Stability study for dolutegravir bulk drug was performed and the degradation products formed were identified by chromatography (HPLC, LCMS) and spectroscopy (ESI-MS, FTIR, 1H and 13C NMR) techniques. Degradation of the drug in acidic and peroxide environment yielded one common major degradant f ((2,4-difluorophenyl) methanamine) with a mass peak at m/z = 182.44. In addition to this, five more minor degradation products (a, b, c, d and e) were formed. The structure interpretation showed that the drug degraded to its synthetic precursor and no extra structures were formed. Hence, it was suggested that drug dolutegravir should be kept away from acidic and oxygen rich conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Dolutegravir (DG) is an antiviral drug that acts by inhibiting integrase enzyme in HIV (Fig. 1) [1]. It was approved by FDA in 2013 under the class of integrase inhibitors for HIV therapy. WHO has included DG as first line drug for the treatment of AIDS in 2016. The clinical trials with DG were found to be effective, well tolerated, and showed less adverse effects than efavirenz [2]. Further, DG is associated with less drug interactions and good generic potential. Its safety and pharmacokinetics in pregnant women were studied and it was reported that DG was well tolerated by pregnant women and no congeniality was observed in newborns, but achieved HIV suppression [3]. Although DG does not exhibit severe toxicity, it is necessary to study the toxicity of its degradants which may form during long-term storage. Several analytical methods using UV spectroscopy and HPLC were reported for the analysis of DG in bulk, formulations, and combinations [4, 5]. Few stability indicating methods for DG have been reported [6, 7]. Stability studies as per ICH guidelines indicated stress conditions that favored degradation of drug compounds [8]. Usage of antiviral drugs is increasing because of an increase in viral infected patients worldwide.
Thus there is a need in studying the stability of drugs, identifying possible degradants, and assessing their toxicity profiles. Drug storage is an important aspect where cause of degradation is high and may be ingested unknowingly. This may lead to further incidence of toxicity in humans. In the present study we focused on the identification, isolation and characterization of degradants formed during DG storage under stress conditions.
2. MATERIALS AND METHODS
2.1. Materials and Reagents
Pharmaceutical grade pure dolutegravir sodium was obtained as a gift sample from Mylan Laboratories (Hyderabad, India), and was stored under refrigerated cool conditions.
The general synthetic route was reported in the literature [9]. HPLC grade acetonitrile and water were procured from Merck Pvt. Ltd. (Mumbai, India). Silica Gel-G 200# was procured from Sigma Aldrich (Mumbai, India). AR grade petroleum ether, chloroform, ethyl acetate, and methanol for purification of stressed samples by column chromatography were purchased from SDFine Ltd. (Hyderabad, India). Precoated F256 Aluminum TLC plates 20 20 cm with were purchased from Merck Pvt. Ltd. (Mumbai, India).
2.2. HPLC Instrumentation and Conditions
The chromatographic separation was performed using Agilent G4286A series compact LC system equipped with isocratic pump, variable wavelength UV detector, and an injector containing 20 μL fixed loop (Agilent Tech, Bangalore, India). The data were obtained and processed using EZ-Chrome Elite software. A reverse phase C18 column (Agilent ODS UG 5 column, 250 mm 4.6 mm] was used for HPLC studies. The isocratic elution used solvent A and solvent B as mobile phases containing water and acetonitrile, respectively. The optimized mobile phase composition for the study comprised solvent A: solvent B in a ratio of 40:60. The mobile phase was filtered through 0.22 μm membrane and sonicated for 15 min prior to use. Chromatographic separation was achieved at a flow rate of 1 mL/min with UV detection at 260 nm. The column was maintained at room temperature.
2.3. Validation of RP-HPLC Method
The developed RP-HPLC method for DG determination has been validated as per the ICH guidelines. The parameters studied for validation included linearity, system suitability, intra-day and inter-day precision, limit of detection (LOD), limit of quantitation (LOQ), robustness of the instrument, and ruggedness. The obtained data were within the acceptable limits and the values of parameters were as reported in Table 1.
2.4. Stability Studies
The drug was exposed to various stress conditions as per the ICH guidelines to know its degradation process and stability. The conditions of acid (0.1 N HCl), base (0.1 N NaOH), peroxide (3% v/v H2O2), UV (254 nm), and thermal (60°C) stress were used. The drug upon exposure to particular conditions for 48 h was withdrawn and neutralized.
