Chaga can be extracted with EtOAc to afford ~0.88% extracted substances, of which ~58% are lipophilic compounds with the remainder being phenolic compounds. Tetracyclic triterpenes, sterols and their esters, sesquiterpenes (azulenes), bicyclic monoterpenes (iridoids), phenolcarboxylic acids, simple phenols, and flavonoids were identified in the extract. Stilbene-type compounds, a phenanthrene derivative, and 9(11)-dihydroergosteryl benzoate were observed in chaga for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ethyl acetate (EtOAc) has been used to extract colloids from the aqueous decoction of chaga in order to broaden the compositions of lipophilic and phenolic compounds and to detect for the first time in aqueous chaga decoctions biologically active compounds such as iridoids and azulenes [1, 2]. The composition of hydrophobic substances of chaga could not be accurately evaluated in this investigation because most of them remained in the disperse phase of the aqueous decoction, i.e., melanin, and in the pulp [3].
The goal of the present work was to compare the compositions of biologically active substances extracted by EtOAc directly from chaga raw material and its aqueous decoctions.
This research was critical because it allowed the number of fungal hydrophobic substances to be revised and the composition to be broadened.
Experimental Part
Chaga raw material purchased in a pharmacy chain (batch 100412 IP Gordeev M. V.) met the corresponding requirements and was used in the work [4]. The extract was obtained by treating ground chaga with EtOAc in a Soxhlet apparatus [5] for 10 h with a raw-material—extractant ratio of 1:40. The obtained extract was evaporated in vacuo at (40 ± 5)°C. The yield was (0.88 ± 0.03)%. The dry residue was worked up (3×) with petroleum ether (bp 40 – 70°C).
The petroleum-ether extracts were combined and evaporated in vacuo (fraction 1). The residue that was insoluble in petroleum ether was dissolved in EtOAc (fraction 2).
The number of extracted compounds was determined spectrophotometrically, i.e., simple phenols and phenolcarboxylic acids using 4-aminoantipyrine [6]; flavonoids, AlCl3 solution [7, 8], tetracyclic triterpenes, vanillin [9]; sterols and their esters, FeCl3 solution [10]. Experimental data were processed using the Statistica 6.0 program.
Gas-chromatography—mass-spectrometry (GC-MS) studies used a GCMS 2010 Plus instrument (Shimadzu) with electron-impact ionization, ion-source temperature 220°C, Slb-5ms capillary column (30 m × 0.32 mm, 0.32 μm, Supelco), and He carrier gas. The chromatogram recording conditions were injector temperature 280°C, carrier-gas flow rate through the column 0.67 mL/min, flow split 1:3. Analyses were performed at constant thermostatted column temperature 280°C. Compounds were identified using the NIST-11 mass spectra database.
Results and Discussion
Compounds extracted by EtOAc from chaga raw material and its aqueous decoction were compared. Extraction of chaga by EtOAc could yield up to (0.88 ± 0.03)%, which is 3.1 times greater than the yield of extracted compounds obtained via EtOAc work up of the aqueous decoction (0.28 ± 0.05)% [1]. The EtOAc extract of the raw material reached up to (57.97 ± 2.34)% lipophilic compounds of the total extracted compounds but only (7.24 ± 0.05)% from the aqueous decoction. Correspondingly, the number of phenolic compounds in the EtOAc extract of the aqueous chaga decoction was twice that in the EtOAc extract of raw material. This agreed well with the fact that lipophilic compounds were more accessible in the raw material whereas lipophilic and phenolic compounds in the aqueous decoctions were associated more with melanin so that they had limited extractability.
Most lipophilic and phenolic compounds can remain in the raw material during aqueous work up. For example, the degree of extraction from the raw material increased to 0.4%; from its aqueous decoction, up to 20%, if petroleum ether was used to extract lipophilic compounds from the samples [11]. Changing to a less polar solvent could extract two times less lipophilic compounds from chaga; three orders of magnitude more, from its aqueous decoction, as compared with EtOAc. This was indirectly confirmed the hypothesis about the different availabilities of lipophilic and phenolic constituents in the fungal cells and melanin and their specific extraction by solvents with different polarities and other physicochemical properties.
The EtOAc extract was fractionated using petroleum ether to give fractions 1 and 2.
