Abstract
In this study, plasma activated water (PAW) has been prepared by using atmospheric pressure air dielectric barrier discharge (DBD) at the PAW temperature of 3–90 °C. The effects of PAW temperature, pH values and O3 concentration on the E. coli inactivation have been studied, and the chemical reactivity of PAW has been analyzed by using the chemical probe. It is found that both the biological and chemical reactivities of PAW are strongly dependent on the PAW temperature, however, their dependences on temperature are not consistent. When the PAW temperature decreases from 90 to 3 °C, the chemical reactivity of PAW is significantly increased due to an increase in the concentration of activated oxygen in PAW. Decreasing the temperature from 30 to 3 °C or increasing the temperature from 45 to 90 °C leads to an obvious increase in the biological reactivity of PAW. Our analysis shows that an obvious increase in the biological reactivity of low-temperature (≤ 15 °C) PAW is due to the synergistic effect of acidic solutions and a high concentration of activated oxygen in PAW. The high biological reactivity of PAW at the temperature of ≥ 60 °C can be attributed to the synergistic effect of acidic solutions, heat and the activated oxygen, such as O3 and HOONO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plasma-activated water (PAW) is usually produced during the direct or indirect interactions between the atmospheric pressure air plasmas and the water to be treated [1, 2], which simulate the generation of reactive oxygen and nitrogen species (RONS) in the lighting and their subsequent dissolution into rainwater, rivers, lakes or seas. During these interactions, the discharge energy is stored into PAW, and the PAW containing various RONS is chemically unstable. Thus, PAW is a natural product reacting with the living things on the planet. PAW has been used for decreasing the viability of cancer (NOS2) cells [3], inactivation of S. aureus on strawberry [4], promoting the seed germination and seeding growth [5], inhibiting SARS-CoV-2 infection [6], wound healing [7], and nitrogen fixation [8].
PAW contains short-lived (S-lived) and long-lived (L-lived) RONS. The main components of S-lived RONS with their lifetimes of < 1–2 ms include hydroxyl radicals (\({\text{OH}}\)), singlet oxygen (\({\text{O}}_{2}\) (1Δg)), superoxide anions (\({\text{O}}_{2}^{-}\)) and water anions (\({\text{H}}_{2}{{\text{O}}}^{-}\)), nitric oxide radicals (\({\text{NO}}\), \({\text{N}}{\text{O}}_{2}\)) while L-lived RONS include hydrogen peroxide (\({\text{H}}_{2}{{\text{O}}}_{2}\)), peroxynitrite (\({\text{HOONO}}\)), ozone (\({\text{O}}_{3}\)), nitrate (\({\text{HN}}{\text{O}}_{3}\)), and nitrite (\({\text{HN}}{\text{O}}_{2}\)). The \({\text{HOONO}}\) lifetime is about 1 s at the room temperature, and they can convert into \({\text{HN}}{\text{O}}_{3}\) [9]. The S-lived RONS in the aqueous solution are mainly produced during the interaction between air plasma and water, resulting in their solution. The S-lived RONS in the aqueous solution tend to convert into L-lived RONS, accompanied by the release of chemical energy. L-lived RONS can be generated due to the dissolution of L-lived RONS in the gas phase or the chemical reactions related to S-lived RONS in water. It has been widely accepted that the \({\text{HOONO}}\) in the aqueous solution can decompose into S-lived RONS, such as \({\text{OH}}\) and \({\text{N}}{\text{O}}_{2}\), which plays a crucial role in controlling the chemical reactivity of PAW. \({\text{HOONO}}\) can be generated via the \({\text{H}}_{2}{{\text{O}}}_{2}\) reaction with \({\text{HN}}{\text{O}}_{2}\) in the acidic PAW, as shown below.
Both \({\text{H}}_{2}{{\text{O}}}_{2}\) and \({\text{HN}}{\text{O}}_{2}\) are L-lived RONS, thus \({\text{HOONO}}\) can be one of the most important RONS in PAW.
