Abstract
Pathways for NH3 decomposition to N2 and N2H4 by atmospheric pressure nonthermal plasma are analyzed using a combination of molecular beam mass spectrometry measurements and zero-dimensional kinetic modeling. Experimental measurements show that NH3 conversion and selectivity towards N2 formation scale monotonically with the specific energy input into the plasma with ~ 100% selectivity to N2 formation achieved at specific energy inputs above 0.12 J cm−3 (3.1 eV (molecule NH3)−1). The kinetic model recovers these trends, although it underpredicts N2 selectivity at low specific energy input. These discrepancies can be explained by the underestimation of reaction rate coefficients for reactions that consume N2Hx species in collisions with H radicals and/or radial nonuniformities in power deposition, gas temperature, and species concentrations that are not represented by the plug flow approximation used in the model. The kinetic model shows that N2 formation proceeds through N2Hx decomposition pathways rather than NHx decomposition pathways in low temperature, atmospheric pressure plasma. Higher selectivity toward N2 production can be achieved by operating at higher NH3 conversion and with a higher gas temperature. The high energy cost of NH3 decomposition by atmospheric pressure nonthermal plasma found in this work (25–50 eV (molecule NH3 converted)−1; 17–33 eV (molecule H2 formed)−1) is a result of the energy requirement for electron-impact dissociation of NH3 and the significant re-formation of NH3 by three-body recombination reactions between NH2 and H.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NH3 can potentially serve as a platform molecule to store renewable energy in a medium that can be easily stored and transported [1]. However, before energy conversion by combustion or in fuel cells, NH3 must be partially or fully decomposed into N2 and H2 because NH3 has a low flammability, is difficult to ignite, and NH3 fuel cell technology is less developed than H2 fuel cell technology [2]. NH3 decomposition to N2 and H2 is an endothermic reaction with a positive entropy of reaction (ΔH = 46 kJ (mol NH3)−1 = 0.48 eV (molecule NH3)−1; ΔS = 99 J (mol NH3)−1 K−1) [3]. To operate at process conditions that favor NH3 decomposition, gas-phase NH3 pyrolysis typically occurs at temperatures above 1000 K [4]. Heterogeneous catalysts can be utilized to lower operating temperatures for NH3 decomposition to ~ 700 K; however, currently the most effective catalysts are expensive noble metals (Ru) [5]. NH3 decomposition can be carried out catalytically at lower temperatures by oxidative reforming processes, although this method sacrifices some H2 to form H2O to make the overall reaction more exothermic [5].
Nonthermal plasma provides an opportunity to drive NH3 decomposition at low temperatures utilizing high-energy electrons to excite or dissociate N–H bonds in NH3. Qiu et al. [6], Gao et al. [7], and Ruiz-Martín et al. [8] demonstrate that atmospheric pressure, nonthermal plasma can drive NH3 decomposition in Ar/NH3 or pure NH3 discharges without external heating, indicating that the plasma can drive NH3 decomposition via nonthermal processes or in-situ heating. Kinetic models to describe NH3 decomposition in Ar/NH3 microdischarges at 100 torr developed by Arakoni et al. [9] show that > 95% of NH3 dissociation is induced by electron impact processes at select process conditions, indicating that NH3 decomposition can be driven by nonthermal processes. They also investigated how changing operating conditions can optimize the energy cost of H2 production, noting an optimal energy cost of H2 production equal to 3.3 eV (molecule H2 consumed)−1. They, as well as Qiu et al. [6], note that this energy cost is higher than the heat of combustion of H2 (− 2.5 eV (molecule H2 consumed)−1 [3]), which is the amount of energy that can be recovered from H2 oxidation in a fuel cell. These studies do not, however, analyze the chemical or physical processes that dictate the energy cost in order to identify methods to lower the energy cost of the plasma-driven NH3 decomposition.
N2 and H2 are not the only products that can form from NH3 decomposition. Fateev et al. [10] note that N2H4 (hydrazine), a toxic compound and a strong reductant [11], was a side product of NH3 decomposition in an Ar/NH3 DBD at 300 K. They posit that N2H4 was formed from three-body collisions between NH2 radicals but do not perform extensive experiments or kinetic modeling to validate this claim. Bang et al. [12] investigated possible mechanisms for NH3 decomposition in a 1% NH3 / 99% N2 atmospheric pressure dielectric barrier discharge using a combination of experimental measurements and kinetic modeling. According to their proposed reaction mechanism, N2H4 is formed from three-body collisions between NH2 radicals, although they do not measure N2H4 in their experiments to help validate their proposed reaction pathways. Their mechanism shows that N2H4 may be an intermediate in NH3 decomposition but that NH3 decomposition can also proceed through NHx decomposition pathways mediated by the presence of N2-derived species in their plasma.
This work utilizes a combination of molecular beam mass spectrometry and zero-dimensional kinetic modeling to assess energy consumption and reaction pathways involved in NH3 decomposition and formation of N2 and N2H4 in an RF-driven atmospheric pressure plasma jet. Experimental measurements show that NH3 conversion and selectivity towards N2 formation increases with the specific energy input into the system. Kinetic modeling results recover these trends but underpredict selectivity toward N2. These underpredictions can be resolved by considering potential inaccuracies associated with reaction rate constants and/or radial nonuniformities in power deposition, gas temperature, and species concentrations that cannot be described with a plug-flow approximation. Results of the kinetic model demonstrate that N2H4 is an intermediate in N2 formation from NH3 decomposition at atmospheric pressure. N2H4 production can be mitigated by operating the plasma jet at higher NH3 conversion and higher gas temperature. The kinetic model demonstrates that NH3 dissociation is induced by electron impact dissociation, which has an energy threshold of 5.72 eV [13]. This gives a lower bound on energy cost for H2 production to be 3.8 eV (molecule H2 produced)−1 if electron impact dissociation is the dominant method of NH3 dissociation. This value is higher than the heat of combustion of H2, indicating that plasma-driven NH3 decomposition via solely electron impact dissociation processes would cost too much energy to be utilized to produce H2 for energy storage applications. Recombination reactions between NH2 and H to form NH3 further increase the energy cost of low temperature plasma-driven NH3 decomposition at atmospheric pressure.
