresponding iminoester hydrochlorides (1a-f) (0.012 mol) in 1,4-dioxane (20 mL) was taken in a round bottom flask. The solution was stirred for 1 h at room temperature and then refluxed for 7 h. The product was precipitated by the addition of water, filtered off, washed with water, and recrystallized from ethanol.
2-(4-Methylbenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2a). Yield 75%, mp: 198 – 199°C. 1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 2.24 (3H, s, CH3); 3.88 (2H, s, CH2); 7.11 (2H, d, J = 7.2 Hz, Ar H); 7.20 (2H, d, J = 7.2 Hz, Ar H); 7.31 (1H, d, J = 6 Hz, Ar H); 7.46 (1H, d, J = 6 Hz, Ar H); 12.57 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 21.07 (CH3); 40.27 (CH2); 115.60; 121.28; 122.76; 123.46; 125.34; 129.23; 129.35; 129.69; 133.71; 136.56; 157.40 (Ar C); 158.80 (C=N); 165.29 (C=O). LC-MS, m/z: 256.92 [M+H]+.
2-(3-Methylbenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2b). Yield 70%, mp: 165°C (dec.).1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 2.23 (3H, s, CH3); 3.90 (2H, s, CH2); 7.06 – 7.09 (1H, m, Ar H); 7.17 – 7.24 (3H, m, Ar H); 7.33 (1H, d, J = 6 Hz, Ar H); 7.42(1H, d, J = 6 Hz, Ar H); 12.37 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 21.40 (CH3); 41.02 (CH2); 115.72; 121.20; 122.56; 124.02; 126.04; 126.43; 128.98; 129.71; 133.65; 136.45; 157.38 (Ar C); 158.76 (C=N); 165.27 (C=O). LC-MS, m/z: 256.96 [M+H]+.
2-(4-Chlorobenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2c). Yield 77%, mp: 211 – 212°C.1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 3.94 (2H, s, CH2); 7.32 – 7.36 (5H, m, Ar H); 7.48 (1H, s, Ar H); 12.61 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 39.91 (CH2); 121.83; 121.89; 123.63; 128.69; 128.92; 131.34; 132.09; 135.69; 156.82 (Ar C); 158.75 (C=N); 165.14 (C=O). LC-MS, m/z: 276.87 [M (Cl35)+H]+, 279.01 [M (Cl37)+H]+
2-(3-Chlorobenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2d). Yield 73%, mp: 204 – 205°C.1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 3.96 (2H, s, CH2); 7.30 – 7.37 (4H, m, Ar H); 7.89 – 7.93 (2H, m, Ar H); 12.98 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 39.87 (CH2); 115.70; 116.45; 121.80; 123.24; 127.00; 128.52; 129.31; 132.12; 132.74; 146.58 (Ar C); 156.75 (C=N); 162.11 (C=O). LC-MS, m/z: 276.88 [M (Cl35)+H]+, 279.02 [M (Cl37)+H]+
2-(4-Bromobenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2e). Yield 80%, mp: 220 – 221°C.1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 3.93 (2H, s, CH2); 7.21 – 7.30 (4H, m, Ar H); 7.49 – 7.51 (2H, m, Ar H); 12.61 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 39.90 (CH2); 120.57; 121.81; 122.33; 123.61; 131.67; 131.83; 132.34; 156.74 (Ar C); 158.73 (C=N); 165.12 (C=O). LC-MS, m/z: 320.70[M(Br79)+H]+; 322,73 [M(Br81)+H]+.
2-(3-Bromobenzyl)thieno[2,3-d]pyrimidin-4(3H)-one (2f). Yield 76%, mp: 213 – 214°C.1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 3.90 (2H, s, CH2); 7.14 – 7.25 (4H, m, Ar H); 7.50 – 7.53 (2H, m, Ar H); 12.74 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 39.92 (CH2); 116.49; 118.67; 122.23; 122.60; 124.82; 130.60; 132.03; 132.37; 154.74 (Ar C); 158.63 (C=N); 165.17 (C=O). LC-MS, m/z: 320.70[M(Br79)+H]+, 322.73 [M(Br81)+H]+.
