The effect of melatoninergic drugs [melaxen and agomelatine (Valdoxan)] on the activity of glutathione antioxidant system enzymes and NADPH suppliers was investigated for experimental hyperthyroidism in rats. The administration of these protectors at the pathology was accompanied by a change of the specific activity of glutathione reductase (EC 1.6.4.2), glutathione peroxidase (GP, EC 1.11.1.9), glutathione transferase (GT, EC 2.5.1.18), NADP-isocitrate dehydrogenase (NADP-IDH, EC 1.1.1.41), and glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49) in rat liver, heart, and blood serum toward the control values. Apparently, this effect was related to a decreased load on the glutathione antioxidant system due to effective interaction of melatonin with free radicals upon correction of its level by melaxen and agomelatine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thyroxine and triiodothyronine are known to be necessary for normal development, growth, and functioning of living systems. These hormones regulate the basal metabolism level of all cells [1]. The stimulating action of thyroid hormones (TH) on the rate of oxygen demand (calorigenic effect) by the whole organism and separate tissues and subcellular fractions is just as important [2].
High TH concentrations during thyrotoxicosis syndrome can increase the oxygen demand and stimulate free-radical (FR) production [3]. Thus, TH assist in increasing mitochondrial respiration by changing the activity of components of the mitochrondrial electron-transport chain (ETC) [4]. Acceleration of mitochondrial electron transport by TH increases superoxide anion-radical (O • −2 ) generation involving ubiquinone. In turn, O • −2 can produce other reactive oxygen species (ROS), including hydroxyl radical (OH•), which launches quickly lipid peroxidation (LPO) processes. The effect of TH on lipid exchange in tissues, in particular on LPO processes was reported. It was shown that hyperthyroidism enhances LPO processes by increasing the number of protein-binding carbonyls [5]. Increased FR formation in vivo and the enhanced LPO processes related to it disrupt biological membranes and cell functions, which can lead to the development of various diseases [6–8].
The cellular antioxidant system is the main protector against activation of LPO processes. An important component of this system is the glutathione-dependent link, which includes glutathione (GSH) and enzymes dependent on it such as glutathione peroxidase (GP, EC 1.11.1.9), which catalyzes peroxide decomposition without forming FR by using GSH as a hydrogen donor; glutathione transferase (GT, EC 2.5.1.18), which uses this thiol to metabolize hydrophobic substances; and glutathione reductase (GR, EC 1.6.4.2), which reduces oxidized glutathione to its reduced form [9]. NADPH is required as a source of the reducing equivalents for the GR reaction. One of the principal NADPH suppliers for operation of the glutathione system is known to be the pentose-phosphate pathway, a key enzyme of which is glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49), which catalyzes transformation of glucose-6-phosphate into 6-phosphogluconolactone [10]. A reaction catalyzed by NADP-isocitrate dehydrogenase (NADP-IDH, EC 1.1.1.41) during which isocitrate is oxidatively decarboxylated to 2-oxoglutarate was reported to be an alternative source of NADPH [11].
It was found that antioxidants in complex therapy of pathologies, including hyperthyroidism, can nullify depletion of the intrinsic antiradical activity and facilitate normalization of in vivo FR homeostasis [12].
The mechanism of action of natural antioxidants is known to involve their capability to react vigorously with peroxide radicals, as a result of which they are transformed from active phenols into inactive quinones [13].
Melatonin (N-acetyl-5-methoxytryptamine, C13H16N2O2) (Fig. 1) is chemically derived from the biogenic amine serotonin that, in turn, is synthesized from the amino acid tryptophan ingested with food. Its antioxidant, antitoxic, and antistress effects are now experimentally demonstrated [14].
Therefore, research on the protector effect of melatoninergic drugs (melaxen and Valdoxan) on experimental hyperthyroidism (EH) is indubitably of interest.
Melaxen (MW 232.3) is a chemical analog of the biogenic amine melatonin (Fig. 1). It is soluble in H2O, alcohol, and lipids. It passes well the blood—brain barrier. It is synthesized from plant amino acids.
Valdoxan or agomelatine [C15H17NO2, 2-(7-methoxynaphthalen-1-yl)ethylacetamide, MW 243.301] (Fig. 1) is an agonist of melatoninergic receptors MT1 and MT2 and an antagonist of serotonin 5-HT2 C-receptors [15].
