A quantitative HPLC method was developed for analyzing mefebut in pharmacokinetic studies. The method was highly sensitive and selective and could be used for determinations in bioassays. The optimized sample-preparation method did not significantly influence the standard error of the quantitative determination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The search for efficacious and safe neuroprotector drugs remains a high-priority problem of modern neuropsychopharmacology. Natural metabolites of the nervous system are highly interesting to pharmacologists. Among them, γ-aminobutyric acid (GABA) and its derivatives that exhibit high neuroprotective activity are especially attractive [1,2,3].
A new GABA structural analog, i.e., the methyl ester of phenibut (methyl ester of 4-amino-3-phenylbutanoic acid hydrochloride, mefebut), was prepared in the Department of Organic Chemistry, A. N. Gertsen Russian State Pedagogical University (St. Petersburg, Russia) (Fig. 1).
The pharmacological properties of this compound were studied in the Department of Pharmacology, Volgograd State Medical University. The compound was confirmed to possess pronounced nootropic, neuroprotective, anticonvulsant, antihypertensive, and actoprotective properties [4].
The goal of the present study was to develop a highly sensitive and selective quantitative method for determining mefebut in order to conduct pharmacokinetic studies.
Experimental Part
The research used a Shimadzu liquid chromatograph (Japan) with a fluorescence detector at absorption wavelength 201 nm and emission wavelength 280 nm and a SUPELCOSIL LC-18 column (150 × 4.6 mm, 5 μm).
Methyl-4-amino-3-phenylbutanoate hydrochloride. A solution of 4-amino-3-phenylbutanoic acid hydrochloride (35 g, 0.16 mol) in MeOH (224 mL) was cooled to 5 – 10°C, stirred, treated in portions with thionylchloride (11.9 mL, 0.16 mol), held at the same temperature, heated on a water bath at 60 – 70°C for 2 h, and cooled to 18 – 20°C. The crystalline product was filtered off to isolate methyl-4-amino-3-phenylbutanoate hydrochloride. The filtrate was evaporated to 2/3 the initial volume. An additional amount of product was separated. Yield 34.6 g (93.5%), mp 175 – 176°C (MeOH). C11H16NO2Cl. IR spectrum (ν, cm–1, KBr): 3030 – 2785 (NH3+); 1725 (C=O). PMR (DMSO-d6, δ, ppm): 7.21 – 7.27 (5H, C6H5), 2.46 – 3.06 (5H, 2CH2, CH), 3.43 (3H, OCH3), 8.17 (3H, NH3+).
Mefebut was extracted from plasma and proteins were precipitated simultaneously using TFA (10%, 1:0.2), which provided adequate degrees of sample extraction and purification. Rat plasma (40 mL) was used for validation and calibration curve construction.
The compound was identified and its concentration calculated using the absolute standard method. The standard was mefebut drug substance synthesized at the Department of Organic Chemistry, A. N. Gertsen Russian State Pedagogical University (St. Petersburg, Russia). The dependence of peak area on mefebut concentration was analyzed using regression analysis. Results were statistically processed using the Microsoft Excel program [5].
Results and Discussion
Ion-pair chromatography that was developed by us earlier for phenibut and other GABA derivatives was used to optimize the retention time of highly polar mefebut and to improve the system selectivity [6, 7]. The modifier was heptanesulfonic acid at concentrations of 0.025 – 0.12%. The molarity and pH of the buffer were also varied. The heptanesulfonic acid concentration turned out to be the most important factor affecting the retention time and system selectivity.
As a result, a mobile phase containing MeCN (UF210, Russia) and a buffer of KH2PO4 (50 mM, pH 2.5) with added heptanesulfonic acid (0.12%) was selected as optimal. The MeCN:buffer ratio was 12:88 (v/v).
Chromatograms of mefebut standard solution were obtained using the developed chromatographic conditions. The retention time was 7.5 – 8.0 min (Fig. 2).
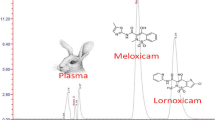
Mefebut was extracted from plasma. Samples were treated with TFA (10%, 1:0.2 ratio), shaken for 10 min in an ultrasonic bath to precipitate proteins, and centrifuged for 15 min at 3,000 rpm and 25°C in an Eppendorf centrifuge. The supernatant liquid was injected into the chromatography system. The degree of mefebut extraction was ≥ 90%. Figure 3 shows a chromatogram of the studied compound after extraction from a biological sample.
Absolute calibration was used for quantitative determination of the compound. The dependence of peak area on mefebut concentration was analyzed by regression analysis for concentrations of 0.1 – 1 μg/mL. Each concentration was tested in five parallel measurements. As a result, the calibration curves were linear with an approximation coefficient (R2) of 0.998 (Fig. 4).
The method was validated according to the Guideline on Bioanalytical Method Validation (EMEA 2012) [8]. The accuracy and precision (average measurement error) were calculated from the results. Table 1 presents the data.
The accuracy averaged 97.78%; precision, 8.32%.
Intraday percent variations (method repeatability) were determined and were % in the studied concentration ranges. Interday percent variations (method reproducibility) averaged <7% for the studied compound. The method sensitivity (limit of quantitation) was 100 ng/mL. The detection limit was 40 ng/mL (Table 2).
The average absolute percent variations were in the same ranges for repeated analysis after storage for 72 h of aqueous solutions of the compound at room temperature, indicating that the studied compound was stable.
The effects of freeze–thaw cycles showed that the average absolute percent variations for the studied compound were in the same ranges, determining that the compounds were stable to these factors.
Thus, the validation confirmed that the analytical characteristics of the quantitative HPLC method for determining mefebut complied with regulatory values (deviations from nominal concentrations and accuracy and precision should not exceed ± 20%).
Thus, the quantitative determination of mefebut was adequate for solving the stated problems of sensitivity and selectivity. The extraction method was optimal and practically did not affect the standard measurement error of the quantitative chromatographic determination method.
The developed method could be used for pharmacokinetic studies of mefebut and its possible metabolites in laboratory animals.
References
T. A. Voronina and S. B. Seredenin, Eksp. Klin. Farmakol., 70(4), 44 – 58 (2007).
I. N. Tyurenkov, L. E. Borodkina, and A. V. Voronkov, Vestn. Volgograd. Gos. Med. Univ., No. 11, 24 – 26 (2004).
I. N. Turenkov, M. N. Bagmetov, and V. V. Epishina, Eksp. Klin. Farmakol., 70(2), 24 – 29 (2007).
V. V. Bagmetova, L. E. Borodkina, I. N. Tyurenkov, et al., Fundam. Issled., 10(3), 467 – 471 (2011).
K. Doerffel, Statistik in der analytischen Chemie, Deutscher Verlag fur Grundstoffindustrie, Leipzig (1990).
I. N. Tyurenkov, V. N. Perfilova, L. A. Smirnova, et al., Khim.-farm. Zh., 44(12), 68 – 70 (2010).
I. N. Tyurenkov, V. N. Perfilova, L. A. Smirnova, et al., Khim.-farm. Zh., 47(3), 55 – 56 (2013).
Guideline on Bioanalytical Method Validation (EMEA 2012); http: /www.ema.europa.eu.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 3, pp. 58 – 60, March, 2019.
Rights and permissions
About this article
Cite this article
Smirnova, L.A., Ryabukha, A.F., Suchkov, E.A. et al. Quantitative HPLC Determination of γ-Aminobutyric Acid Derivative Mefebut in Bioassays. Pharm Chem J 53, 272–274 (2019). https://doi.org/10.1007/s11094-019-01991-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-01991-4