General procedure for the synthesis of compounds 3a-f. A mixture of 2-amino-4,5,6,7-tetrahydrobenzo(b)thiophene- 3-carboxamide (0.010 mol) and corresponding iminoester hydrochlorides (1a-f) (0.012 mol) in 1,4-dioxane (20 mL) was taken in a round bottom flask. The solution was stirred for 1h at room temperature and then refluxed for 7 h. The product was precipitated by the addition of water, filtered off, washed with water, and recrystallized from ethyl acetate-petroleum ether, 3:1.
2-(4-Methylbenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3a). Yield 85%, mp: 195 – 196°C.1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.72 – 1.75 (4H, m, CH2, cyclohexyl); 2.23 (3H, s, CH3); 2.66 – 2.69 (2H, m, CH2, cyclohexyl); 2.81 – 2.84 (2H, m, CH2, cyclohexyl); 3.83 (2H, s, CH2); 7.09 (2H, d, J = 8 Hz, Ar H); 7.18 (2H, d, J = 8 Hz, Ar H); 12.38 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 21.07 (CH3); 22.22; 22.94; 24.83; 25.69 (4xCH2, cyclohexyl); 39.35 – 40.60 (DMSO-d6+ CH2); 120.84; 129.14; 130.96; 131.72; 133.84; 136.31; 156.54 (Ar C); 158.98 (C=N); 163.51 (C=O). LC-MS, m/z: 311.02 [M+H]+.
2-(3-Methylbenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3b). Yield 72%, mp: 181 – 183°C. 1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.71 – 1.74 (4H, m, CH2, cyclohexyl); 2.27 (3H, s, CH3); 2.65 – 2.68 (2H, m, CH2, cyclohexyl); 2.79 – 2.82 (2H, m, CH2, cyclohexyl); 3.86 (2H, s, CH2); 7.13 – 7.22 (5H, m, Ar H); 12.63 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 21.03 (CH3); 22.14; 22.82; 24.80; 25.65 (4xCH2, cyclohexyl); 39.32 (CH2); 116.79; 118.23; 121.04; 128.95; 129.11; 130.99; 132.78; 136.62; 156.53 (Ar C); 158.96 (C=N); 163.63 (C=O). LC-MS, m/z: 311.03 [M+H]+.
2-(4-Chlorobenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3c). Yield 88%, mp: 160 – 161°C [23, 24]. 1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.66 – 1.70 (4H, m, CH2, cyclohexyl); 2.56 – 2.60 (2H, m, CH2, cyclohexyl); 2.62 – 2.65 (2H, m, CH2, cyclohexyl); 3.77 (2H, s, CH2); 7.18 (2H, d, J = 8 Hz, Ar H); 7.35 (2H, d, J = 8 Hz, Ar H); 12.43 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 22.64; 23.18; 24.59; 26.05 (4xCH2, cyclohexyl); 35.65 (CH2); 115.83; 123.80; 127.46; 128.69; 131.11; 131.74; 131.83; 134.34; 146.53 (Ar C); 159.45 (C=N); 164.43 (C=O). LC-MS, m/z: 330.91 [M (Cl35)+H]+, 332.97 [M (Cl37)+H]+.
2-(3-Chlorobenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3d). Yield 85%, mp: 220°C (dec.) (CAS number: 1082465-79-9). 1H NMR (400 MHz, DMSO-d6): 1.67 – 1.70 (4H, m, CH2, cyclohexyl); 2.55 – 2.61 (2H, m, CH2, cyclohexyl); 2.60 – 2.63 (2H, m, CH2, cyclohexyl); 3.75 (2H, s, CH2); 7.17 – 7.27 (4H, m, Ar H); 12.58 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 22.62; 23.13; 24.55; 26.02 (4xCH2, cyclohexyl); 35.69 (CH2); 115.78; 116.90; 123.45; 128.02; 128.79; 130.98; 132.04; 133.07; 134.24; 146.62 (Ar C); 159.53 (C=N); 164.51 (C=O). LC-MS, m/z: 330.94 [M (Cl35)+H]+, 332.98 [M (Cl37)+H]+.