Methods
Plasma Jet and MBMS Setup
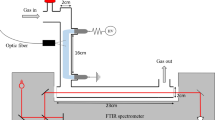
An RF-driven (13.8 MHz) atmospheric pressure plasma jet with a feed gas containing mixtures of 5% NH3 in Ar (Airgas), N2 (HP, Airgas, 99.998% purity), 4% H2 in Ar (HP, Airgas, 99.995% purity), and Ar (UPC, Airgas, 99.9993% purity) at total flow rates ranging from 0.5 to 6.0 standard liters per minute (slm) was utilized in this work (Fig. 1). The jet consisted of a 3 mm outer diameter, 2 mm inner diameter quartz tube with two electrodes: an inner 1 mm diameter tungsten needle electrode and an outer copper ring electrode (5.5 mm long), with the tip of the needle in the center of the ground electrode [14]. The distance between the end of the ground electrode and the nozzle of the plasma jet was kept at 10 mm throughout this work. The sampling plate of the mass spectrometer was placed adjacent to the nozzle (< 0.2 mm). Methods used for the RF power measurement are described in more detail by Hofmann et al. [15]. The plasma-on power was varied from 2.2 to 4.05 W in this work, with an optional 20 kHz, 50% duty cycle modulation utilized to lower the average power by a factor of two. The waveform was modulated with an additional 10 Hz modulation cycle to enable multi-channel scaler measurements with the MBMS (Sect. “Molecular Beam Mass Spectrometry (MBMS) Methods”.).
Molecular Beam Mass Spectrometry (MBMS) Methods
MBMS was utilized in this work to detect and quantify densities of N2, H2, NH3, and N2H4. Details about the setup and operation of the MBMS are described in more detail in previous work [16, 17]. Table 1 displays the MBMS methods used in this work to detect and quantify these species. NH3 was detected at an electron energy of 16 eV to reduce the background signal coming from the dissociative ionization of H2O (\({m}_{{\text{NH}}_{3}^{+}}={m}_{{\text{OH}}^{+}}\)= 17) in the last stage of the MBMS, and N2H4 was detected at an electron energy of 15 eV to potentially reduce the background signal coming from the ionization of O2 (\({m}_{{{\text{N}}_{2}\text{H}}_{4}^{+}}={m}_{{\text{O}}_{2}^{+}}\)= 32) in the last stage of the MBMS. N2 was utilized to calibrate N2H4 densities due to their similar mass [18]. An additional correction was made when calibrating for N2H4 to account for the differences in ionization cross sections between N2 and N2H4 [19]. Attempts to measure the densities of N, H, NH, NH2, N2H, N2H2/H2NN, and N2H3 were also pursued using methods described in previous work [17]. Densities of these species, aside from possibly N2H3, were below the detection limit (~ 1013–1014 cm−3 for N, NHx, and N2Hx; ~ 1015 cm−3 for H) for the investigated operating conditions [17]. A signal for N2H3 (m/z = 31 at 15 eV) could be detected but was consistently smaller than the N2H4 signal. It is possible that this signal could arise from dissociative ionization of N2H4 in the ionizer of the mass spectrometer, so the detection and enumeration of this species was not investigated in detail in this work.
A multichannel scaler measurement with a plasma modulation at 10 Hz was utilized to quantify changes in the MBMS signal when the plasma was on and off, which can occur due to chemical reactions that consume/form species, vibrational excitation (for N2) [20, 21], or gas heating if the species is present in the feed gas [22]. For Ar/NH3 plasmas, H2 formation (\(\Delta {c}_{{\text{H}}_{2}}\)) and N2H4 formation (\(\Delta {c}_{{{\text{N}}_{2}\text{H}}_{4}}\)) were quantified, and NH3 consumption (\(\Delta {c}_{{\text{NH}}_{3}}\)) and N2 formation (\(\Delta {c}_{{\text{N}}_{2}}\)) due to chemical reactions were calculated using H- (Eq. 1) and N- (Eq. 2) atom balances, assuming that N2, H2, and N2H4 were the only products formed:
This method eliminates confounding effects that gas heating may have on the NH3 signal and that vibrational excitation may have on the N2 signal.
NH3 fractional conversion (\({X}_{{\text{NH}}_{3}}\)) and N2 selectivity (\({S}_{{\text{N}}_{2}}\)) are computed to compare results between experiments and simulations among different process conditions. NH3 fractional conversion is calculated using Eq. 3:
In Eq. 3, \({c}_{{\text{NH}}_{3,inlet}}\) is the concentration of NH3 at the inlet of the reactor, and \({T}_{g,out}\) is the gas temperature at the outlet. The \(\frac{{T}_{g,out}}{300 K}\) term in Eq. 3 accounts for the decrease in the inlet concentration of NH3 due to gas heating. \({T}_{g,out}\) is calculated using Eq. 7 described in Sect. “Zdplaskin Model”. N2 selectivity is calculated using Eq. 4, assuming that N2 and N2H4 are the most abundant nitrogen-containing products of NH3 decomposition:
Zdplaskin Model
A kinetic model was developed using zdplaskin to determine the important pathways and reactions relevant to NH3 decomposition in the experiments. Zdplaskin [23] concurrently solves species concentration balances, the energy balance for heavy species, and the Boltzmann equation for the electron energy distribution using Bolsig [24]. Electron–electron and electron–ion collisions were not considered when using Bolsig due to the low degree of ionization (< 10–7). The inlet gas composition, gas flow rate, inlet gas temperature, pressure, and power deposition profile were used as inputs for the model. A finite but small seed electron density (107 cm−3) and reduced electric field (0.001 Td) were used as inlet conditions to initiate the simulations.