Very complex chemical reactions occurred in PAW, which are thus affected by the PAW temperature. The study by Tsoukou et al. [10] indicated that the concentrations of chemical species in PAW were affected by the storage at different temperatures. The PAW became very stable when stored at the temperature of ≤ -80 °C, and it achieved 6 log reduction of S. aureus and E. coli after 18 months. Arda et al. found that maintaining PAW at low temperatures was not an adequate recourse to preserve the reactive species [11]. However, the conservation of reactive species and restoration of bactericidal activity of PAW was achieved via low-temperature storage and pH adjustment. To address the limitations of the short lifetime of the RONS in PAW, plasma-activated hydrogels were developed by Chen et al. to act as reactive species carriers that allow good storage and controlled slow-release of RONS to preserve its antimicrobial reactivity for more than 14 days [12]. The study by Shen J et al. [13] showed that PAW stored at -80 °C retained bactericidal activity, and attributed to the fact that low temperatures contribute to the maintenance of \({\text{H}}_{2}{{\text{O}}}_{2}\) and \({\text{N}}{\text{O}}_{2}^{-}\) concentrations. Similarly, PAW prepared from the DBD source used in the study by Subramanian G et al. [14] retained a large proportion of its potency against cancer cells after 14 days of refrigerated storage at − 20 °C. Rathore et al. [15] found that storing PAW at 4 °C for two weeks resulted in small changes in physicochemical properties, \({\text{N}}{\text{O}}_{3}^{-}\) and \({\text{H}}_{2}{{\text{O}}}_{2}\) concentrations, but significantly decreases in \({\text{N}}{\text{O}}_{2}^{-}\) and \({\text{O}}_{3}\). And the study showed the interaction of the stirrer speed and temperature had a significant effect on the ORP of PAW. Pang B et al. [16] demonstrated that the chemical activity of activated water prepared using the plasma jet was higher at 25 °C than at 40 °C, 70 °C and 4 °C, which was related to the high surface tension at 25 °C.
This study by Choi et al. evaluated the effect of a sequential combination of washing treatments using PAW and mild heating at 60 °C on the inactivation of background microbiota and inoculated foodborne pathogens of shredded salted Chinese cabbages [17]. The treatment with PAW led to 2.0, 2.2, 1.8, and 0.9 log CFU/g reduction in mesophilic aerobic bacteria, lactic acid bacteria, yeast, moulds and coliforms, respectively. The subsequent mild heating treatment decreased the counts of lactic acid bacteria, yeast and moulds below the detection limit. The synergistic effect of PAW and mild heat on the inactivation of S. cerevisiae on grapes was evaluated by Xiang et al. [18]. The maximum inactivation efficiency of S. cerevisiae cells was 5.85 Log CFU/g after the PAW treatment at 55 °C for 30 min, which was much higher than that of PAW treatment at 25 °C (2.39 Log CFU/g). After the mild heat at 25–55 °C for 30 min, the concentrations of \({\text{H}}_{2}{{\text{O}}}_{2}\) and \({\text{N}}{\text{O}}_{2}^{-}\) in PAW significantly decreased, accompanied by an increase in \({\text{N}}{\text{O}}_{3}^{-}\) content. It was suggested that the synergistic effect of PAW and mild heat increased the membrane permeability, which contributed to the leakage of intracellular components, such as nucleic acids and proteins [19]. In the study by Tian Y et al. [20], temperatures of 31.5 °C and 38.1 °C were not critical for S.aureus sterilization. Wang B et al. [21] found that PAW combined with mild heating (40–60 °C for 4 min) exhibited enhanced antibacterial activity against L. monocytogenes and S. typhimurium. Okyere A et al. [22] found that increasing the temperature of PAW improves the hydration properties of starch and gelatinization temperatures.