2-(4-Bromobenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3e). Yield 90%, mp: 175 – 176°C. 1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.70 – 1.75 (4H, m, CH2, cyclohexyl); 2.66 – 2.70 (2H, m, CH2, cyclohexyl); 2.81 – 2.83 (2H, m, CH2, cyclohexyl); 7.26 (2H, d, J = 8 Hz, Ar H); 7.49 (2H, d, J = 8 Hz, Ar H); 12.43 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 22.20; 22.54; 24.83; 25.68 (4×CH2, cyclohexyl); 33.42 (CH2); 120.53; 120.95; 131.01; 131.61; 131.82; 131.92; 145.77; 155.91 (Ar C); 158.94 (C=N); 164.73 (C=O). LC-MS, m/z: 374.88[M(Br79)+H]+, 376.91 [M(Br81)+H]+.
2-(3-Bromobenzyl)-5,6,7,8-tetrahydrobenzo[4,5]thien o[2,3-d]pyrimidin-4(3H)-one (3f). Yield 80%, mp: 188 – 190°C. 1H-NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.70 – 1.74 (4H, m, CH2, cyclohexyl); 2.67 – 2.71 (2H, m, CH2, cyclohexyl); 2.82 – 2.84 (2H, m, CH2, cyclohexyl); 3.74 (2H, s, CH2); 7.25 – 7.33 (4H, m, Ar H); 12.58 (1H, s, NH). 13C-NMR (100 MHz, DMSO-d6), δ, ppm: 22.22; 22.51; 24.80; 25.65 (4×CH2, cyclohexyl); 33.47 (CH2); 116.78; 118.67; 120.05; 120.74; 129.89; 131.27; 131.75; 132.03; 144.91; 155.79 (Ar C); 158.90 (C=N); 165.07 (C=O). LC-MS, m/z: 374.88[M(Br79)+H]+, 376.91 [M(Br81)+H]+.
3.3. Antimicrobial Activity Assay
The antimicrobial activity of compounds was tested using the modified agar dilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI) [21, 22]. The minimum inhibitory concentration (MIC) for each compound was determined against standard bacterial and fungal strains. Bacterial strains of S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922, P. aeruginosa ATCC 27853 was obtained from American Type Culture Collection (ATCC) Rockville, MD. Fungal strains of General procedure for the synthesis of compounds C. albicans and C. tropicalis strains were used as described by the Ege University Medical Faculty Microbiology Department. The bacterial strains were grown in Muller Hinton broth (HiMedia Laboratories Pvt. Ltd. Mumbai-India), and the fungal strains were grown on RPMI 1640 Broth (Sigma-Aldrich Chemie GmbH Taufkirchen, Germany). To obtain the standard inoculum, the blurs of bacteria and fungi were prepared according to the McFarland 0.5 conjugate. Test solutions of all compounds were prepared in DMSO. All sample dilutions were made with distilled water. The serial concentrations of test compounds were 800, 400, 200, 100, 50, 25, 12.5 and 6.25 μg/mL.
Fluconazole for fungi and piperacillin-tazobactam for bacteria were used as standard drugs. The standard inoculant of bacteria and fungi (106 CFU/mL) were seeded on agar plates with sterile plastic ring nose (0.01 mL). All plaques were evaluated at 35°C for 16 – 20 h for bacteria and 48 h for fungi. The minimum concentrations of compounds, which prevented the proliferation of test bacteria and fungi were defined as their minimum inhibitory concentration (MICs).
References
T. Eicher and S. Hauptmann, The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications, 2nd Edition, Wiley-VCH Verlag GmbH (2005), pp. 1 – 4.