2.5. LC-ESI/MS Instrumentation and Conditions
The analysis was performed on Waters ACQUITY series XEVO TQD LC/MS (CA, USA) equipped with ESI and used for identification of degradants products. The LC study was performed on Waters UPLC system containing quaternary pump, a column oven compartment, and a photodiode array (PDA) detector. ACQUITY UPLC BEH C18, 2.1 × 50 mm, 1.7 μm column maintained at 40°C was used for separation. A gradient mobile phase consisting of solvent A (5% acetonitrile in water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid) was used. The gradient regime was set as follows (time in minutes/%B): 0.01/5, 0.25/5, 7/95, 8/95, 9/5, and 10/5. The flow rate at 0.3 mL/min with a run time of 5 minutes was set. The mass spectroscopy (MS) instrument was Agilent 6520 series Q-TOF LC/MS run in electrospray ionization using both positive and negative ion mode at were an acquisition time of 0.2 sec. The ion source parameters set as follows: nitrogen gas temperature, 350°C; gas flow rate, 8 L/min; nebulizer pressure, 30 psi; and capillary voltage, 3500 V. The mass spectra were acquired within a range of m/z = 100 – 1700.
2.6. Isolation and Characterization of Dolutegravir Degradants
The stressed samples of DG were studied by HPLC and the observed degradants were isolated using column chromatography over a stationary phase (silica 200 #). Petroleum ether and ethyl acetate were used as gradient mobile phase for separating and isolating the degradants in pure form. The eluted compounds were analyzed on TLC plates using UV cabinet detector at 256 nm (Toshniwal Electronics). The collected samples from column chromatography were concentrated under vacuum using rotary evaporator. The purified degradants were characterized using spectroscopic techniques. The (1H, 13C) NMR spectra were obtained with 400 MHz Bruker (Switzerland) NMR spectrometer at 25°C in CDCl3. The FTIR spectra were recorded on ALPHA (Bruker) spectrometer. Axis LC-GC electronic weighing balance AGN-204 PO Model was used. UV-Vis spectrophotometer Lab India 3000+ was used to measure optical absorption maxima of isolated compounds.
3. RESULTS AND DISCUSSION
3.1. RP-HPLC Method Development and Validation
The proposed chromatographic method showed linearity in a range of 100 – 600°C/mL with 0.997 regression coefficient. The method was validated and the parameters of system suitability, linearity, precision, LOD, LOQ, accuracy, and robustness were found to be within the acceptable limits that are summarized in Tables 1, 2, and 3.
3.2. HPLC Detection of Degradants
The samples of DG stressed under various conditions as mentioned in Section 2.4 were analyzed by HPLC method described in Section 2.2. The retention time for the pure DG was 4.6 min (Fig. 2a ). It was fund that the drug was unstable in peroxide and acid environment. The drug was found to be stable under the action of base, UV (254 nm), and thermal (60°C) stress conditions. The retention peaks of degradants were observed after 2.5 min (Fig. 2b ). The stressed samples were also studied using TLC in UV chamber at 254 nm.
3.3. Isolation of Degradants
The drug was stressed in acid and peroxide environment as mentioned above in bulk quantity (200°C). The samples after stipulated time were neutralized and loaded on silica gel G (200#) packed column. Chloroform and methanol were used as mobile phase in gradient regime. The chromatographic separation of degradants was achieved. The eluent was collected and observed under UV cabinet. The pure isolated degradation products were concentrated under vacuum using rotary evaporator.
3.4. Data of 1 H and 13 C NMR Spectroscopy
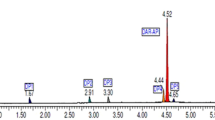
The isolated impurities were found to be pure and had amorphous character. Two degradants were isolated from the acid and peroxide stressed samples. They were characterized by MS and NMR techniques. The mass spectral data showed that pure drug had M+ at 419.72 with a retention time of 3.76 min. The isolated degradants showed m/z of 59.74, 120.68, 122.7, 182.63, 182.44, and 264.62 (Figs. 4 and 5) at retention times of 0.876, 2.844, 2.853, 5.125, 5.125 and 5.168 min, respectively (Fig. 3).