Clearly, the stability or reactivity of PAW is strongly dependent on the storage temperature and the temperature of pathogens to be treated by PAW. An increase in the temperature increases the reaction rate of RONS in PAW, thus affecting the inactivation process of pathogens in PAW. The PAW reactivity can be also dependent on the PAW temperature during the PAW production. The gas-phase RONS from the plasma will dissolve into water or react with water to generate PAW. Both Henry’s law constant of the gas-phase RONS and the rate constant of chemical reactions in PAW are significantly affected by PAW temperature. The physical and chemical properties of PAW will be analyzed as a function of PAW temperature. Due to the low cost of gaseous materials, the method of air PAW has potential application prospects. Currently, complete inactivation E. coli of 104—106 CFU/mL by air PAW usually takes minutes of time [23,24,25]. However, the effect of PAW temperature during generation on their reactivity is not entirely understood. In this study, the PAW was prepared by using atmospheric pressure air DBD, and the biological and chemical reactivities of PAW will be evaluated by varying the temperature of treated water from 3 to 90 °C. By controlling the PAW temperature during generation, complete inactivation of E. coli (~ 104 CFU/mL) can be achieved in the order of seconds. The factors affecting the biological and chemical reactivities of PAW will be clarified in this study.
Material and Methods
Experimental Setup for Generating PAW
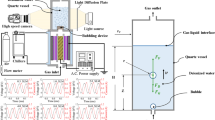
The plasma treatment system for generating PAW is schematically illustrated in Fig. 1. The system mainly consists of a dielectric barrier discharge reactor, a bubbling device, and an A.C. power supply. The DBD reactor includes a copper rod with its diameter of 54 mm, a quartz tube (inside diameter: 54 mm and outside diameter: 58 mm), and a stainless-steel tube with its inside diameter of 60 mm. The water-cooled stainless-steel tube acts as the ground electrode. The copper rad acts as the high-voltage electrode embedded in the quartz tube with its length of 750 mm. The compressed air (\({\text{H}}_{2}{\text{O}}\)≤3 ppm) or O2 (purity: 99.99%, \({\text{H}}_{{2}} {\text{O}}\) ≤ 3 ppm) flows through the plasma device at a flow rate of 10 standard liter per minute (SLM). The DBD is driven by the sine-wave voltage at a frequency of 10 kHz. The peak-to-peak voltage (VPP) can be set within the range of 0 to 12 kV, leading to an increase in the discharge power (P) from 0 to 350 W, as measured by Lissajous figure [26]. The Lissajous figure is obtained by measuring the charges across the capacitor (2.2 μF) in series to ground electrode and the applied voltage across the plasma device. The air or \({\text{O}}_{{2}}\) DBD is produced in the gas spacing of 1.0 mm, and the DBD plasma is 0.14 L in volume. The exhaust gas containing O3 or NOX flows into the bubbling device to generate the bubbles in the sink. The bubbling device is made up of porous ceramics. The diameter of these bubbles in water is typically in the range of 1 to 3 mm, and their retention time is 0.2 s.
Before the discharge, the air or O2 feeds through the DBD reactor for 45 min at a flow rate of 10 SLM. The temperature of 600 mL deionized (DI) water in the sink is controlled by the water bath outside the sink, which is typically 3 °C, 7.5 °C, 15 °C, 30 °C, 45 °C, 60 °C, 75 °C or 90 °C. The gas-phase RONS in the bubbles dissolve into DI water and react with each other, leading to the formation of air PAW or \({\text{O}}_{{2}}\) PAW. The plasma treatment time (t) varies from 0 to 320 s at a given P. To generate the consistent \({\text{O}}_{{3}}\) concentration of PAW, the air and \({\text{O}}_{{2}}\) plasma treatments were performed at P = 300 W and 270 W, respectively.
E. coli Sample Preparation and Treatment
The biological reactivity of PAW was evaluated by measuring the inactivation efficiency of E. coli. The E. coli was cultured in a lysogeny broth (LB) growth medium, and the initial bacterial concentration was ~ 107 CFU/mL. Four experiments were designed to evaluate the dependence of E. coli inactivation on the temperature (T) of DI water treated by the air or \({\text{O}}_{{2}}\) plasma.
-
2.2.1 E. coli inactivation by DI water at different temperature. The E. coli inactivation was also performed by changing DI water temperature from 3 to 90 °C. 600 μL of DI water with different temperatures was immediately added to the cuvette containing 20 μl of bacterial suspension. Then, the cuvette was immersed in the constant temperature (30 °C) bath for 5 min. After that, 300 μL of the mixed solutions was spread onto the LB agar medium. After overnight culture at 37 °C, colony counting was performed to determine the number of E. Coli survival in aqueous solution (CFU/mL). Data represent the mean and standard deviation of three independent biological replicates.