A. J. Folkes, K. Ahmadi, W. K. Alderton, et al., J. Med. Chem., 51(18), 5522 – 5532 (2008).
F. E. M. El-Baih, H. A. S. Al-Blowy, H. M. Al-Hazimi, Molecules, 11(7), 498 – 513 (2006).
R. V. Chambhare, B. G. Khadse, A. S. Bobde, et al., Eur. J. Med. Chem., 38(1), 89 – 100 (2003).
N. T. Pokhodylo, O. Y. Shyyka, R. D. Savka, et al., Phosphorus, Sulfur, Silicon, 185, 2092 – 2100 (2010).
M. R. Bhadane, J. N. N. Sharath Chandra, and L. V. G. Nargund, Der Pharma Chemica, 3(4), 238 – 244 (2011).
P. Rashmi, L. V. G. Nargund, K. Hazra, et al., Arch. Pharm. Chem. Life Sci., 344, 459 – 465 (2011).
V. K. Akbari, P. D. Patel, and K. C. Patel, Int. J. Chem. Tech. Res., 5(1), 142 – 155 (2013)
X. Ji, T. Peng, X. Zhang, et al., Bioorg. Med. Chem., 22(7), 2366 – 2378 (2014).
J. A. S. Mulla and M. I. A. Khazi, Med. Chem. Res., 23(6), 3235 – 3243(2014).
S. Saxena, G. Samala, J. Renuka, et al., Bioorg. Med. Chem., 23(7), 1402 – 1412 (2015).
J. W. De Schutter, J. Park, C. Y. Leung, et al., J. Med. Chem., 57(13), 5764 – 5776 (2014).
X.-F. Wu, S. Oschatz, M. Sharif, et al., Tetrahedron, 70(1), 23 – 29 (2014).
X. Yang, G. Cheng, J. Shen, et al., Org. Chem. Front., 2, 366 – 368 (2015).
M. R. Prasad, A. R. Rao, P. S. Rao, et al., J. Chem. Res. (S), 5 (2002); J. Chem. Res. (M), 0149 (2002).
S. Hesse, E. Perspicace, and G. Kirsch, Tetrahedron Lett., 48(30), 5261 – 5264 (2007).
V. Goudar, P. Rashmi, U. Shantharam, et al., J. Chem. Pharm. Res., 4(6), 3100 – 3106 (2012).
L. Ouyang, L. Zhang, J. Liu, et al., J. Med. Chem., 60, 9990 – 10012 (2017).
A. Kumar Verma, A. Martin, R. Kant, et al., J. Pharm. Res., 8(11), 1677 – 1681 (2014).
A. S. Abd El-All, S. M. Sh. Atta, H. M. F. Roaiah, et al., Arch. Pharm. Chem. Life Sci., 349, 202–210 (2016).
A. Pinner, Dieimidoether und ihre Derivate, 1. Auflage, Oppenheim, Berlin (1892).
E. Menteşe, H. Bektaş, S. Ülker, et al., J. Enzym. Inhib. Med. Chem., 29(1), 64 – 68 (2014).
National Committee for Clinical Laboratory Standarts (NCCLS). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 8th ed., M 07-A8. Clinical and Laboratory Standards Institute, Wayne, PA (2008).
National Committee for Clinical Laboratory Standarts (NCCLS). Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed., M 27-A3. Clinical and Laboratory Standards Institute, Wayne, PA (2008).
M. R. Prasad and D. P. Kishore, Chem. Pharm. Bull., 55(5), 776 – 780 (2007).
C. J. Shishoo, M. B. Devani, S. Ananthan, et al., Indian J. Chem., Sect. B, 28B(12), 1039 – 1045 (1989).
Acknowledgements
This study was supported by the Scientific Research Project Coordination Unit of Karadeniz Technical University. Project number: FBA-2016-5461.
We would like to thank Assist. Prof. Dr. Hasan SAĞLAMEL for the English review. We would like to thank Statistician Tuðba GÜVEN
KURT, KTÜ Farabi Hospital, Statistical Unit for the statistical data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kahveci, B., Doğan, İ.S., Menteşe, E. et al. Synthesis and Antibacterial and Antifungal Activity of New Thieno[2,3-d]Pyrimidin-4(3H)-One Derivatives. Pharm Chem J 54, 647–653 (2020). https://doi.org/10.1007/s11094-020-02252-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02252-5