Fraction 1 contained tetracyclic triterpenes in addition to sterols and their esters in the amounts of (25.80 ± 1.09)% and (10.74 ± 0.26)% of the total compounds, respectively.
TLC on Sorbfil PTSKh-AF-A-UF plates identified the other compounds in fraction 1 by comparison with standards as mono- and 1,2-diglycerides, sterols, lanosterol, trialkyl glycerin esters, sterol esters, hydrocarbons (paraffins and olefins), and waxes (petroleum-ether—Et2O—HOAc, 90:10:1, FeCl3) [12]. All compounds identified in fraction 1 were observed earlier in the EtOAc extract of chaga aqueous decoction [2].
Separation of the compounds in fraction 1 using C6H6 and subsequent treatment of the chromatograms with Stahl reagent detected three spots (Rf 0.04, 0.23, 0.69) of grayish-violet and blue colors typical of sesquiterpenes (azulenes) [13]. Only one azulene compound was detected previously by analyzing the EtOAc extract of the aqueous chaga decoction [1].
Fraction 2 was analyzed to give phenolic compounds such as phenolcarboxylic acids and simple phenols in the amount of (2.56 ± 0.07)% of the total compounds and flavonoids in the amount of (1.86 ± 0.03)% of the total compounds. These compounds accounted for up to 11% of the total compounds of fraction 2. Apparently, the other compounds were more complicated polyphenols, e.g., tannins, or ballast compounds of a different nature that were very soluble in EtOAc [14].
The qualitative composition of phenolcarboxylic acids, simple phenols, and flavonoids in fraction 2 was studied using TLC on Sorbfil PTSKh-AF-A-UF plates and comparisons with standards. Phenolcarboxylic acids such as gallic, protocatechoic, m-hydroxybenzoic or p-coumaric or β-resorcylic, vanillic, veratric (C6H6—EtOH—HOAc, 96:16:8, diazo reagent) were also detected in it [13]. Simple phenols included pyrocatechol or homopyrocatechol, resorcinol or hydroquinone, indophenol, and phenol (C6H6, UV light, phosphomolybdic reagent) [6]. The flavonoid composition comprised quercetin, kaempferol or myricetin (EtOAc—HOAc—H2O, 5:1:1, UV light, AlCl3 solution) [15]. All detected phenolic compounds were identified earlier in chaga itself and its aqueous and organic extracts [1, 16,17,18,19,20]. The spectrum of phenolcarboxylic acids and flavonoids was broader in the aqueous chaga decoction than in fraction 2 [1].
Fraction 2 also contained bicyclic monoterpenes (iridoids). One spot (Rf 0.72) was detected in it using CHCl3—EtOH (25:1) and subsequent treatment of the chromatogram with Trim—Hill reagent to give the rose-lilac color corresponding to iridoids [21]. Analysis of the EtOAc extract of the aqueous chaga decoction for iridoids also detected one compound of this class [1].
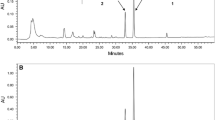
GC-MS analysis of the EtOAc extract of chaga identified six compounds (Fig. 1).
Three lipophilic compounds were identified as compound 3, inotodiol (lanosta-8,24-diene-3,22-diol) [tr 17.10, (M+) 355], which was detected earlier in aqueous chaga decoctions [22] and in the raw material [23] and possessed proven antitumor activity against cancer cells [24]. Compound 4, palmitic acid [tr 20.95, (M+) 236] and compound 6, 9(11)-dihydroergosteryl benzoate [tr 26.89, (M+) 498], were detected in chaga for the first time.
The phenolic compounds included a stilbene, compound 1 [tr 9.61, (M+) 253.08], and a flavone [compound 2, tr 10.90, (M+) 342]. Compound 5 was a phenanthrene derivative [tr 22.54, (M+) 316.4]. The phenanthrene derivative and the stilbene were detected in chaga for the first time. According to the literature [25], the identified compounds possessed anti-inflammatory and analgesic properties, were effective antioxidants, and were used to prevent cancer and treat the cardiovascular system.
Thus, EtOAc was able to extract from chaga ~0.88% of extractable substances as represented by lipophilic and phenolic compounds with biological activity. EtOAc extracted three times more hydrophobic substances from chaga raw material than from the aqueous chaga decoction. Their qualitative compositions differed.