Compounds isolated from acid and peroxide stressed samples were also studied by 1H and 13C NMR. The 1H NMR spectrum of acid stressed sample (Fig. 6) displayed peaks at (ä, ppm) 8.131 (t, J = 4.2 Hz, 2H), 7.629 (t, J = 7.4 Hz, 1H), and 7.496 (t, J = 7.8 Hz, 2H). All the five protons resonate in the aromatic region. Similarly, the same product shown signals at (ä, ppm) 130.60, 130.15, and 128.45 in 13C NMR spectrum (Fig. 7). The degradant isolated from peroxide stress also showed 1H NMR peaks at 8.15 (t, J = 7.2 Hz, 2H), 7.644 (t, J = 7.4 Hz, 1H), and 7.508 ppm (t, J = 7.8 Hz, 2H). The position and number of hydrogens present in the isolated product suggested the presence of substituted benzene ring. The chemical structure of compound f was supported by the mass peak at m/z = 182.13 in positive mode ESI, having a retention time of 5.07 min in LC. The isolated compound f was analyzed by FTIR spectroscopy (Fig. 8) and showed peaks at (KBr, cm-1) 3200.17 (N-H str.), 3003.21 (Ar-H str.), 1567.75 (C=C stretching), 1489 (strong, CH2 bending), 1290.45 (strong, C-F stretching).
Thus, the obtained data show that the main degradant is f, which is a side chain molecule getting detached in acidic and peroxide environment conditions. Among the other degradants observed in LCMS, compound a with mass peak at 264.62 was a major component. The stressed sample also showed a degradant which present in trace amount, compound e, with mass peak at 59.74 (N-methylformamide). It is also a starting reactant in DG synthesis. It was found that compounds b (M+ 182.63) and f (M+ 182.44) have almost the same molecular mass with slight difference. This is attributed do the formation of potassium complex ion (M+39) with compound f, which is due to the ESI mode of ionization. The other compounds observed are c (5,6-dihydro-4-methyl-2H-1,3-oxazine) and d (1,4-dihydropyridin-3-ol) with mass peaks at 122.7 and 120.68 respectively. Both have complex ions with sodium in mass spectra (M+Na) and are attributed due to the ESI mode of ionization. Compounds a and b, which are found to possess an aldehyde functional group, were not formed under the stress conditions, but were observed after the column chromatographic separation. The reason for this might be further instability of products in the presence of silica in the stationary phase. There is a general tendency of silica groups to convert amide to an aldehyde and was a drawback of silica packed column. This was observed in LCMS data, but not in HPLC chromatograms, indicating the possible affect of stationary phase used in column separation. The parameters of degradants are tabulated in Table 4 and schematically shown in Fig. 9.
Thus, our present study has predicted possible degradants of dolutegravir, whose toxicity upon storage has to be determined. Further study requires knowledge of the toxicity of degradants and the probable side effects thereof associated with long-storage samples.
References
British National Formulary: BNF 69 (69th Edition), British Medical Association (2015), 429.ISBN 9780857111562.
S. Kanters, M. Vitoria, M. Doherty, et al., Lancet HIV, 3, 510–520 (2016).
N. Mulligan, B. Best, E. Capparelli, et al., in: Conference on Retroviruses and Opportunistic Infections (2016, Boston, MA, USA), Abstract 438.
G. B. Bhavar, S. S. Pekamwar, K. B. Aher, et al., Sci. Pharm., 84(2), 305 – 320 (2016).
G. B. Bhavar, K. B. Aher, R. S. Thorat, et al., Malay. J. Anal. Sci., 19(6), 1156 – 1163 (2015).
N. M. Rao and D. G. Sankar, Future J. Pharm. Sci., 1(2), 73 – 77 (2015).
X. Wang, S. D. Penchala, A. Amara, et al., Ther. Drug Monitor., 38(3), 327 – 31 (2016).
ICH Q2 (R1), Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization, IFPMA, World Health Organization: Geneva (2009).
M. Nenad, Lovro Selic, and C. Anja, Patent WO2016113372A1 (2016).
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, T.N.V.G., Vidyadhara, S., Narkhede, N.A. et al. Study of Dolutegravir Degradation and Spectroscopic Identification of Products by LCMS, 1H and 13C NMR Techniques. Pharm Chem J 53, 368–375 (2019). https://doi.org/10.1007/s11094-019-02007-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02007-x