The goal of the present work was to study the effect of these melatoninergic drugs on the specific activity of enzymes of the glutathione antioxidant system and enzymes that supply the reducing equivalents as NADPH in several tissues of rats with EH.
Experimental Part
We used male laboratory white rats (total number n =100) (Rattus norvegicus, 200 – 250 g). All experimental procedures complied with requirements of international rules on humanitarian use of animals that are incorporated into health rules for selection and maintenance of experimental biological clinics (vivariums) (RF Criminal Code St. 245. Harsh treatment of animals).
Hyperthyroidism was induced by i.p. injection of triiodothyronine (Sigma, USA, crystalline drug substance). The hormone in normal saline (0.9% NaCl) was injected into healthy animals at a dose of 100 μg per 100 g of body mass [16] three times per day for six days.
The animals were divided into six experimental groups. The first group (n =19) was maintained on a standard vivarium regime. The second group (n =9) had induced hyperthyroidism. The third and fourth groups (n =18) were injected i.p. with melaxen (Unipharm, USA, coated tablets) at doses of 5 and 10 mg/kg of animal body mass daily in the morning for three days. The fifth and sixth groups (n =18) of rats with EH were injected i.p. with Valdoxan (Laboratoires Servier Industrie, France, film-coated tablets) at doses of 5 and 10 mg/kg of animal body mass. The melaxen and Valdoxan solutions were prepared in a ceramic mortar by grinding the tablets and adding NaCl solution (0.9%) immediately before use. Analytical samples were taken on the seventh day after the start of the experiment.
Liver was excised by performing a laparotomy on the rats. Then, a ligature was placed on the portal vein, which was incised and cannulated 10 mm below the sinus. The anterior vena cava was severed near the diaphragm and perfused with cold isotonic saline at 5 mL/min for 5 min. The liver was reserved for further testing.
Liver tissue homogenate was prepared by grinding tissue pieces in a porcelain mortar with four-fold the volume of cold isolation medium containing Tris-HCl buffer (50 mM, pH 7.6), EDTA (10 mM), and mercaptoethanol (0.5 mM).
The extract from the homogenization was filtered through a layer of nylon with square pores (0.1 mm) and centrifuged at 5,000 g for 10 min to separate intact tissue elements. The supernatant was used in further testing.
Heart was excised after multiple rinsings with cold normal saline. The rinsed heart was dried on filter paper and thoroughly ground with scissors. The ground tissue was weighed on a torsion balance and homogenized in a porcelain mortar with three-fold the volume of cold isolation medium.
The extract from the homogenization was filtered through a layer of nylon with square pores (0.1 mm) and centrifuged at 3,500 g for 10 min to separate intact tissue elements and cardiomyocyte membranes. The supernatant was used to determine the studied parameters.
Venous blood was collected into a clean glass tube without anticoagulant and placed for 0.5 h into a thermostat at 37°C. After the phases separated, the supernatant was collected and centrifuged at 4,000 g for 10 min. The obtained serum was used for further testing.
Enzyme activity was measured on a Hitachi U1900 spectrophotometer with software. GP activity was measured in potassium phosphate buffer (50 mM, pH 7.4) containing EDTA (1 mM), NADPH (0.12 mM), reduced glutathione (0.85 mM), H2O2 (0.37 mM), and GR (1 U/mL). The control did not contain reduced glutathione. GR activity was measured in the same buffer containing EDTA (1 mM), NADPH (0.16 mM), and oxidized glutathione (0.8 mM). GT activity was determined with 1-chloro-2,4-dinitrobenzene substrate in medium consisting of potassium phosphate buffer (0.1 M, pH 7.4) containing EDTA (1 mM), 1-chloro-2,4-dinitrobenzene (1 mM), and GSH (5 mM). The medium for spectrophotometry of G6PDH was Tris-HCl buffer (0.05 mM, pH 7.8) containing glucose-6-phosphate (3.2 mM) and NADP (0.25 mM). The NADP-IDH activity was determined in Tris-HCl buffer (50 mM, pH 7.8) containing isocitrate (1.5 mM) and NADP (0.25 mM). The rates of the enzymatic reactions were judged from the optical density change at 340 nm [17]. The enzyme activity was expressed as the specific activity because the total protein content could change during modeling of EH and use of drugs capable of correcting pathological disorders. This enabled modifications of the enzyme activity to be analyzed most adequately. A unit of enzyme activity (U) was taken as the amount of enzyme catalyzing the formation of 1 ìmol of reaction product per min at 25°C. Total protein was determined by the biuret method.