-
2.2.2 E. coli inactivation by air and \({\text{O}}_{{2}}\) PAW at different temperatures. After the air or \({\text{O}}_{{2}}\) plasma treatments at different temperatures, 600 μL of air or \({\text{O}}_{{2}}\) PAW was immediately added to the cuvette containing 20 μL of bacterial suspension. Then, the cuvette was immersed in the constant temperature (30 °C) bath for 5 min. After that, 300 μL of the mixed solution was spread onto the LB agar medium. After overnight culture at 37 °C, colony counting was performed to determine the number of E. Coli survival in aqueous solution (CFU/mL). Data represent the mean and standard deviation of three independent biological replicates.
-
2.2.3 E. coli inactivation by air and \({\text{O}}_{{2}}\) PAW at 30 °C. The air or \({\text{O}}_{{2}}\) PAW was first prepared at different temperatures. Then, 50 mL of PAW was immediately added to the cuvette containing the quartz beads with their diameter of 5 mm. The quartz beads were used to rapidly decrease or increase the PAW temperature to 30 °C. The quartz beads were previously heated or cooled, depending on PAW temperature. After that, 600 μL of 30 °C PAW was immediately added to the cuvette containing 20 μL of bacterial suspension, and the cuvette was immersed in the constant temperature (30 °C) bath for 5 min. 300 μL of the mixed solution was spread onto the LB agar medium. After overnight culture at 37 °C, colony counting was performed to determine the number of E. Coli survival in aqueous solution (CFU/mL). Data represent the mean and standard deviation of three independent biological replicates.
-
2.2.4 E. coli inactivation by \({\text{O}}_{{2}}\) PAW at the pH value of 2.8. To analyze the synergistic effect of aqueous \({\text{O}}_{{3}}\) and pH value on the E. coli inactivation, the O2 plasma treatments of DI water were performed at the pH value of 2.8 to generate the acidic \({\text{O}}_{{2}}\) PAW. The \({\text{H}}_{{2}} {\text{SO}}_{{4}}\) solution was used to control the pH value of DI water. After the \({\text{O}}_{{2}}\) plasma treatments at different temperatures, 600 μL of PAW was immediately added to the cuvette containing 20 μL of bacterial suspension. Subsequently, the cuvette was immersed in the constant temperature (30 °C) bath for 5 min. Then, 300 μL of the mixed solution was spread onto the LB agar medium. After overnight culture at 37 °C, colony counting was performed to determine the number of E. Coli survival in aqueous solution (CFU/mL). Data represent the mean and standard deviation of three independent biological replicates.
NO2 − and NO3 − Concentrations of Air PAW
To measure the \({\text{NO}}_{{3}}^{ - }\) concentration, 1 mL of PAW was immediately added to 5 mL nickel sulfamate solution (purity > 98%, 50 g/L) after plasma treatment. Then, 3 mL of the mixed solution was measured by using UV/VIS-spectrophotometer (UV-1900, Shimadzu, Japan) at 219 nm. To measure the \({\text{NO}}_{{2}}^{ - }\) concentration, 60 μL of sulphanilamide solution (purity > 98%, 10 g/L) as the diazotizing reagent was immediately added to 3 mL of air PAW after the air plasma treatment. Then, the mixed solution was incubated at room temperature for 2 min. Subsequently, 60 μL of N-(1-Naphthyl)-ethylenediamine hydrochloride (purity > 98%, 1 g/L) as the coupling reagent was added into the mixed solution. After reaction for 20 min at room temperature, the \({\text{NO}}_{{2}}^{ - }\) concentration was measured by using the UV-spectrophotometer at 540 nm [27].
O3 Concentration of Air and O2 PAWs
The concentration of \({\text{O}}_{{3}}\) in air and O2 PAWs was determined by the iodometric titration method [28]. 100 ml of PAW was immediately added to 20 mL KI solution (purity > 98%, 200 g/L). Then the mixed solution was acidified with 5 ml \({\text{H}}_{{2}} {\text{SO}}_{{4}}\) solution (3 mol/L) immediately. After 5 min, \({\text{Na}}_{{2}} {\text{S}}_{{2}} {\text{O}}_{{3}}\) solution (purity > 98%, 0.05 mol/L) was used to titrate the mixed solution until it was pale yellow. After that, 1 mL starch solution (5 g/L) was added. The titrate was continued until it was colorless. The \({\text{O}}_{{3}}\) concentration was measured by calculating the total amount of sodium thiosulfate titration solution used.