The power density profile used in simulations is displayed in Fig. 1 and was assumed to rise from the start of ground electrode to the end of the inner electrode, stay constant from the end of the inner electrode to the end of the ground electrode, and fall from the end of the ground electrode to the end of the plasma plume, which depends on the experimental operating condition (Table S6). Integration of the power density profile over the volume of the reactor yields the experimentally measured power at the corresponding operating condition. For experiments that utilized 20 kHz modulation with a 50% duty cycle, corresponding simulations did not employ power modulation but rather operated with continuous power deposition decreased by a factor of two. This method has been demonstrated to reasonably approximate the effects of power modulation [25]. Power density (\({P}_{dens}\)) was converted to reduced electric field (E/N; electric field divided by gas density), the input required for zdplaskin, at each timestep using Eq. 5 and Eq. 6:
In Eq. 5 and Eq. 6, \(J\) is the current density, \(E\) is the electric field strength, \({n}_{e}\) is the electron density, and \({v}_{drift}\) is the electron drift velocity. Zdplaskin calculates the current density by calculating the electron density and drift velocity iteratively at each timestep in the plasma. The model assumes an effective DC electric field in contrast to the RF field used in experiments. Gas heating due to power deposition was considered in the model using Eq. 7:
In Eq. 7, \(\tau\) is the residence time in the reactor, \(R\) is the gas constant, \(p\) is the gas pressure (105 Pa), \({C}_{p,\text{Ar}}\) is the heat capacity of Ar at constant pressure [J mol−1 K−1], and \(t\) is time. This method provides an upper bound on the gas temperature increase through the reactor but is a reasonable estimate because only a small fraction of the energy deposited into the plasma goes into changing the end chemical composition of the system (see Sect. “Assessment of the Energy Efficiency of Plasma-Driven NH3 Decomposition”.) and because gas residence times are short (< 5 ms) so that radial heat transfer away from the plasma jet is insignificant [26]. Outlet gas temperatures are listed in Table S6 for each simulation and range from 313 to 617 K.
Time, the independent variable in zdplaskin, was converted to distance (\(z\)) using Eq. 8 by considering the inlet volumetric flow rate (\(F\)), the cross sectional area of the reactor (\(A\)), and changes in the volumetric gas flow rate due to gas heating.
The change in volumetric flow rate that would occur due to molar expansion (an increase in molecules due to the stoichiometry of NH3 decomposition) was neglected due to the high degree of dilution used in this work (99% Ar).
The source code for the reaction set was developed using the reaction set made available by Bang et al. [12]. The reaction set was modified to include reactions involving Ar-derived species and by replacing rate coefficients that were potentially inaccurate. A detailed list of reactions and their rate constants is presented in the supporting information (section S.1.). Electron-impact reactions involving NH3 and Ar were taken from the Hayashi database, and electron-impact reactions involving N2 and H2 were taken from the Lisbon database [13, 27]. Vibrational excitation of N2, H2, and NH3 by electron impact was considered in the model for purposes of determining the electron energy distribution function (EEDF), although concentrations of the vibrationally excited states of these species were assumed to remain at zero in the model. Electron impact reactions involving NH2, NH, N, and H were not considered because the addition of these reactions caused segmentation faults when trying to run zdplaskin. It is assumed that the inclusion of these reactions would not significantly change the EEDF or concentrations of these species because they accumulate to densities that are smaller than the densities of Ar, N2, H2, and NH3. Reaction rate coefficients for ion recombination reactions, charge transfer reactions, and chemistry related to Ar- and N-based excited states were taken from Bang et al. [12], Arakoni et al. [9], and Van Gaens & Bogaerts [28]. Reaction rate coefficients involving ground state neutral species were mostly taken from the NH3 pyrolysis mechanism from Alturaifi et al. [29]. As noted by Bang et al. [12], some of the reaction rate coefficients for three-body recombination reactions presented by Alturaifi et al. [29] extrapolate to unrealistic values near 300 K. For these reactions, rate constants were taken from alternative sources [30,31,32,33]. Reaction rate coefficients for the reverse reactions of those listed by Alturaifi et al. [29] and alternative sources [30,31,32,33] were calculated using the principle of detailed balance with thermodynamic data from the NIST database [3] for all species aside from N2Hx species. For N2Hx species, thermodynamic data was retrieved from the Burcat database [34].
Results
MBMS measurements were performed to assess the influence that power and flow rate have on NH3 conversion and formation of N2 and N2H4 for 1% NH3 in Ar plasma (Figure S1). These data were reformulated to plot NH3 consumption and selectivity towards N2 formation as a function of specific energy input (SEI, the power divided by the flow rate) as shown in Fig. 2. This figure shows that NH3 conversion and N2 selectivity (with N2H4 being the other N-containing product) monotonically increase with SEI. The observation that NH3 fractional conversion increases with SEI is consistent with previous studies of plasma-driven NH3 decomposition [9].
A comparison between SEI and a NH3 fractional conversion and b N2 selectivity measured in experiments and predicted by kinetic modeling simulations. Experimental conditions: 1% NH3 in Ar, 0.5–6 slm, 13.8 MHz, 2.2–4.05 W plasma-on power, 10 Hz, 50% duty cycle modulation for MBMS measurements. For the 1.1 W data, the plasma was run with 2.2 W plasma-on power with 20 kHz, 50% duty cycle modulation. Error bars represent 95% confidence intervals. 1 J cm−3 = 26 eV (NH3 molecule) −1. The lines in a and b serve as a guide to the eye to describe the trend of the kinetic modeling simulation results
Increasing the SEI will increase both the electron density and the gas temperature of the plasma. Therefore, the increase in NH3 fractional conversion and N2 selectivity with higher SEI can result from nonthermal (electron-driven) and/or thermal (temperature-driven) processes. The correlation between the higher selectivity toward N2H4 formation at lower NH3 conversions also suggests that N2H4 could be a primary product of NH3 decomposition that then reacts further to form N2. Measurements were made to compare N2H4 production in Ar/NH3 plasma and Ar/N2/H2 plasma with the same N- and H- content and operating conditions to determine whether N2H4 is a product of NH3 decomposition or is formed from reactions involving plasma-derived N2/H2 species (Fig. 3(a)). N2H4 formation is measureable in Ar/NH3 plasma, whereas N2H4 formation is below the detection limit in Ar/N2/H2 plasma, indicating that N2H4 is indeed a product of NH3 decomposition rather than reactions involving plasma-derived N2/H2 species. These data also show that the plasma jet induces a greater change in NH3 density in Ar/NH3 plasma than in Ar/N2/H2 plasma by > 20 × (Fig. 3(b)). This observation demonstrates that at these investigated operating conditions, the atmospheric pressure RF plasma jet more effectively channels energy towards reactions that decompose NH3 than reactions that synthesize NH3.
