Extraction of lipophilic substances from shells and spines of green sea urchins (Strongylocentrotus droebachiensis) was studied. It was found that the extraction time had the greatest influence on the yield of lipophilic substances whereas temperature and extraction modulus had insignificant effects. Ethanol (95%) was chosen as the extractant affording the maximum amount of lipophilic substances. Optimization of the defatting step established that the maximum yield of lipophilic substances (1.2%) was attained after extraction for 3 h at 55°C with a 1:8 raw-material—extractant ratio. Free fatty acids (57%), phospholipids (~7%), diglycerides, sterols, triglycerides, and hydrocarbons were detected in the lipophilic extract. Phospholipids in the lipophilic extract were represented mainly by phosphatidylcholine. Lysophosphatidylcholine, lysophosphatidylethanolamine, phosphatidylinositol, and phosphatidylethanolamine were detected in trace quantities. The (poly)hydroxynaphthoquinone pigment content after purifying the raw material of lipophilic substances was 98.5% according to HPLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Several biologically active compounds (BACs) isolated from marine animals, algae, and bacteria possess high pharmacological activity [1]. Sea urchins are bottom dwellers that inhabit practically all (with the exception of low-salt) seas and oceans and belong to the class Echinoidea. Several sea urchin species are commercial products. Mainly their gonads, the mass of which is ~10% of the sea-urchin mass, are used as food. Extracts of sea-urchin gonads exhibit anti-inflammatory and antidiabetic properties [2]. Wastes from commercial processing (shells and spines) could be a rich source of unique BACs [3]. Shells are calcareous structures. Pigments, i.e., naphthoquinone derivatives, give the shells different colors. The total contents of polyhydroxylated 1,4-naphthoquinone pigments (PHNQPs) in shells is 121 – 163 mg/g [4]. They differ from naphthoquinones of other marine animals by several free hydroxyls in the quinone molecule. As a result, they have clearly pronounced antioxidant properties [5]. The preparation Histochrome, which possesses antimicrobial, cardioprotective, and antioxidant activity, is based on echinochrome A, which was first isolated from the flat echinoid Scaphechinus mirabilis [6]. Spinochromes B and D and dimers based on them exhibited antiradical [7], antiallergic [8], and antidiabetic properties [9].
Therefore, the development of the optimal technology for isolating pure dimeric PHNQPs from green sea urchin shells is critical. Preliminary experiments showed that the extract obtained via demineralization of native shells contained significant amounts of lipid impurities that destabilized the pigment. The goal of the present work was to optimize the extraction of PHNQPs from S. droebachiensis shells by removing extraneous lipid impurities and increasing the purity of the final product.
Experimental Part
Shells and spines of green sea urchin S. droebachiensis gathered in Barents Sea in 2015 were studied. Shells from dissection of the animals were rinsed with tap water to remove traces of internal organs, dried in air for 1 d, ground, and used for further studies.
Extraction of lipophilic substances. Dried and ground shells with spines (20 g) were treated with extractants (160 mL) of different polarity (95% EtOH, 2-PrOH, Me2CO, hexane), stirred, and left to extract at room temperature for 3 h. When the extraction was finished, the extractant was separated, concentrated in vacuo in a rotary evaporator, and dried to constant mass. The yield of lipophilic substances was estimated gravimetrically. The remaining shells were dried in air at room temperature and used for further isolation of PHNQPs.
Contents of total free fatty acids calculated as oleic acid were determined by titration [10].
Contents of total phospholipids calculated as phosphatidylcholine were determined spectrophotometrically on a UV-1700 spectrophotometer (Shimadzu, Japan) at 475 nm with phosphatidylcholine (Sigma, USA) as a standard using the reaction with ammonium ferrothiocyanate [11].
The qualitative lipid composition was determined by TLC on Silica gel 60 F254 plates (Merck, Germany) using solvent systems petroleum-ether—Et2O—HOAc (8:2:0.1, v/v) for analysis of neutral lipids [12] and CHCl3—MeOH—HOAc—H2O (7:2:0.8:0.5, v/v) for analysis of phospholipids [13]. Solutions of test compounds (20 mg/mL) were prepared in CHCl3—MeOH (2:1, v/v); of standards (1 mg/mL), in MeOH.
