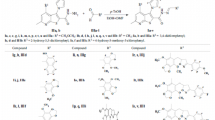
Interaction of 1,4,5-trisubstituted tetrahydropyrrol-2,3-diones with hydrazine hydrate was used to synthesize 3,4-diaryl5-(4-guanidylsulfonylphenyl)-4,6-dihydropyrrolo[3,4-c]pyrazol-6-ones. The antibacterial activity of these compounds was investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pyrazole and its derivatives have a wide spectrum of biological activities – antimicrobial, antiviral, anti-inflammatory, and analgesic [1]. Substituted tetrahydropyrrol-2,3-

diones are known to have antimicrobial, anti-inflammatory, analgesic, nootropic, and other types of activity [2, 3].
As 4-aroyl-3-hydroxy-3-pyrrolin-2-one molecules have two electrophilic centers – the carbonyl group in position 3 of the heterocycle and the side chain carbonyl group – tetrahydropyrrol-2,3-diones can react with binucleophiles, which can lead to the formation of condensed heterocyclic systems [4].
Continuing our studies of the reactivity of 3-hydroxy-3-pyrrolin-2-ones, we have investigated the reactions of previously prepared 5-aryl-4-aroyl-3-hydroxy-1-(4-guanidylsulfonylphenyl)-3-pyrrolin-2-ones [5] with hydrazine hydrate.
Boiling of the starting reagents for 1 – 2 h in glacial acetic acid was found to form 3,4-diaryl-5-(4-guanidylsulfonylphenyl)-4,6-dihydropyrrolo[3,4-c]pyrazol-6-ones (I – VII) (Table 1).
Compounds I – VII were obtained as white or pale yellow crystalline substances, soluble in dimethylsulfoxide (DMSO) and dimethylformamide and, with heating, dioxane and glacial acetic acid; compounds were insoluble in water.
The 1H NMR spectra of compounds I – VII contained signals from aromatic protons as multiplets at 7.05 – 8.26 ppm, a singlet from the methine proton at position 4 at 6.84 – 7.09 ppm, a singlet from the four amino group protons of the guanidine fragment at 6.60 – 6.63 ppm, and a singlet from the proton at the nitrogen atom of the pyrazole ring at 13.70 – 14.15 ppm.
The IR spectra of compounds I – VII contained absorption bands for stretch oscillations from amino groups at 3450 – 3176 cm-1, the lactam carbonyl at 1700 – 1716 cm-1, and the sulfonyl group in two ranges, i.e., 1384 – 1344 cm-1 and 1148 – 1136 cm-1. The spectral characteristics of compounds I – VII are shown in Table 2.
Compounds I – VII did not give the characteristic cherry coloration with ethanolic iron (III) chloride which, along with the spectral data, confirms the structure shown.
Experimental Chemical Section
1H NMR spectra were recorded on a Bruker AM-300 instrument (working frequency 300 MHz) in DMSO-d6, using tetramethylsilan as internal standard. IR spectra were taken on a Specord M-80 instrument in Vaseline paste. Elemental analysis data obtained on a Perkin Elmer 2400 instrument were consistent with calculated values. The melting temperatures of the compounds synthesized here were measured on an M-565 Melting Point apparatus.
3,4-Diaryl-5-(4-guanidylsulfonylphenyl)-4,6-dihydropyrrolo[3,4-c]pyrazol-6-ones (I – VII). A suspension of 0.01 mol of 5-aryl-4-aroyl-3-hydroxy-1-(4-gyanidylsulfonylphenyl)-3-pyrrolin-2-one in 15 – 20 ml of glacial acetic acid was supplemented with 0.012 mol of hydrazine hydrate. The reaction mix was boiled for 1 – 2 h. The precipitate forming on cooling was collected by filtration and recrystallized from glacial acetic acid.
Experimental Biological Section
The antibacterial activity of the compounds synthesized here against test strains of Staphylococcus aureus and Escherichia coli was measured by two-fold serial dilutions in liquid nutritive medium with a bacterial loading of 250,000 microbial units/ml of solution [6]. One active dose was taken as the minimum inhibitory concentration (MIC). MIC was established from the absence of signs of growth on nutritive medium and the last tube with inhibited growth (transparent solution) was the MIC of the compound against the strain being studied. Bacteriostatic effects of compounds were compared with the actions of dioxidine and chloramine B. Study results are shown in Table 3.
The results in Table 3 show that 3,4-diaryl-5-(4-guanidylsulfonylphenyl)-4,6-dihydropyrrolo[3,4-c]pyrazol-6-ones had intermediate antibacterial activity in relation to both strains.
The rather higher activity of compounds I, III, IV, and VII against St. AUreus appeared to result from the presence of one or more halogen molecules in these substances.
References
R. Mallikarjuna Rao, J. Sreeamulu, L. K. Ravindranath, et al., J. Chem. Pharm. Res., 4(1), 272 – 278 (2012).
V. L. Gein, V. V. Yushkov, T. A. Silina, et al., Khim.-Farm. Zh., 42(5), 24 – 26 (2008); Pharm. Chem. J., 42(5), 255 – 257 (2008).
V. L. Gein, L. F. Gein, N. Yu. Porseva, et al., Khim.-Farm. Zh., 31(5), 33 – 36 (1997); Pharm. Chem. J., 31(5), 251 – 254 (1997).
M. A. Mar’yasov and V. L. Gein, Tetrahydropyrrol-2,3-diones [in Russian], PGFA, Perm (2013).
V. L. Gein, I. V. Kovtonogova, O. V. Bobrovskaya, et al., Zh. Obshch. Khim., 84(2), 271 – 274 (2014).
G. N. Pershin, Methods in Experimental Chemotherapy [in Russian], Meditsinskaya Literatura, Moscow (1971), pp. 100, 109 – 117.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 50, No. 1, pp. 17 – 18, January, 2016.
Rights and permissions
About this article
Cite this article
Bobrovskaya, O.V., Kovtonogova, I.V., Gein, V.L. et al. Synthesis and Antibacterial Activity of 3,4-Diaryl-5-(4-Guanidylsulfonylphenyl)-4,6-Dihydropyrrolo[3, 4-C]Pyrazol-6-Ones. Pharm Chem J 50, 16–18 (2016). https://doi.org/10.1007/s11094-016-1390-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1390-5