Results were processed using the Student t-criterion. Differences were considered statistically significant for p <0.05.
We used melatonin, isocitrate, NADP, NADPH, EDTA, and 1-chloro-2,4-dinitrobenzene (Sigma, USA); Tris-HCl buffer (Serva, Germany); oxidized and reduced glutathione and glucose-6-phosphate (ICN, USA). The other reagents were purchased domestically and were chemically or analytically pure.
Results and Discussion
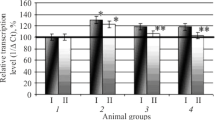
It was discovered during the course of the experiments that 1administration of melaxen and Valdoxan to rats with EH was associated with a change of GP and GR specific activity in the liver, heart, and blood serum toward the control values (Figs. 2 and 3).
Activity of glutathione peroxidase expressed as U per mg of protein in rat liver (a), heart (b), and blood serum (c) under normal conditions (1) and experimental hyperthyroidism (2) after administration of melaxen at doses of 5 and 10 mg/kg (3, 4) and Valdoxan at doses of 5 and 10 mg/kg (5, 6). Statistically significant (p ≤ 0.05): * compared with normal; ** compared with pathology.
Activity of glutathione reductase expressed as U per mg of protein in rat liver (a), heart (b), and blood serum (c) under normal conditions (1) and experimental hyperthyroidism (2) after administration of melaxen at doses of 5 and 10 mg/kg (3, 4) and Valdoxan at doses of 5 and 10 mg/kg (5, 6). Statistically significant (p ≤ 0.05): * compared with normal; ** compared with pathology.
Thus, the GR specific activity in blood serum decreased in the pathological state by 29%. Injecting melaxen at doses of 5 and 10 mg/kg increased it by 21 and 22% (Fig. 3 c). The GP specific activity increased by 19 and 20% compared with that for enzyme from blood of animals with EH (Fig. 2 c). Furthermore, the GR and GP activity in rat liver decreased with pathology by 1.3 times. Injecting melaxen at doses of 5 and 10 mg/kg increased it by 17 and 20% (for GR) (Fig. 3 a) and 10 and 19% (for GP) (Fig. 2 a), respectively. It was found that GR specific activity in rat heart increased in the pathological state by 22%. Injecting melaxen at doses of 5 and 10 mg/kg decreased it by 6 and 13%, respectively (Fig. 3 b). GP activity in rat heart increased by 7% compared with the EH group for melaxen at the studied doses (Fig. 2 b).
The observed changes of enzyme activity were probably related to the antioxidant function of the melatonin incorporated into melaxen, which exhibited a pronounced capability to bind FR [18]. Such a mechanism of action would ameliorate LPO processes and, as a result, decrease the functional load on the GP/GR system.
The effect of Valdoxan at doses of 5 and 10 mg/kg increased the GP specific activity in rat blood serum by 8 and 13% compared with the second group of animals (Fig. 2 a). The GR activity increased by 19 and 20%, respectively (Fig. 3 a). Injecting Valdoxan at the studied doses into animals with pathology increased the GP and GR activity in rat liver by 10 and 14%, respectively (Fig. 2 a and 3a, respectively), compared with animals with EH. It was found that the GR specific activity in rat heart decreased by 11 and 20% for injection of Valdoxan at doses of 5 and 10 mg/kg (Fig. 3 b) whereas GP activity increased by 14 and 21%, respectively (Fig. 2 b).
The effect of Valdoxan was probably related to its structure, in which the melatonin indole ring is replaced by a naphthalene ring. Because of this, it is highly selective for melatonin receptors (MT1 and MT2) [19], the principal part of which is concentrated in suprachiasmatic nuclei [19]. This is responsible for its ability to affect the level of melatonin, which can act as a FR trap and thereby reduce the load on the in vivo antioxidant system.
It was found that development of EH was accompanied by a reduction of GT specific activity in the liver by 1.4 times; in the heart, by 2.1 times; and in rat blood serum, by 2.2 times compared with the control level (Fig. 4).