Plates were detected by immersing in phosphomolybdic acid solution (2%) in MeOH for 5 sec, drying horizontally to remove the excess of solvent from the surface, and heating on a TLC Plate Heater at 120°C. Detection used a TLC Scanner 3 spectrodensitometer (Camag, Switzerland). Contents of identified compounds were calculated using internal normalization.
PHNQP purity was estimated by HPLC with a diode-array detector using a linear gradient of trifluoroacetic acid solution (0.03%) and MeCN at flow rate 1.0 mL/min. The sample volume was 20 μL. The detection wavelength was 330 nm. Chromatograms were recorded and processed using LabSolution software (Shimadzu, Japan). The PHNQP contents were estimated using internal normalization [14].
Optimization of lipophilic substance extraction used Box—Wilson experimental design. The optimization was performed using a fractional replica (1/4) with two levels of five variables in order to check the significance of effects from the factors. The independent variables were X1, the degree of grinding (fractions passing through a sieve of the corresponding size) (mm); X2, the duration of the extraction (h); X3, ultrasound effect; X4, temperature regime; and X5, raw-material—extractant ratio (extraction modulus). The process yield (Y) was defined as the yield of lipophilic substances (percent of raw-material weight). Table 1 lists the main factors and their variation ranges.
Multivariate regression analysis of the obtained results used the Statgraphics 5.0 program (Statpoint Technologies, Inc., USA).
Results and Discussion
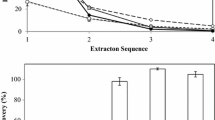
Solvent polarity effects on the yield of lipophilic substances was studied first (Fig. 1).
It was found that a nonpolar solvent (hexane) extracted less (~0.35%) lipophilic substances from sea-urchin shell than polar solvents (2-PrOH, 95% EtOH) (up to 0.7 – 0.9%). The lipid extraction efficiency depended largely on the chemical classes of the lipophilic substances and the types of complexes formed by them in this system. Polar solvents destroyed H-bonds and weakened electrostatic interactions of the lipids with proteins, which facilitated the most efficient extraction of the lipids [15]. Figure 1 shows that 95% EtOH had the maximum extraction capacity for this system. This solvent extracted the maximum amount of lipophilic substances. Therefore, it was chosen for further experiments.
The optimization consisted of determining the process parameters that gave the maximum yield of lipophilic substances. Table 2 presents results from executing the experimental design matrix.
Statistical processing of results from the factorial experiment produced a regression equation in actual units:
The model was informative and significant according to the Fisher criterion (F exp = 56.60 > F table = 4.9). The determination coefficient of Y was 97.14%; significance level p = 0.0003.
A check of the significance of the coefficients in these equations using the Student t-criterion at confidence probability 0.95 showed that coefficients X1 and X3 were statistically insignificant for the equation (Y), i.e., the degree of grinding and ultrasound effect did not significantly affect the yield of lipophilic substances.
The equation gave an impression about the quantitative effect of each significant factor on the yield of lipophilic substances. Dispersion analysis found that the extraction time (factor X2) had the greatest effect on Y whereas the effects of temperature (X4) and extraction modulus (X5) were not as important.
The lack of an effect from the degree of grinding the raw material (X1) on the yield of lipophilic substances was interesting. This probably occurred because lipophilic substances in the shell could accumulate only in the film lining its inside [16]. As a result, only a thin layer of the shell inner surface was accessible for lipid extraction. In this instance, grinding did not affect the surface area in contact with the extractant that was required to attain complete extraction. This was observed experimentally.
The lack of an effect from another process parameter, i.e., ultrasound (factor X3), could also be related to this structural feature of sea-urchin shell.
The optimal process conditions that resulted from the steepest ascent method gave the maximum yield of lipophilic substances for treatment of the raw material for 3 h at 55°C with raw-material—extractant modulus 1:8. Under these conditions, the extract gave a yield of ~1.2% lipophilic substances. A comparison of the experimental yields of lipophilic substances from the shells with the literature (0.76%) [4] led to the conclusion that the extraction under the optimized conditions gave the most complete extraction of lipids from the shell.