To determine which reactions contribute to NH3 decomposition, N2H4 production, and N2 production, kinetic modeling was performed with the model described in Sect. “Zdplaskin Model”. to simulate the process conditions reported in Fig. 2. The results from these simulations are plotted in Fig. 2 alongside the experimental data. The simulations quantitatively agree with the experimental data relating SEI to NH3 conversion. Though the simulations systematically underestimate N2 selectivity, especially at lower SEI, they do accurately reproduce the observation that N2 selectivity increases with SEI.
Axial changes in concentrations of intermediates and reaction rates predicted by the model are analyzed to assess reaction pathways and chemical reactions relevant for NH3 decomposition and N2/N2H4 formation. Axial variations in power density deposition (as the input, see Sect. “Zdplaskin Model”.), electron density, electron temperature, gas temperature, and concentrations of products (N2H4, N2, H2) and intermediates (N, H, NH, NH2, N2H, N2H2, H2NN, N2H3) are displayed in Fig. 4. The electron density is proportional to the power deposition profile in the plasma and then decays in the afterglow. The electron temperature in the plasma predicted by the model (~ 2 eV) is slightly higher and the electron density (~ 1011 cm−3) is lower than what would be expected for atmospheric pressure RF plasma jets, which typically have electron temperatures around 1–2 eV and electron densities ~ 1012–1013 cm−3 [15, 35, 36]. This has been previously observed in global models that spatially average plasma parameters and is ascribed to not considering secondary emission from metal electrodes or photoionization as ionization sources in such models [25, 37].
Axial profiles of a power density, electron density, electron temperature, and gas temperature; b NH3, N2, H2, and N2H4 densities; c N2H4, N2H3, H2NN, N2H2, and N2H densities; and d H, N, NH, and NH2 densities predicted by the kinetic model for 1% NH3 in Ar, 1 slm, 2.2 W. 1 cm of distance corresponds to between 1.2 and 1.8 ms of gas residence time (Figure S3)
Species density profiles shown in Fig. 4(b) demonstrate that N2H4 density peaks in the plasma at around 0.44 cm (650 µs) and then decays simultaneously with a rise in N2 density, suggesting that N2H4 forms over shorter timescales in the plasma and then is consumed to form N2 over longer timescales. The decrease in NH3 density exhibited in Fig. 4(b) mostly occurs due to gas rarefaction, since the gas temperature increases from 300 to 448 K due to gas heating (Fig. 4(a)). The data in Fig. 4(c) show that concentrations of N2Hx species decrease as the degree of hydrogenation decreases, indicating that these species become more unstable as more hydrogen is removed. Axial profiles of NH2 reported in Fig. 4(d) show that NH2 concentrations are highest in the plasma but then drop by over two orders of magnitude in < 100 µs in the spatial afterglow of the plasma because of its high reactivity.
Axially resolved rates for formation and consumption of NH3, N2H4, and N2 were assessed to identify the reaction steps that contribute most to formation and consumption of these species (Fig. 5). For reactions that do not involve electrons or excited states of atoms/molecules, the net rates (\({r}_{net}={r}_{forward}-{r}_{reverse}\)) are plotted to express the net rates at which the species of interest are formed or consumed.
Figure 5(a) demonstrates that NH3 dissociation to form NH2 and H is dominated by electron impact, which is consistent with the inferences of Arakoni et al. [9]. NH3 is re-formed from the three-body recombination reaction involving NH2 and H at rates that are on the same order of magnitude as electron impact dissociation of NH3, indicating that a large fraction of energy deposited into dissociation of NH3 is wasted to re-form NH3.
Figure 5(b) shows that N2H4 is mainly formed from the three-body recombination reaction involving NH2 radicals in the plasma and through N2H3 disproportionation reactions in the afterglow. N2H4 consumption is dominated by H abstraction reactions in and after the plasma. Figure 5(c) shows that the dominant pathway for N2 formation is through collision-mediated dissociation of N2H. N2H is formed from successive dehydrogenation of N2H4, N2H3, and N2H2/H2NN. N2 formation from NH2 + N occurs at a rate over an order of magnitude lower than N2 formation from N2H, indicating that N2 formation mostly occurs through N2Hx dehydrogenation pathways rather than solely NHx dehydrogenation pathways. Simulations that eliminate reactions that form/consume N2Hx species predict NH3 conversion approximately an order of magnitude lower than those observed in experiments, affirming that the majority of N2 formation occurs from N2Hx dehydrogenation pathways (Fig. 6). N2 consumption occurs primarily by dissociative collisions with Ar* species, with maximum rates ~ 1014 cm−3 s−1. Figure 5(d) demonstrates that H2 is mostly formed from H abstraction from N2Hx species, demonstrating that the presence of N2Hx species increases the rate of H2 formation. H2 consumption occurs mostly by electron-impact dissociation, with maximum rates ~ 1015 cm−3 s−1.