H2O2 Concentration of Air PAW
For the \({\text{H}}_{{2}} {\text{O}}_{{2}}\) concentration measurements, 2 mL \({\text{TiSO}}_{{4}}\) (purity > 98%, 50 g/L) solution was immediately transferred to 2 mL air PAW after the air plasma treatment. Then, 4 mL \({\text{H}}_{{2}} {\text{SO}}_{{4}}\) solution was added to the mixed solution. After that, 3 mL solution was measured by using UV/VIS-spectrophotometer (UV-1900, Shimadzu, Japan) at 407 nm [29].
Conductivity and pH Value of Air and O2 PAW
Both the pH value and conductivity of PAW were measured by a pH and conductivity analyzer (SI Analytics Co., model Lab-850, Germany).
Chemical Reactivity of Air and O2 PAW
The chemical reactivity of air and \({\text{O}}_{{2}}\) PAWs is measured by using the chemical probe of terephthalic acid (TA). Terephthalic acid (TA) can be oxidized into 2-hydroxyterephthalic acid (HTA) by the activated oxygen in PAW, and HTA can be identified by fluorescence measurement [30, 31]. When the HTA solution is irradiated by UV light (λ = 310 nm), HTA molecules emit light at λ = 425 nm. The aqueous solution of TA (MΛCKLIN, purity > 98%) was prepared by dissolving TA in NaOH solution. The initial concentrations of TA and NaOH were 2 and 5 mM, respectively. 2 mL of TA solution was immediately added to 1 mL of PAW. The fluorescence measurements were performed by using a fluorescence spectrometer (Cary Eclipse 2018A43C). To quantify the concentration of HTA generated in the mixed solution, a calibration curve was obtained by using the standard HTA solution (MΛCKLIN, purity > 99%).
Results
The Effect of DI Water Temperature on E. coli Inactivation
The E. coli inactivation has been performed by using the DI water at different temperatures. The E. coli survival is almost independent on the DI water temperature varying from 3 to 45 °C (Fig. 2). However, the number of E. coli survival greatly decreases when the DI water temperature is higher than 45 °C. The survival rates of E. coli at 60 °C, 75 °C, and 90 °C are 37%, 13%, and 0.1%, respectively.
E. coli Inactivation by Air PAW
Figure 3 shows the E. coli inactivation by the air PAW prepared at different temperatures. The number of E. coli survival is strongly dependent on the plasma treatment time (t) varying from 0 to 320 s. At the temperature (T) of 3 °C, the E. coli is completely inactivated at t ≥ 20 s. The times required for the complete inactivation of E. coli are 40 s at T = 7.5 °C, 80 s at T = 15 °C, and 320 s at T = 30 °C. At the T = 45 °C, the number of E. coli survival slowly decreases from 3.8 Log CFU/mL to 3.1 Log CFU/mL when t varies from 0 to 320 s. The biological reactivity of air PAW is greatly decreased when T increases from 3 to 45 °C. Compared to the other remote plasma treatment methods [1, 32], our bubbling device is effective for rapidly increasing the biological reactivity of PAW. Plenty of small bubbles are formed in the water, which contributes to an increase in the gas–liquid interface and the diffusion of gas-phase RONS into the water.
The times for the complete E. coli inactivation at T = 60 °C, T = 75 °C, and T = 90 °C are 80 s, 20 s, and 5 s, respectively (Fig. 3). Clearly, the biological reactivity of PAW is significantly increased when the PAW temperature increases from 45 to 90 °C. An increase in the biological reactivity can be closely related to an increase in the temperature of PAW. The study by Zhang et al. [19] indicates that the synergistic effect of PAW and mild heat can cause significant increases in membrane permeability, resulting in the leakage of intracellular components, such as nucleic acids and proteins. This effect could also increase the intracellular levels of reactive oxygen species. Decreasing PAW temperature from 45 to 3 °C is very helpful for increasing the biological reactivity of air PAW. After the low-temperature (≤ 15 °C) PAW is heated to 30 °C, the PAW reactivity is greatly decreased, as shown in Fig. 4. This suggests that increasing the temperature leads to a decrease in the stability of activated oxygen or their release from PAW.