The lipophilic extract produced under the optimized conditions contained ~57% free fatty acids and ~7% phospholipids. TLC identified in the lipid extract neutral lipids (Table 3) and phospholipids (Table 4). Pure compounds or groups were identified based on the literature [12,13,14,15].
The lipid composition of sea-urchin gonads included phospholipids, cholesterol, lecithin, free fatty acids, di- and triglycerides, and sterol esters [2, 12].
Free fatty acids were primarily identified in the lipid extract of shells and spines under the conditions for analyzing neutral lipids. Sterols, triglycerides and diglycerides, and hydrocarbons were detected in trace quantities. The studies of the lipid extract showed that it contained phosphatidylcholine, phosphatidylethanolamine, lysophosphatidylcholine, and lysophosphatidylethanolamine. The dominant constituent was phosphatidylcholine.
Defatted shell that was purified of lipophilic substances was hydrolyzed. A series of pigments was produced and characterized by HPLC as a highly pure (98.5%) dimeric polyhydroxynaphthoquinone (Fig. 2).
Thus, an optimized process for isolating a purified dimeric polyhydroxynaphthoquinone was developed and the optimum conditions for isolating lipophilic substances were found. The pigment obtained after purifying the raw material of lipophilic substances was 98.5% pure according to HPLC.
References
K. B. Glaser and A. M. Mayer, Biochem. Pharmacol., 78(5), 440 – 448 (2009).
O. N. Pozharitskaya, A. N. Shikov, I. Laakso, et al., J. Funct. Foods, 17, 227 – 234 (2015).
T. A. Rutskova, A. A. Artyukov, E. V. Kupera, et al., Vestn. DVO RAN, No. 1, 174 – 183 (2014).
R. Amarowicz, J. Synowiecki, and F. Shahidi, Food Chem., 133(3), 822 – 826 (2012).
R. Kuwahara, H. Hatate, T. Yuki, et al., LWT – Food Sci. Technol., 42(7), 1296 – 1300 (2009).
N. P. Mishchenko, S. A. Fedoreev, and V. L. Bagirova, Khim.-farm. Zh., 37(1), 49 – 53 (2003); Pharm. Chem. J., 37(1), 48 – 52 (2003).
O. N. Pozharitskaya, S. A. Ivanova, A. N. Shikov, and V. G. Makarov, Chromatographia, 76(19 – 20), 1353 – 1358 (2013).
O. N. Pozharitskaya, A. N. Shikov, M. N. Makarova, et al., Planta Med., 79(18), 1698 – 1704 (2013).
M. A. Kovaleva, S. A. Ivanova, M. N. Makarova, et al., Eksp. Klin. Farmakol., 76(8), 27 – 30 (2013).
P. J. Ke and A. D. Woyewoda, Anal. Chim. Acta, 99(2), 387 – 391 (1978).
J. C. M. Stewart, Anal. Biochem., 104(1), 10 – 14 (1980).
A. N. Shikov, I. Laakso, O. N. Pozharitskaya, et al., Planta Med., 77(12), 1357 – 1358 (2011).
S. A. Ivanova, I. N. Urakova, O. N. Pozharitskaya, et al., Planta Med., 76(12), 1333 (2010).
A. N. Shikov, V. I. Ossipov, O. Martiskainen, et al., J. Chromatogr. A, 1218(50), 9111 – 9114 (2011).
M. A. Sysoeva, V. R. Khabibrakhmanova, V. S. Gamayurova, and A. Kh. Tazeeva, Khim. Rastit. Syr’ya, No. 3, 119 – 122 (2008).
A. C. Giese, Physiol. Rev., 46(2), 244 – 298 (1966).
Acknowledgments
The work was supported financially under State Contract GK No. 14411.2049999.19.052 “Preclinical drug research based on a bisnaphthazarin for the treatment of allergic and inflammatory eye diseases.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 5, pp. 56 – 59, May, 2017.
Rights and permissions
About this article
Cite this article
Krishtopina, A.S., Urakova, I.N., Pozharitskaya, O.N. et al. Optimization of (Poly)Hydroxynaphthoquinone Extraction from Shells of Strongylocentrotus Droebachiensis Sea Urchins. Pharm Chem J 51, 407–410 (2017). https://doi.org/10.1007/s11094-017-1623-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1623-2