Anti-complement activity is a measure of the specific safety of human immunoglobulins characterizing one of the unwanted effect - spontaneous activation of the complement system. In compliance with the specifications of the European Pharmacopeia and WHO recommendations, the anti-complement activity of human immunoglobulin preparations must not be greater than 50%, which corresponds to a maximum of 1 CH50/mg of immunoglobulin. The measurement method was based on the ability of human immunoglobulins to produce “nonspecific” (in the absence of an antigen-antibody complex) binding of complement, preventing lysis of sensitized sheep red blood cells. We present here the results of analysis of the critical parameters of the method for measuring anti-complement activity, along with assessment of existing methodological approaches to its optimization. We report experimental studies of the effects of components of the hemolytic system and complement in guinea pigs on the results of measurements of anti-complement activity. The optimum conditions for the method of measuring the anti-complement activity of human immunoglobulins are based in evidence and the anti-complement properties of human immunoglobulin preparations for intravenous use of Russian origin are evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Human immunoglobulin preparations for intravenous use (IGIV) have a stringent requirement for specific safety, one parameter of which is its anti-complement activity [1]. According to the specifications of the European Pharmacopeia (Eur. Ph.) and the recommendations of the World Health Organization (WHO), the anti-complement activity (ACA) of IGIV preparations must not be greater than 50%, which corresponds to a maximum of 1 CH50/mg of IgG [2, 3]. This level of ACA prevents patients from developing potential adverse reactions associated with effects on the complement system and supports the therapeutic efficacy of IGIV preparations [4]. The need to obtain significant values of ACA for IGIV preparations, allowing clinical results to be predicted, makes the present study relevant.
The aim of the present work was to optimize the conditions for measuring the anti-complement activity of human immunoglobulin preparations for intravenous use.
Experimental Section
Guinea pig hemolytic sera and complement were obtained from Siemens, Germany, ZAO ÉKOlab, and FGUP NPO Mikrogen, Russian Ministry of Health; pooled (in-house) guinea pig complement [3] was used; experiments used normal human immunoglobulin with protein contents of 50 mg/ml in therapeutic formulations: lyophilisate for preparation of solutions for intravenous administration; solution for intravenous administration; solution for infusions, and standard human immunoglobulin BRP (Biological Reference Preparation) batch 1 (Y0001504).
ACA was measured as described in the Eur. Ph. (monograph 01/2010:20617) [3] and Methodological Instructions MUK 3.3.2.1063 – 01 [5]. Experimental data were processed using statistical methods in Microsoft Office Excel. Results are presented as mean and standard deviation (\( \overline{x} \) ± s x ).
Studies used a PD-303S digital spectrophotometer (Apel, Japan), a model 5810R multifunction centrifuge (Eppendorf AG, Germany), an Adventurer AR-5120 precision balance (Ohaus, USA); a BD 115 incubator (Binder, Germany), and a model S220 SevenCompact pH meter (Mettler Toledo AG, China).
Results and Discussion
The method for measuring ACA was based on the ability of human immunoglobulins to bind complement, preventing lysis of sensitized sheep red blood cells. Complement activity was expressed in hemolytic units (CH50). A value of 1 CH50 was the quantity of complement which in defined experimental conditions induces the hemolysis of 50% of 5 × 108 optimally sensitized red blood cells. Binding of complement requires the presence of the required concentrations of Ca2+ and Mg2+ ions in buffer solutions. In addition, CH50 values depend on pH and the ionic strength of the system, the red blood cell concentration, and the quantity of antibodies used for sensitization.
An information-analytical search showed that the critical parameters influencing the final results of measurements of the ACA of IGIV preparations include the activity of the hemolytic serum used, the sheep red blood cells, guinea pig complement, the composition of the buffer solution, and its temperature and pH [6, 7]. Variability in these parameters generates the need to use a standard sample, positive and negative controls for which confirm the significance of the results obtained at different ranges of ACA values. Current world practice uses the BRP human immunoglobulin standard, ratified by the European Directorate for the Quality of Medicines, though WHO experts recommend using national standard samples [3].
Assays of ACA in IGIV preparations in Russia currently employ a method [5, 8] not involving use of a standard. The method described in [8], introduced in 1988, does not evaluate whether immunoglobulins meet current international specifications, i.e., interpret ACA as “retention of the activity of 2 hemolytic units in the presence of 10 mg of immunoglobulin protein.” The method described in [5], in contrast to non-Russian pharmacopoeia methods [3, 9], uses barbital in the buffer solution, dried (commercial) complement (Technical conditions TU 9398-016-14237183-2007), and a large reaction volume, and does not calculate ACA as a percentage.
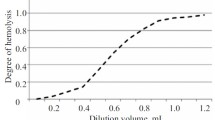
With the aim of optimizing the conditions for determining ACA, the effects of the hemolytic system (the hemolytic serum and the sheep red blood cell suspension) were studied. The method uses a dilution of hemolytic serum containing two minimum hemolytic units per ml (MHU/ml). A value of 1 MHU/ml corresponds to the lowest dilution at which further increases in the quantity of hemolytic serum do not produce any significant increase in the extent of hemolysis. Assessment of three batches of hemolytic serum from different manufacturers did not identify any differences in their activity, i.e., 2 MHU/ml was present in a dilution of 1:250 (Fig. 1).