The previous study has indicated that the sequential combination of washing with PAW followed by mild heating at 60 °C greatly decreases the counts of Listeria monocytogenes and Staphylococcus aureus [17]. Our study shows that the air PAW prepared at the temperature of ≥ 60 °C is very effective for the E. coli inactivation. Figure 5 shows that the biological reactivity of air PAW prepared at T = 60 – 90 °C is greatly reduced after being cooled to 30 °C. After being cooled to 30 °C, the biological reactivity of air PAW slightly increases with an increase in t, which is almost independent on T varying from 60 to 90 °C. This indicates that the concentration of RONS in PAW is greatly decreased at a relatively high PAW temperature. Increasing T decreases both the solubility and stability of RONS in PAW. The number of E. coli survival (N(T)) due to the combined effect of heat and RONS can be estimated as
where N(cooled) is the number of E. coli survival due to the inactivation by the PAW cooled to 30 °C. η(T) is the survival rate of E. coli due to the DI water heated to T. N(T) as a function of t is plotted in Fig. 5. The number of E. coli survivals after 60 – 90 °C PAW treatment is much lower than N(T), which confirms the synergistic effect of PAW and mild heat on the E. coli inactivation.
The E. coli inactivation by 60–90 °C air PAW. The 60–90 °C air PAWs were used for the direct E. coli inactivation or for the E. coli inactivation after being cooled to 30 °C. The E. coli survival (N(T)) due to the combined effect of heat and RONS can be estimated from N(T) = N(cooled) × η(T), where N(cooled) is the E. coli survival due to the inactivation by the PAW cooled to 30 °C. η(T) is the survival rate of E. coli due to the DI water heated to T
Physicochemical Properties of Air PAW
Both the pH value and conductivity of air PAW are strongly dependent on the plasma treatment time, t, as shown in Fig. 6. When t varies from 0 to 320 s, the pH value of PAW significantly decreases, accompanied by an increase in its conductivity. The PAW pH values of 2.4 – 3.2 are consistent with the ones reported previously [10, 17]. To identify the species in the plasma, Optical emission spectrum (OES) and Fourier-transform infrared spectrometer (FTIR) measurements has been conducted. Consistent with previous research [32,33,34], the emission data reveal that the species generated in the air discharge of the air DBD primarily include excited nitrogen molecules, nitrogen ions, oxygen atoms, and nitrogen atoms (figure S1). Further, FTIR spectrum indicates that the gas-phase species are mainly O3 and N2O (figure S2). A decrease in the pH value is primarily due to the dissolution of gas-phase NO, NO2, N2O3, and N2O5 into DI water, and their subsequent reactions, leading to the formation of \({\text{HNO}}_{{2}}\) and \({\text{HNO}}_{{3}}\) in PAW [1, 2]. At a given t, decreasing T leads to a decrease in the pH value, accompanied by an increase in the conductivity (inserts in Fig. 6), indicating that the temperature can affect the Henry’s law constants (H) of \({\text{NO}}\), \({\text{NO}}_{{2}}\), \({\text{N}}_{{2}} {\text{O}}_{{3}}\), and \({\text{N}}_{{2}} {\text{O}}_{{5}}\), which determines the highest achievable (saturated) concentration of these RONS in PAW. Decreasing T leads to an increase in the constant, thus an increase in the RONS concentration and a decrease in the pH value.
When t increases from 0 to 40 s, the \({\text{NO}}_{{3}}^{ - }\) concentration of air PAW slowly increases, then turns to significantly increase with t (Fig. 7a). However, the \({\text{NO}}_{{2}}^{ - }\) concentration rapidly increases when t varies from 0 to 40 s (Fig. 7b). The \({\text{NO}}_{{2}}^{ - }\) concentration remains almost unchanged when t is higher than 40 s. \({\text{HNO}}_{{2}}\) and \({\text{HNO}}_{{3}}\) can be generated due to the reactions of \({\text{NO}}\), \({\text{NO}}_{{2}}\), \({\text{N}}_{{2}} {\text{O}}_{{3}}\), and \({\text{N}}_{{2}} {\text{O}}_{{5}}\) with water, as shown below [1, 2, 35, 36].