A comparison of experimentally measured NH3 conversion and NH3 conversion predicted by the kinetic model with and without N2Hx species and reactions. Experimental conditions: 1% NH3 in Ar, 1–4 slm, 13.8 MHz, 2.2–4.05 plasma-on power, 10 Hz, 50% duty cycle modulation for MBMS measurements. Error bars represent 95% confidence intervals
Figure 5(e–f) shows the loss pathways of NH2 and H, the primary products of NH3 dissociation. At short times, NH2 mostly reacts to form N2H4, but after NH2 concentration becomes relatively attenuated in comparison to H concentrations over longer (> 300 µs) timescales (Fig. 4(d)), NH2 reacts with H in three-body recombination reactions at faster rates to re-form NH3 (Fig. 5(e)). The reaction between NH2 and H to form NH and H2 occurs at a rate nearly two orders of magnitude lower than the three-body recombination reaction to re-form NH3 from NH2 and H. Figure 5(f) demonstrates that H is consumed by NH2 + H + M and N2Hx + H reactions at similar rates.
Discussion
Possible Sources of Error in the Model
Though the kinetic model can accurately describe NH3 conversion in the plasma, it systematically overestimates N2H4 production relative to N2 production. To assess possible sources of error in the model, a sensitivity analysis was performed.
The kinetic model described in Sect. “Zdplaskin Model”. does not include electron-impact reactions involving N2Hx species, since reaction rate constants for these reactions are unknown/not reported [12]. To probe the role that dissociation of N2Hx species by nonthermal reactions could have on driving N2Hx decomposition to N2, reactions involving dissociation of N2Hx species by electron impact or by collision with excited states of Ar were included in simulations. For reactions that break an N–N bond in N2Hx species, rate constants for N2 dissociation were utilized, and for reactions that break an N–H bond in N2Hx species, rate constants for NH3 dissociation were utilized. Results of these simulations show that inclusion of these reactions does result in an increase in N2 selectivity but not to the N2 selectivity observed in experiments (Fig. 7). N2 selectivity is still underpredicted by as much as ~ 40%.
A comparison of a experimentally measured NH3 conversion and NH3 conversion predicted by the kinetic model with and without nonthermal N2Hx dissociation reactions and b experimentally measured N2 selectivity and N2 selectivity predicted by the kinetic model with and without nonthermal N2Hx dissociation reactions. Experimental conditions: 1% NH3 in Ar, 1–4 slm, 13.8 MHz, 2.2–4.05 plasma-on power, 10 Hz, 50% duty cycle modulation for MBMS measurements. Error bars represent 95% confidence intervals
The kinetic model could also inaccurately predict N2 selectivity if the rate coefficients used in the model are inaccurate. A sensitivity analysis was performed in which rate constants for N2Hx + H reactions (and their associated reverse reactions) were increased by a factor of four (Fig. 8). Results of these simulations show that the model now predicts N2 selectivity accurately within 7%, although the model now systematically overpredicts NH3 conversion. These results demonstrate that H abstraction reactions could occur at faster rates than those reported in the model.
A comparison of a experimentally measured NH3 conversion and NH3 conversion predicted by the kinetic model with and without N2Hx + H reaction rate constants increased 4 × and b experimentally measured N2 selectivity and N2 selectivity predicted by the kinetic model with and without N2Hx + H reaction rate constants increased 4 × . Experimental conditions: 1% NH3 in Ar, 1–4 slm, 13.8 MHz, 2.2–4.05 plasma-on power, 10 Hz, 50% duty cycle modulation for MBMS measurements. Error bars represent 95% confidence intervals
Assumptions associated with the plug flow approximation may also contribute to some of the inaccuracies of the simulation results. The model appears to underestimate the electron density and overestimate the electron temperature for reasons discussed in the results section. The plug flow model also assumes that the electron density, electron temperature, gas temperature, and species concentrations are radially uniform at every axial position of the reactor. In the experiments, it is possible that the plasma is constricted, resulting in regions of the plasma with locally higher gas temperatures and electron densities [38]. Simulations were performed to probe the effect that uneven power deposition may have on dictating the N2 selectivity and NH3 conversion. In these simulations, 1/3 of the gas stream is exposed to plasma with 3 × the experimental power density. This stream was then mixed with 2/3 of the unreacted gas stream at the outlet of the reactor to yield a stream with the same SEI as those in experiments. Results of these simulations show that the model now predicts higher N2 selectivity, especially for the experiments with 4 slm gas flow rates (Fig. 9). Though these simulations are an oversimplification because they do not capture mixing between fluid elements that are exposed to different degrees of power deposition over the length of the reactor and do not consider a continuous gradient in power deposition over the radial coordinate, these simulations demonstrate that uneven power deposition, not considered in the plug-flow model, could contribute to the low N2H4 selectivity observed in experiments. Locally higher gas temperatures in a constricted plasma lead to a greater extent of N2Hx decomposition, since rate constants for N2Hx + H reactions increase with gas temperature, while rate constants for the other reactions that consume H (NH2 + H + M, H + H + M) are temperature-independent or decrease with temperature (Fig. 5(f), Table S5).
A comparison of a experimentally measured NH3 conversion and NH3 conversion predicted by the kinetic model with and without nonuniform power deposition and b experimentally measured N2 selectivity and N2 selectivity predicted by the kinetic model with and without nonuniform power deposition. Experimental conditions: 1% NH3 in Ar, 1–4 slm, 13.8 MHz, 2.2–4.05 plasma-on power, 10 Hz, 50% duty cycle modulation for MBMS measurements. Error bars represent 95% confidence intervals
Assessment of the Energy Efficiency of Plasma-Driven NH 3 Decomposition
Experimental data and kinetic modeling results show that NH3 conversion increases with SEI (Fig. 2(a)). For the 1% NH3 in Ar plasma investigated in this work, SEI can be converted to energy cost per NH3 molecule using Eq. 9:
An examination of the slope of the trend in Fig. 2(a) demonstrates ~ 25–50 eV are required to convert one molecule of NH3. This far exceeds the enthalpy of reaction for NH3 decomposition (NH3 → 0.5N2 + 1.5H2, ΔH = 46 kJ (mol NH3)−1 = 0.48 eV (NH3 molecule)−1; NH3 → 0.5N2H4 + 0.5H2, ΔH = 94 kJ (mol NH3)−1 = 0.97 eV (NH3 molecule)−1) [3].