Specific activity of glutathione transferase in rat liver (a), heart (b), and blood serum (c) under normal conditions (1) and experimental hyperthyroidism (2) after administration of melaxen at doses of 5 and 10 mg/kg (3, 4 and Valdoxan at doses of 5 and 10 mg/kg (5, 6). Statistically significant (p ≤ 0.05): * compared with normal; ** compared with pathology.
GT is known to play the main role in deactivation of LPO secondary products and biotransformation of xenobiotics [20]. Glutathione is conjugated to the toxic products during the reaction. Thus, the observed change of GT activity could be connected to depletion of the pool of reduced glutathione under conditions where ROS are extensively formed during the development of hyperthyroidism.
Injection of melaxen at doses of 5 and 10 mg/kg to rats with EH increased GT specific activity in blood serum by 1.5 and 1.8 times, respectively; Valdoxan, by 1.7 times compared with the pathological level (Fig. 4 c). The enzyme specific activity in rat liver with pathology after injection of melaxen at the studied doses increased by 20 and 22%, respectively; of Valdoxan, by 16 and 17% compared with EH (Fig. 4 a). The GT activity in heart after injection of melaxen at a dose of 5 mg/kg increased by 37%; at a dose of 10 mg/kg, by 39%; after injection of Valdoxan, by 25 and 30%, respectively (Fig. 4 b).
It is noteworthy in this respect that the change of GT activity through the action of melaxen and Valdoxan toward the control values was obviously indicative of the antioxidant properties of melatonin, owing to which it interacted efficiently with FR to form mildly toxic or non-toxic metabolites [21]. Furthermore, it is known from the literature that melatonin removes directly FR and can affect the mitochondrial respiratory chain [22].
NADP-IDH activity in liver and blood serum of animals with EH decreased whereas significant changes compared with the control were not observed in heart [23]. Apparently, this was the consequence of organ-specific changes in metabolism at high doses of TH.
NADP-IDH specific activity in liver increased by 1.5 and 1.6 times (Fig. 5 a), in blood serum by 1.3 and 1.4 times (Fig. 5 c) for injection of melaxen at doses of 5 and 10 mg/kg, respectively, into animals with EH. However, statistically significant changes of this parameter were not observed for heart tissue (Fig. 5 b). The effect of Valdoxan at the studied doses on the specific activity of this enzyme was characterized by similar trends (Fig. 5).
Activity of NADP-isocitrate dehydrogenase expressed as U per mg of protein in rat liver (a), heart (b), and blood serum (c) under normal conditions (1) and experimental hyperthyroidism (2) after administration of melaxen at doses of 5 and 10 mg/kg (3, 4) and Valdoxan at doses of 5 and 10 mg/kg (5, 6). Statistically significant (p ≤ 0.05): * compared with normal; ** compared with pathology.
G6PDH specific activity in rat heart decreased after injection of melaxen at doses of 5 and 10 mg/kg by 1.5 and 2.0 times; through the action of Valdoxan at the same doses, by 1.5 and 2.1 times, respectively (Fig. 6 c). Injection of the studied melatoninergic drugs to animals with EH decreased G6PDH specific activity in blood serum after it increased by 1.8 times with pathology (Fig. 6 c). Thus, the enzyme activity decreased by 1.2 times after injection of melaxen at doses of 5 and 10 mg/kg to rats with EH; of Valdoxan at the same doses, by 1.1 and 1.2 times, respectively (Fig. 6 c). Statistically significant changes of G6PDH activity were not observed in liver of experimental animals of the second group that were injected with melaxen and Valdoxan (Fig. 6 a). Judging from the results, it was assumed that G6PDH was responsible for supplying most of the reducing equivalents to the glutathione system under the studied conditions.
Specific activity of glucose-6-phosphate dehydrogenase in rat liver (a), heart (b), and blood serum (c) under normal conditions (1) and experimental hyperthyroidism (2) after administration of melaxen at doses of 5 and 10 mg/kg (3, 4) and Valdoxan at doses of 5 and 10 mg/kg (5, 6). Statistically significant (p ≤ 0.05): * compared with normal; ** compared with pathology.