References
M. A. Sysoeva, V. R. Khabibrakhmanova, G. I. Kyyamova, et al., Khim. Rastit. Syr’ya, No. 4, 117 – 122 (2009).
M. A. Sysoeva, V. R. Khabibrakhmanova, V. S. Gamayurova, et al., Khim. Rastit. Syr’ya, No. 1, 111 – 114 (2008).
A. N. Shivrina, Biosynthetic Products of Higher Fungi and Their Use [in Russian], Leningrad, Moscow (1966).
State Pharmacopoeia of the USSR, No. 2, Meditsina, Moscow (1990).
State Pharmacopoeia of the USSR, No. 2, Meditsina, Moscow (1987).
M. N. Zaprometov, Principles of the Biochemistry of Phenolic Compounds [in Russian], Vysshaya Shkola, Moscow (1974).
E. V. Beketov, A. A. Abramov, O. V. Nesterov, et al., Vestn. Mosk. Univ., Ser. 2, 46(4), 259 – 262 (2005).
A. A. Lobanova, V. V. Budaeva, and G. V. Sakovich, Khim. Rastit. Syr’ya, No. 1, 47 – 52 (2004).
E. N. Zhukovich, M. Yu. Semenova, L. A. Sharikova, et al., Khim.-farm. Zh., 44(3), 35 – 37 (2010); Pharm. Chem. J., 44(3), 144 – 146 (2010).
L. V. Antipov and N. N. Bezryadin, Physical Methods for Control of Meat-Industry Raw Materials and Products [in Russian], GI-ORD, St. Petersburg (2006).
M. A. Sysoeva, V. R. Khabibrakhmanova, V. S. Gamayurova, et al., Khim. Rastit. Syr’ya, No. 3, 119 – 122 (2008).
M. Kates, Techniques of Lipidology: Isolation, Analysis, and Identification of Lipids, Elsevier, New York (1972), 610 pp.
E. Stahl (ed.), Thin-Layer Chromatography: A Laboratory Handbook [translated from the German], Academic, New York (1965), 553 pp.
S. E. Severin and G. A. Solov’ev, Practicum for Biochemistry [in Russian], Izd. MGU, Moscow (1989).
M. M. Anisimova, V. A. Kurkin, and V. N. Ezhkov, Izv. Samar. Nauchn. Tsentra RAN, 1(8), 2011 – 2014 (2010).
G. L. Ryzhova, S. S. Kravtsova, and S. A. Matasova, Khim.-farm. Zh., 31(10), 44 – 47 (1997); Pharm. Chem. J., 31(10), 551 – 554 (1997).
M. A. Burmasova and M. A. Sysoeva, Khim. Rastit. Syr’ya, No. 1, 149 – 152 (2012).
W. Mazurkiewicz, Acta Pol. Pharm., No. 67, 397 – 406 (2010).
W.-F. Zheng, Mycosystema, No. 27, 575 – 581 (2008).
Y. J. Kim, J. Korean Soc. Appl. Biol. Chem., No. 54, 287 – 294 (2011).
L. R. Ivanova, L. I. Butenko, and L. V. Ligai, Khim. Rastit. Syr’ya, No. 4, 131 – 133 (2010).
A. N. Shivrina and E. V. Lovyagina, Feed Proteins and Physiologically Active Substances for Animal Husbandry [in Russian], Moscow-Leningrad (1965), pp. 59 – 64.
R.-S. Ludwiczak and U. Wrecino, Rocz. Chem., No. 36, 497 – 502 (1962).
E. V. Lovyagina and A. N. Shivrina, Biokhimiya, No. 5, 794 – 800 (1962).
A. Blazhei and L. Shutyi, Phenolic Compounds of Plant Origin [in Russian], Mir, Moscow (1977).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 12, pp. 21 – 23, December, 2017.
Rights and permissions
About this article
Cite this article
Kyyamova, G.I., Khabibrakhmanova, V.R. & Sysoeva, M.A. Hydrophobic Constituents Extracted from Chaga by Ethylacetate. Pharm Chem J 51, 1085–1087 (2018). https://doi.org/10.1007/s11094-018-1745-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1745-1