Figure 4(a) demonstrates that electron-impact dissociation is the dominant process driving NH3 decomposition. The energy threshold for this reaction is 5.72 eV [13], indicating that plasma-driven NH3 decomposition by electron impact dissociation requires 10 × more energy than the enthalpy of reaction for NH3 decomposition to form N2 and H2 [3]. Therefore, 5.72 eV is the lower bound on energy cost for NH3 decomposition by nonthermal plasma if electron-impact dissociation is the main reaction driving NH3 dissociation. This value is equivalent to 3.8 eV (molecule H2 produced)−1 if NH3 is decomposed to N2 and H2. As noted by Qiu et al. [6] and Arakoni et al. [9], an energy cost of H2 production above 2.5 eV (molecule H2 produced)−1 exceeds the amount of energy that can be recovered from H2 combustion (2.5 eV (molecule H2 consumed)−1) [3]. Therefore, plasma-driven NH3 decomposition solely from electron impact dissociation has an energy cost too high for H2 production for energy storage purposes. Plasma-driven processes would need to rely on lower energy processes (excitation-driven or thermal-driven) to drive NH3 decomposition with a suitable energy cost. If the extra dissipated electron energy beyond 0.48 eV (NH3 molecule)−1 utilized to dissociate NH3 is effectively utilized to heat the plasma, NH3 decomposition can be driven by thermal processes in the gas phase [39] or over a catalyst [40, 41].
The 5.72 eV (NH3 molecule)−1 lower bound is still lower than 25–50 eV (NH3 molecule)−1 predicted by experimental measurements and kinetic modeling simulations, so energy dissipation processes are analyzed below to determine where energy is wasted. A comparison between the electron energy deposition into NH3 dissociation compared to the electron energy deposition into all processes can be analyzed using Eq. 10:
In Eq. 10, \({E}_{\text{I}+\text{e}}\) is the energy dissipated in a collision between species I and an electron, \({r}_{\text{I}+\text{e}}\) is the rate at which these collisions occur, and \({\tau }_{end}\) is the residence time associated with the outlet of the reactor (1.55 cm). For the process condition analyzed in Figs. 4–5, this fraction is equal to 0.93, indicating that the plasma very efficiently directs electron energy towards NH3 dissociation in comparison to elastic collisions, ionization, excitation, and other dissociation processes.
To assess whether the products of NH3 electron impact dissociation are effectively converted to desired products, the fraction of NH2 molecules that are converted to N2H4 vs NH2 converted to N2H4 or NH3 can be analyzed using Eq. 11:
For the process condition analyzed in Figs. 3 and 4, this fraction is equal to 0.41, indicating that more than half of the NH2 molecules formed from electron-impact dissociation of NH3 are re-converted to NH3. Limiting the extent to which NH3 is re-formed from three-body collisions between NH2 and H is critical to improving the utilization of NH3 dissociation products for N2/H2 formation. To do this, process conditions must be chosen that increase the rates at which NH2 and H react to form N2H4 or N2 products vs recombine to form NH3. The ratio of the rate of N2H4 formation vs NH3 formation by three-body recombination takes the form of Eq. 12:
An analysis of this ratio shows that the branching ratio between N2H4 formation and NH3 formation is not easily manipulated by varying process conditions. Lower temperature would favor N2H4 formation over NH3 formation, although as the gas temperature increases and NH2 concentrations become relatively lower than H concentrations as observed in Fig. 4, NH2 consumption to form NH3 becomes more pronounced.
Although not a dominant reaction in these experiments, the reaction between NH2 and H to form NH and H2 can consume NH2 and H to eventually form N2 and H2 instead of re-forming NH3. The ratio of the rates of these processes takes the form of Eq. 13:
This ratio is dependent on both gas temperature and pressure, with higher temperatures and lower pressures favoring NH2 + H conversion to NH and H2 instead of NH3. At atmospheric pressure, 0.1 bar, and 0.01 bar, the ratio of these reaction rates becomes greater than 1 at 1330 K, 630 K, and 386 K, respectively. Operating at lower pressure could thus improve energy efficiency by mitigating re-formation of NH3 by three-body recombination reactions involving NH2 and H. Additionally, the mechanism for N2 formation from NH3 decomposition could shift from the N2Hx formation/decomposition pathway exhibited at atmospheric pressure to a NHx decomposition pathway as rates of three body recombination reactions between two NH2 molecules to form N2H4 become relatively attenuated at reduced pressures. As a result, operating at lower pressure could also improve the selectivity towards N2 formation, even at lower temperatures and NH3 conversions.
Conclusions
Reaction pathways and energy consumption for NH3 decomposition in an RF-driven atmospheric pressure plasma jet are assessed using a combination of molecular beam mass spectrometry and zero-dimensional kinetic modeling. MBMS measurements show that N2 and N2H4 are the main products of NH3 decomposition and that the extent of NH3 conversion and the selectivity towards N2 formation scales monotonically with the specific energy input into the plasma. Kinetic modeling shows that N2H4 is an intermediate in N2 formation from NH3 decomposition and rates of N2H4 decomposition increase with gas temperature, which explains why N2H4 selectivity is relatively higher at lower NH3 conversion and lower gas temperatures. Though the kinetic model systematically underestimates N2 selectivity at low specific energy inputs, a sensitivity analysis demonstrates that this underestimation can be explained by the underestimation of rate constants for reactions that consume N2Hx species in the model and/or the inaccurate assumption of radially uniform power deposition, gas temperature, and species concentrations associated with the plug-flow approximation of the model.