The sheep red blood cell suspension could be prepared from sheep blood defibrinated with sodium citrate. Because of the instability of defibrinated sheep blood, it could be used for only 14 days, after which spontaneous hemolysis started, so there was a preference to use sheep blood defibrinated with sodium citrate.
Studies of the effects of guinea pig complement from different manufacturers, (two commercial and pooled (in-house) complement) showed that the ACA of the BRP human immunoglobulin standard corresponded to the verified values only when pooled (in-house) complement was used (Table 1).
Assessment of the ACA of human immunoglobulin preparations using the method described in [5], as regulated by the normative documentation, provided evidence that of all the samples studied, the greatest ACA was obtained using pooled (in-house) complement (Table 2).
Use of dried guinea pig complement as specified in MUK 3.3.2.1063-01 did not detect significant levels of ACA and cast doubt on the significance of the results obtained for predicting the safety of using preparations. The results of these studies confirm the need to use pooled (in-house) complement in the ACA measurement method.
The use of sodium barbital in the buffer solution is currently restricted by the need for the relevant licensing. Methodological recommendations [8] previously required the use of a gelatin-salt solution. Evidence was obtained for the suitability of using this in the method harmonized in the EU by finding ACA values for the BRP human immunoglobulin standard which corresponded to the verified values (Fig. 2).
The studies reported here provided evidence for the optimum conditions for ACA determinations and for developing a general pharmacopeia monograph “Determination of the anti-complement activity of medicinal formulations of human immunoglobulins for intravenous administration” [10]. Studies of the anti-complement properties of IGIV preparations from Russian manufacturers using the optimized method confirmed their compliance with international safety and efficacy requirements (Table 3).
Thus, the experiments reported here provide evidential grounds for the optimum conditions for determining ACA in IGIV preparations and developing the general pharmacopeia monograph “Determination of the anti-complement activity of medicinal formulations of human immunoglobulins for intravenous administration” which uses sheep red blood cells defibrinated with sodium citrate, hemolytic serum at the appropriate condition, pooled (in-house) guinea pig complement, and gelatin-salt solution as an alternative to gelatin-barbital buffer solution. Russian-manufactured IGIV preparations were shown to comply with international specification in terms of the ACA level when the optimized method was used.
References
O. G. Kornilova, M. A. Krivykh, N. D. Bunyatyan, et al., Farmatsiya, No. 2, 43 – 46 (2015).
WHO Expert Committee on Biological Standardization: forty-third report. Geneva, 1992; WHO Technical Report Series 840; [online resource] URL: http://whqlibdoc.who.int/trs/WHO_TRS_840.pdf (download date: March 21, 2014).
European Pharmacopoeia, edition 8.1; [Online resource] URL: http://online6.edqm.eu/ep801 (download date: 21.03.2014).
A. G. Israfilov, L. K. Lapteva, and É. Yu. Kudasheva, Proceedings of All-Russian Scientific Conference “Medical Immunobiological Preparations in the XXI Century: Development, Manufacture, and Application” [in Russian], Ufa (2005), Chapter 2, pp. 172 – 178.
Determination of the Anti-Complement Activity of Immunoglobulin Preparations for Intravenous Administration, Methodological Instructions MUK 3.3.2.1063 – 01 [in Russian], Moscow (2001).
Ann-Louise Borg, Investigation of Method for Determination of Anti-Complementary Activity (ACA) in Octagam ® (2009), pp. 1 – 52; [Online resource] URL: http://liu.diva-portal.org/smash/get/diva2:241409/fulltext01.pdf (download date: 21.03.2014).
A. Buchacher, P. Schluga, J. Mullner, et al., Vox. Sang., 98, 209 – 218 (2010).
Determination of the Anti-Complement Activity of Immunoglobulin Preparations: Methodological Recommendations of the RSFSR Ministry of Health [in Russian], Gor’kii (1988).
Indian Pharmacopoeia, Volume III (2010).
General Pharmacopeia Monograph “Determination of the anti-complement activity of medicinal formulations of human immunoglobulins for intravenous administration” Approved by the Russian Ministry of Health No. 768 of November 21. 2014 [in Russian], [Online resource] URL: http://www.rosminzdrav.ru (download date: 03.02.2015).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 49, No. 7, pp. 39 – 42, July, 2015.
Rights and permissions
About this article
Cite this article
Krivykh, M.A., Kornilova, O.G., Bunyatyan, N.D. et al. Optimization of the Conditions for Assay of the Anti-Complement Activity of Human Immunoglobulin Preparations for Intravenous Administration. Pharm Chem J 49, 473–476 (2015). https://doi.org/10.1007/s11094-015-1308-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1308-7