Thus, melaxen and Valdoxan could be used to correct the functioning of enzymes in the glutathione redox cycle and also enzymes generating NADPH for this link of the antioxidant system. This was indicative of the positive effect of melatoninergic drugs on the in vivo antioxidant system with hyperthyroidism.
References
J. H. Oppenheimer, H. H. Samuels, and J. W. Apriletti, Molecular Basis of Thyroid Hormone Action, Academic Press, New York (1983), p. 485.
Yu. A. Parkhisenko, A. I. Zhdanov, and A. Yu. Tsurkan, Thyroid Diseases: Student Aide [in Russian], Izd. VGMA, Voronezh (2006), p. 116.
S. N. Kumari, N. Sandhya, and K. M. Damodara Gowda, Al Ameen J. Med. Sci., 4(1), 49 – 53 (2011).
F. Goglia, E. Silvestri, and A. Lanni, Biosci. Rep., 22(1), 17 – 32 (2002).
M. Sugawara, Y. Sugawara, K. Wen, et al., Exp. Biol. Med., 227(2), 141 – 146 (2002).
M. Aglarz, R. V. Jouyz, E. C. Viel, et al., Am. J. Hypertens., No. 17, 597 – 603 (2004).
J. Nordberg and E. S. J. Arner, Free Radical Biol. Med., 31(11), 1287 – 1312 (2001).
M. Martiner-Cayuela, Biochimie, 77(3), 147 – 161 (1995).
E. G. Gorozhanskaya, V. B. Larionova, G. N. Zubrikhina, et al., Biokhimiya, 66(2), 273 – 278 (2001).
N. A. Zherebtsov, T. N. Popova, and V. G. Artyukhov, Biochemistry [in Russian], Izd. VorGU, Voronezh (2002), pp. 335 – 338.
L. V. Medvedeva, T. N. Popova, V. G. Artyukhov, et al., Biokhimiya, 67(6), 838 – 849 (2002).
S. S. Popov, A. N. Pashkov, V. I. Zoloedov, et al., Biomed. Khim., 56(3), 397 – 403 (2010).
R. S. Tishenina, T. A. Filonenko, A. V. Dreval’, et al., Probl. Endokrinol., 46(6), 26 – 28 (2000).
K. S. El’bek’yan, A. B. Murav’eva, and E. V. Pazhitneva, Fundam. Issled. (Fundam. Res.), No. 9, Part 1, 178 – 181 (2013).
V. Srinivasan, R. Zakaria, Z. Otman, et al., J. Neuropsychiatry Clin. Neurosci., 24(3), 290 – 308 (2012).
V. Fernandez, K. Simizu, and S. B. M. Barros, Endocrinol., 129(1), 85 – 91 (1991).
A. A. Agarkov, T. N. Popova, A. N. Verevkin, et al., Byull. Eksp. Biol. Med., 157(2), 158 – 162 (2014).
R. J. Reiter, D.-X. Tan, J. C. Mayo, et al., Acta Biochim. Pol., 50(4), 1129 – 1146 (2003).
M. Zupancic and C. Guilleminault, CNS Drugs, 20(12), 981 – 992 (2006).
P. Morini, E. Casalino, C. Sblano, et al., Int. J. Biochem., 23(10), 1025 – 1030 (1991).
R. Hardeland, Int. J. Biometeorol., 41(2), 47 – 57 (1997).
P. Solis-Munoz, J. A. Solis-Herruzo, D. Fernandez-Moreira, et al., J. Pineal Res., 51(1), 113 – 123 (2011).
S. S. Popov, A. N. Pashkov, T. N. Popova, et al., Probl. Endokrinol., No. 3, 47 – 51 (2008).
Acknowledgments
The work was supported by the Ministry of the Russian Federation for Education and Science under the auspices of State Support to Higher Educational Institutions for Scientific Activities in 2014 – 2016, Project No. 1090.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 48, No. 8, pp. 14 – 19, August, 2014.
Rights and permissions
About this article
Cite this article
Popova, T.N., Agarkov, A.A., Gorbenko, M.V. et al. Effect of Melatoninergic Drugs on the Specific Activity of Glutathione Redox-Cycle Enzymes in Experimental Hyperthyroidism. Pharm Chem J 48, 499–504 (2014). https://doi.org/10.1007/s11094-014-1138-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-014-1138-z