An analysis of the energy deposition processes shows that NH3 dissociation is dominated by electron-impact dissociation in this study, which has an energy threshold of 5.72 eV. Therefore, 5.72 eV is the lower bound on the energy cost for NH3 decomposition if electron-impact dissociation is the dominant process for NH3 dissociation in plasma. This energy cost is ~ 10 × higher than the enthalpy of NH3 decomposition to N2 and H2 (0.48 eV (molecule NH3 consumed) −1). This value is also higher than the energy that can be derived from H2 combustion (2.5 eV (molecule H2 consumed)−1), indicating that plasma-driven NH3 decomposition using electron impact dissociation inherently has an energy cost too high for energy storage applications. NH3 re-formation from three-body collisions between NH2 and H, the primary products of NH3 dissociation, further lowers the energy efficiency of the process. To produce H2 from plasma-driven NH3 decomposition with an energy cost below 2.5 eV (molecule H2 produced)−1, plasma-driven NH3 decomposition would need to utilize processes other than electron-impact dissociation to induce NH3 dissociation (excitation-driven or thermal-driven) and would need to limit energy loss from NH3 re-forming reactions.
Data Availability
All data presented in this work will be made available upon request to the corresponding author.
References
MacFarlane DR, Cherepanov PV, Choi J, Suryanto BHR, Hodgetts RY, Bakker JM, Ferrero Vallana FM, Simonov AN (2020) A roadmap to the ammonia economy. Joule 4:1186–1205. https://doi.org/10.1016/j.joule.2020.04.004
Valera-Medina A, Xiao H, Owen-Jones M, David WIF, Bowen PJ (2018) Ammonia for power. Prog Energy Combust Sci 69:63–102. https://doi.org/10.1016/j.pecs.2018.07.001
NIST (2010) Standard Reference Database 69: NIST Chemistry WebBook; National Institute of Standards and Technology, (n.d.)
Benés M, Pozo G, Abián M, Millera Á, Bilbao R, Alzueta MU (2021) Experimental study of the pyrolysis of NH3 under flow reactor conditions. Energy Fuels 35:7193–7200. https://doi.org/10.1021/acs.energyfuels.0c03387
Caballero LC, Thornburg NE, Nigra MM (2022) Catalytic ammonia reforming: alternative routes to net-zero-carbon hydrogen and fuel. Chem Sci 13:12945–12956. https://doi.org/10.1039/D2SC04672E
Qiu H, Martus K, Lee WY, Becker K (2004) Hydrogen generation in a microhollow cathode discharge in high-pressure ammonia–argon gas mixtures. Int J Mass Spectrom 233:19–24. https://doi.org/10.1016/j.ijms.2003.08.017
Gao Y, Zhou M, Hu E, Zhao Y, Yin G, Huang Z (2024) Hydrogen generation by dielectric barrier discharge plasma assisted ammonia decomposition. Energy Convers Manag 306:118271. https://doi.org/10.1016/j.enconman.2024.118271
Ruiz-Martín M, Marín-Meana S, Megías-Sánchez A, Oliva-Ramírez M, Cotrino J, González-Elipe AR, Gómez-Ramírez A (2023) H2 Production from NH3 in a BaTiO3 Moderated ferroelectric packed-bed plasma reactor. Plasma Chem Plasma Process 43:2093–2110. https://doi.org/10.1007/s11090-023-10427-7
Arakoni RA, Bhoj AN, Kushner MJ (2007) H2 generation in Ar/NH3 microdischarges. J Phys D: Appl Phys 40:2476. https://doi.org/10.1088/0022-3727/40/8/010
Fateev A, Leipold F, Kusano Y, Stenum B, Tsakadze E, Bindslev H (2005) Plasma chemistry in an atmospheric pressure Ar/NH3 dielectric barrier discharge. Plasma Process Polym 2:193–200. https://doi.org/10.1002/ppap.200400051
Troyan JE (1953) Properties, production, and uses of hydrazine. Ind Eng Chem 45:2608–2612. https://doi.org/10.1021/ie50528a020
Bang S, Snoeckx R, Cha MS (2023) Kinetic study for plasma assisted cracking of NH3: approaches and challenges. J Phys Chem A 127:1271–1282. https://doi.org/10.1021/acs.jpca.2c06919
Hayashi database (2024) (n.d.). www.lxcat.net. Accessed 4 June 2024
Kondeti VSSK, Gangal U, Yatom S, Bruggeman PJ (2017) Ag+ reduction and silver nanoparticle synthesis at the plasma–liquid interface by an RF driven atmospheric pressure plasma jet: mechanisms and the effect of surfactant. J Vac Sci Technol, A 35:061302. https://doi.org/10.1116/1.4995374
Hofmann S, van Gessel AFH, Verreycken T, Bruggeman P (2011) Power dissipation, gas temperatures and electron densities of cold atmospheric pressure helium and argon RF plasma jets. Plasma Sources Sci Technol 20:065010. https://doi.org/10.1088/0963-0252/20/6/065010
Jiang J, Luo Y, Moldgy A, Aranda Gonzalvo Y, Bruggeman PJ (2020) Absolute spatially and time-resolved O, O3, and air densities in the effluent of a modulated RF-driven atmospheric pressure plasma jet obtained by molecular beam mass spectrometry. Plasma Process Polym 17:1900163. https://doi.org/10.1002/ppap.201900163
Bayer BN, Bruggeman PJ, Bhan A (2023) Species, pathways, and timescales for NH3 formation by low-temperature atmospheric pressure plasma catalysis. ACS Catal 13:2619–2630. https://doi.org/10.1021/acscatal.2c05492
Benedikt J, Hecimovic A, Ellerweg D, von Keudell A (2012) Quadrupole mass spectrometry of reactive plasmas. J Phys D: Appl Phys 45:403001. https://doi.org/10.1088/0022-3727/45/40/403001
Singh H, Coburn JW, Graves DB (2000) Appearance potential mass spectrometry: discrimination of dissociative ionization products. J Vac Sci Technol, A 18:299–305. https://doi.org/10.1116/1.582183
Jiang J, Richards C, Adamovich I, Bruggeman PJ (2022) Molecular beam mass spectrometry measurements of vibrationally excited N2 in the effluent of an atmospheric plasma jet: a comparison with a state-to-state kinetic model. Plasma Sources Sci Technol 31:10LT03. https://doi.org/10.1088/1361-6595/ac954c
