Experiments on cell cultures addressed the antiviral properties of 2-anilino-4,6-di-tert-butylphenol and N-(3,5-di-tert-2-hydroxyphenyl)acetamide, both of which are o-aminophenol derivatives, and 3,4-dihydroxy-benzoic acid against herpes simplex virus and in experimental cutaneous herpes in white mice. Assessment of the data obtained here indicates that all three study compounds were significantly less active than acyclovir in cell cultures. In experiments on laboratory animals, the efficacy of ointment containing N-(3,5-di-tert-2-hydroxyphenyl) acetamide was comparable with that of acyclovir ointment, while ointments containing 2-anilino-4,6-di-tert-butylphenol and 3,4-dihydroxybenzoic acid were less active.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The ability of viruses to acquire resistance to drugs largely determines the need to develop new effective antiviral compounds with original mechanisms of action. Current methods for creating new antiviral substances are based on information on interactions between viruses and cells and the patterns of biochemical processes underlying virus viability. The main property of the most effective inhibitors of various viral infections (herpes, influenza, HIV) is their ability to take part in inactivating key enzymes responsible for virus replication [1]. However, these substances have, along with their virus-inhibiting properties, undesirable side effects as a result of possible actions on the genetic apparatus of host cells. This accounts for the attraction of the search for substances able to inhibit the processes of virus multiplication at stages not associated with the encoding and utilization of viral genetic information. These properties can be found with compounds able to regulate the probability and direction of free-radical processes in biological systems. This occurs because the production of reactive oxygen species and activation of lipid peroxidation accompany a variety of viral infections [2]. In this light, we have studied the antiviral properties of many natural and synthetic phenolic compounds with antioxidant properties.
The resulting data established that aminophenol derivatives include substances with antiviral properties and effectively suppressing the multiplication of herpes and influenza viruses [3, 4], as well as HIV [5]. Hydroxyl-containing benzoic acid derivatives also included substances with antiviral properties [6]. Aminophenol and benzoic acid derivatives were found to have marked antiherpetic activity and to be effective regulators of free-radical processes involving radical particles of different types [3, 7, 8]. The antiviral properties of these substances correlated with their ability to suppress free-radical reactions damaging cell membrane components and not their ability to influence the generation of reactive oxygen species by phagocytes [3].
In this regard, there is interest in comparing the antiviral properties of 2-anilino-4,6-di-tert-butylphenol (I), N-(3,5-di-tert-2-hydroxyphenyl)acetamide (II), 3,4-dihydroxybenzoic acid (III), and acyclovir (IV) (Fig. 1) in identical conditions, as their virus-inhibiting activities are mediated by different mechanisms of action; this was the subject of the present work.
Aminophenols I and II were synthesized as described in [9, 10]. Compounds III and IV were obtained from Sigma-Aldrich and used without preliminary purification. The purity of the study compounds was at least 97%.
Experimental Section
Studies of the antiviral activity of substances used a human rhabdomyosarcoma cell line (RD) and herpes simplex virus type I (HSV, strain 1C). Manipulations were performed as described previously [11]. The extent of the cytopathic effect of the virus at different cell culture multiplicities of infection was used to determine the virus titer in the presence and absence (virus control) of test substances. The primary criterion for antiviral activity was the presence of differences from the virus control. The concentrations of substances suppressing virus multiplication by 50% (50% effective concentration, ED50) and 90% (ED90) were determined using a computer program based on probit analysis and weighted linear regression [12]. The maximum tolerable concentrations (MTC) of compounds for cell cultures were determined at 48 – 72 h of incubation in an incubator on the basis of the morphology of unstained monolayers.
Experimental samples of ointments containing study compounds for studies of their efficacies in experimental cutaneous herpes were prepared in ointment base containing a mixture of Vaseline with 5% Vaseline oil. Acyclovir ointment (2.5%) prepared by RUP Belmedpreparaty was used for comparison. Experiments used white mongrel mice weighing 20 – 25 g (males, Institute of Bioorganic Chemistry, Belarus National Academy of Sciences) and HSV. Experimental cutaneous herpes in mice was produced as described in [13] with some modifications relating mainly to the means of anesthesia and the virus strain used [14].
Treatment of animals started the day after infection; ointments containing compounds I – IV were used and treatment was continued for five days. Ointment was applied three times a day. Efficacy was assessed in terms of the extent of development of signs of infection as compared with the control group of animals treated with ointment base. The presence of erythema, depending on the severity and area of coverage, was assessed as 0.5 or 1 point; the presence of vesicles, crusts, scabs, and ulcers scored 1 – 2 points (1 – isolated lesions; 2 – confluent lesions). Scores for erythema and the presence of vesicles were summed. Mean scores were calculated for each group of animals.
Results and Discussion
Data obtained from cell cultures indicate that the MTC of compounds increased sequentially from I to IV (Table 1).
All study compounds had antiviral properties, the most active of which was compound II. The feature of this compound was its quite low EC50 and EC90 values and its wide range of nontoxic concentrations with antiviral actions (MTC/EC50 = 87.3, MTC/EC90 = 51.3).
Compounds I and III were less active. The data obtained here provide evidence that test compounds were less active in terms of antiviral activity in cell cultures than acyclovir, which had low EC50 and EC90 values; they were practically nontoxic. Only experiments with compound II gave EC90 values comparable with that of acyclovir.
Testing of ointments containing substances I-IV was performed in experiments on laboratory animals. All groups of animals showed increases in the severity of infection in the 2 – 4 days after infection, with a subsequent decrease. In the control group, treated with ointment base, the severity of the signs of infection reached 1.22 points by day 4 and decreased two-fold on day 5, after which there was a gradual further reduction to a level of 0.27 points at the end of the observation period (10 days).