Bayer BN, Raskar S, Adamovich IV, Bruggeman PJ, Bhan A (2023) Availability and reactivity of N2(v) for NH3 synthesis by plasma catalysis. Plasma Sources Sci Technol 32:125005. https://doi.org/10.1088/1361-6595/ad10f0
Jiang J, Bruggeman PJ (2021) Tuning plasma parameters to control reactive species fluxes to substrates in the context of plasma catalysis. J Phys D: Appl Phys 54:214005. https://doi.org/10.1088/1361-6463/abe89a
Pancheshni S, Eismann B, Hagelaar GJM, Pitchford LC (2008) Computer code ZDPlasKin. http://www.zdplaskin.laplace.univ-tlse.fr
Hagelaar GJM, Pitchford LC (2005) Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci Technol 14:722. https://doi.org/10.1088/0963-0252/14/4/011
Jiang J, Kondeti VSSK, Nayak G, Bruggeman PJ (2022) Experimental and modeling studies of the plasma chemistry in a humid Ar radiofrequency atmospheric pressure plasma jet. J Phys D: Appl Phys 55:225206. https://doi.org/10.1088/1361-6463/ac570a
Bayer BN, Bruggeman PJ, Bhan A (2024) NO formation by N2/O2 plasma catalysis: the impact of surface reactions, gas-phase reactions, and mass transport. Chem Eng J 482:149041. https://doi.org/10.1016/j.cej.2024.149041
Lisbon database (2024) (n.d.). www.lxcat.net. Accessed 4 June 2024
Gaens WV, Bogaerts A (2013) Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J Phys D: Appl Phys 46:275201. https://doi.org/10.1088/0022-3727/46/27/275201
Alturaifi SA, Mathieu O, Petersen EL (2022) An experimental and modeling study of ammonia pyrolysis. Combust Flame 235:111694. https://doi.org/10.1016/j.combustflame.2021.111694
Gordiets B, Ferreira CM, Pinheiro MJ, Ricard A (1998) Self-consistent kinetic model of low-pressure - flowing discharges: I. volume processes. Plasma Sources Sci Technol 7:363–378. https://doi.org/10.1088/0963-0252/7/3/015
Pagsberg PB, Eriksen J, Christensen HC (1979) Pulse radiolysis of gaseous ammonia-oxygen mixtures. J Phys Chem 83:582–590. https://doi.org/10.1021/j100468a006
Zheng J, Rocha RJ, Pelegrini M, Ferrão LFA, Carvalho EFV, Roberto-Neto O, Machado FBC, Truhlar DG (2012) A product branching ratio controlled by vibrational adiabaticity and variational effects: Kinetics of the H + trans-N2H2 reactions. J Chem Phys 136:184310. https://doi.org/10.1063/1.4707734
Altinay G, Macdonald RG (2015) Determination of the rate constants for the NH2(X2B1) + NH2(X2B1) and NH2(X2B1) + H recombination reactions in N2 as a function of temperature and pressure. J Phys Chem A 119:7593–7610. https://doi.org/10.1021/acs.jpca.5b00917
Goos E, Burcat A, Ruscic B (2006) Extended third millenium ideal gas and condensed phase thermochemical database for combustion with updates from active thermochemical tables. http://garfield.chem.elte.hu/Burcat/THERM.DAT (accessed June 6, 2024)
Andersen JA, van’tVeerChristensenØstbergBogaertsJensen KJMMAAD (2023) Ammonia decomposition in a dielectric barrier discharge plasma: insights from experiments and kinetic modeling. Chem Eng Sci 271:118550 https://doi.org/10.1016/j.ces.2023.118550
van Gessel B, Brandenburg R, Bruggeman P (2013) Electron properties and air mixing in radio frequency driven argon plasma jets at atmospheric pressure. Appl Phys Lett 103:064103. https://doi.org/10.1063/1.4817936
Jiang J, Bruggeman PJ (2021) Absolute ion density measurements in the afterglow of a radiofrequency atmospheric pressure plasma jet. J Phys D: Appl Phys 54:15LT01. https://doi.org/10.1088/1361-6463/abdc91
von Keudell A, der Gathen VS (2017) Foundations of low-temperature plasma physics—an introduction. Plasma Sources Sci Technol 26:113001. https://doi.org/10.1088/1361-6595/aa8d4c
Bruggeman PJ, Iza F, Brandenburg R (2017) Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci Technol 26:123002. https://doi.org/10.1088/1361-6595/aa97af
Hsu DD, Graves DB (2005) Microhollow Cathode Discharge Reactor Chemistry. Plasma Chem Plasma Process 25:1–17. https://doi.org/10.1007/s11090-004-8831-8
Wang L, Zhao Y, Liu C, Gong W, Guo H (2013) Plasma driven ammonia decomposition on a Fe-catalyst: eliminating surface nitrogen poisoning. Chem Commun 49:3787–3789. https://doi.org/10.1039/C3CC41301B
Wang N, Otor HO, Rivera-Castro G, Hicks JC (2024) Plasma Catalysis for Hydrogen Production: A Bright Future for Decarbonization. ACS Catal 14(9):6749–6798. https://doi.org/10.1021/acscatal.3c05434
Funding
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Fusion Energy Sciences General Plasma Science program under Award Number DE-SC0020232. The work heavily relied on equipment and methods developed within project DE-SC0001939. The authors also acknowledge partial support from the National Science Foundation under Award Number NSF-CBET 2234270.
Author information
Authors and Affiliations
Contributions
Brian N. Bayer: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing. Aditya Bhan: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing—review & editing. Peter J. Bruggeman: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bayer, B.N., Bhan, A. & Bruggeman, P.J. Reaction Pathways and Energy Consumption in NH3 Decomposition for H2 Production by Low Temperature, Atmospheric Pressure Plasma. Plasma Chem Plasma Process (2024). https://doi.org/10.1007/s11090-024-10501-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11090-024-10501-8