In the group of animals treated with 2.5% acyclovir ointment, the maximum mean assessment was 0.57 points (day 4) with a subsequent sharp decrease by a factor of four or more, while signs of infection in this group on day 7 were already completely absent. In the group treated with ointment containing 1% compound II, the maximum severity of signs of infection reached 0.4 points (days 4 and 5), while there were no signs on day 8. Treatment of animals with ointment containing 1% compound I was slightly less effective. The maximum severity of signs of disease was also reached on day 4 and was 0.68 points. On subsequent days there was a gradual reduction in signs. These were present to the end of the observation period but were less severe than in control group. In the group of animals treated with ointment containing 3% compound III, the maximum mean score was 1.01 pints (day 4) with a subsequent sharp decrease to 0.24 points on day 6.
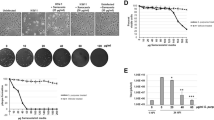
Comparison of the efficacy of the experimental test ointments containing different substances is shown in Fig. 2. Control data were obtained from animals treated with ointment base.
Efficacies of ointments containing I – IV in experimental cutaneous herpes in mite mice (6 – 20 animals per group). The ordinate shows the severity of signs of infection, points; the abscissa shows days after infection. On the abscissa: * statistically significant differences compared with ointment base group.
It follows from these data that among the study sample ointments, the most effective was acyclovir ointment. Ointment containing compound II was almost as effective. These were followed, in decreasing order of efficacy, by compounds I and III. Complete disappearance of signs of infection by the end of the observation period occurred only as a result of using acyclovir ointment and compound II ointment. The same tendency to changes in the antiherpes activity of test substances was seen in experiments on cell cultures.
We believe that particular attention should be paid to the fact that in cell cultures, the activity of the guanosine analog acyclovir, whose antiviral properties are associated with inhibition of viral DNA synthesis, was significantly higher than those of substances I-III. In particular, the EC50 values of compounds I-III were higher than that of EC50 by factors ranging from 8.7 to 71.4. At the same time, differences in the efficacies of ointments containing test compounds I-III and acyclovir IV were less marked in experiments on laboratory animals. Thus, assessment of the signs of disease at the peak of infection (Fig. 2) indicates that acyclovir ointment was more effective than ointment containing compound I by a factor of only 1.2, while it was 1.8 times as effective as ointment containing compound III. Use of ointment containing compound II decreased the severity of signs at the peak of infection by a factor of 1.5 compared with the effect of acyclovir ointment.
The cause of these differences may be, in particular, the conditions for the test compounds to produce virus-inhibiting activity in vitro and in vivo. Our further studies will seek to establish which factors lead to the quantitative differences in properties on testing compounds with different mechanisms of formation of the virus-inhibiting action in experiments on cell cultures and laboratory animals.
References
E. De Clercq, Clin. Microbiol. Rev., 10(4), 674 – 930 (1997).
B. Halliwell, and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Oxford University press, Oxford (2012), pp. 609 – 613.
O. I. Shadyro, G. A. Ksendzova, G. I. Polozov, et al., Bioorg. Med. Chem. Let., 18(7), 2420 – 2423 (2008).
O. I. Shadyro, V. L. Sorokin, G. A. Ksendzova, et al., Khim.-Farm. Zh., 46(7), 27 – 30 (2012); Pharm. Chem. J., 46(7), 414 – 417 (2012).
Patent of the Republic of Belarus 11933; Afitsyiny Byul. Nats. Tséntra Intélekt. Ulasnastsi, No. 3, 42 (2009).
Patent of the Republic of Belarus 17000; Afitsyiny Byul. Nats. Tséntra Intélekt. Ulasnastsi, No. 2, 56 – 57 (2013).
S. N. Samovich, S. D. Brinkevich, and O. I. Shadyro, Rad. Phys. Chem., 82(1), 35 – 43 (2013).
G. A. Ksendzova, V. L. Sorokin, I. P. Edimecheva, and O. I. Shadyro, Free Radic Res., 38(11), 1183 – 1190 (2004).
O. I. Shadyro, V. L. Sorokin, G. A. Ksendzova, et al., Khim.-Farm. Zh., 36(8), 14 – 16 (2002); Pharm. Chem. J., 36(8), 410 – 412 (2012).
O. I. Shadyro, V. L. Sorokin, G. A. Ksendzova, et al., Khim.-Farm. Zh., 37, No. 8, 5 – 7 (2003); Pharm. Chem. J., 37, No. 8, 399 – 401 (2003).
E. I. Boreko, N. I. Pavlova, G. V. Zaitseva, and I. A. Mikhailopulo, Vopr. Virusol., No. 5, 40 – 42 (2001).
K. P. Fung, Comput. Biol. Med., 19, No. 2, 131 – 135 (1989).
M. R. Boyd, T. N. Bacon, and D. Sutton, Antimicrob. Agent Chemother, 32(1), 358 – 363 (1988).
E. I. Boreko, O. V. Savinova, N. I. Pavlova, et al., “Current problems in infectious pathology in humans (epidemiology, clinical features, virology, microbiology, and immunology)” [in Russian], in: Proceedings of the Science Research Institute of Epidemiology and Microbiology for the State Scientific-Technical Program “Infections and Medical Biotechnology” 2001 – 2005 [in Russian], Minsk (2005), pp. 430 – 437.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 7, pp. 45 – 48, July, 2019.
Rights and permissions
About this article
Cite this article
Shadyro, O.I., Sorokin, V.L., Ksendzova, G.A. et al. Comparative Evaluation of the Antiherpes Activity of Compounds with Different Mechanisms of Action. Pharm Chem J 53, 646–649 (2019). https://doi.org/10.1007/s11094-019-02055-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02055-3