In PAW, \({\text{NO}}_{3}^{ - }\) is very stable, however, \({\text{NO}}_{2}^{ - }\) is very unstable. \({\text{NO}}_{2}^{ - }\) can be oxidized to \({\text{NO}}_{3}^{ - }\), as shown below.
Thus, \({\text{NO}}_{{2}}^{ - }\) concentration initially increases with t, then turns to remain unchanged due to the chemical reactions (R(6), R(7), and R(8)), leading to the formation of \({\text{NO}}_{{3}}^{ - }\). This leads to a significant increase in the \({\text{NO}}_{{3}}^{ - }\) concentration at an increasing t. At a given t, both the \({\text{NO}}_{{2}}^{ - }\) and \({\text{NO}}_{{3}}^{ - }\) concentrations decrease with an increase in T, which shows that the solubility of gas-phase RONS is affected by the temperature.
The \({\text{H}}_{{2}} {\text{O}}_{{2}}\) concentration of PAW remains almost unchanged at ~ 7 μmol/L when t varies from 5 to 320 s (Fig. 8a). \({\text{H}}_{{2}} {\text{O}}_{{2}}\) can be generated by a combination of \({\text{OH}}\) radicals from the homolysis of aqueous \({\text{HOONO}}\), as shown below [9].
The \({\text{O}}_{{3}}\) concentration of PAW initially increases with t, then remains almost unchanged when t is higher than 40 s (Fig. 8b). At a given t, the saturated concentration of \({\text{O}}_{{3}}\) in PAW decreases with an increase in T. This suggests that the solubility of \({\text{O}}_{{3}}\) in PAW is determined by the Henry’s law constant, which greatly decreases with an increase in the temperature.
TA can be oxidized by the peroxide (activated oxygen [O]) in PAW, such as \({\text{HOONO}}\), \({\text{H}}_{{2}} {\text{O}}_{{2}}\), and \({\text{O}}_{{3}}\), which is used to evaluate the chemical reactivity of PAW [30, 31, 37, 38]. One TA can be converted into one HTA when one oxygen atom from the activated oxygen molecule is added to TA, as shown in Fig. 9. The oxidation rate is determined by the chemical activity of the peroxide or activated oxygen. Note that one \({\text{OH}}\) radical in PAW will not lead to the complete oxidation of a TA molecule to a HTA molecule. However, the sequential reactions of two \({\text{OH}}\) radicals with a TA molecule could lead to the complete oxidation of a TA molecule to a HTA molecule, accompanied by the formation of a \({\text{H}}_{{2}} {\text{O}}\) molecule. The \({\text{OH}}\) radicals will not play a crucial role in the oxidation process since their concentration is very low. Figure 10 shows that the HTA molecules are generated due to the oxidation of TA by the activated oxygen in air PAW. The HTA concentration initially increases when t varies from 0 to 40 s. Then it remains unchanged when t is higher than 40 s. This indicates that the concentration of activated oxygen in air PAW remains constant when t is higher than 40 s. At a given t, decreasing T leads to a significant increase in the HTA concentration, indicating an increase in the chemical reactivity of PAW.
E. coli Inactivation by O2 PAW
The \({\text{O}}_{{2}}\) PAW is prepared at different temperatures to evaluate the effect of \({\text{O}}_{{3}}\) on the E. coli inactivation. The \({\text{O}}_{{3}}\) concentration of \({\text{O}}_{{2}}\) PAW initially increases with t, then turns to remain unchanged with further increasing t (Fig. 11a), which is quite similar to the variation of \({\text{O}}_{{3}}\) concentration of air PAW with t. The pH value of \({\text{O}}_{{2}}\) PAW remains almost unchanged at 5.8. Decreasing T from 75 to 3 °C contributes to an obvious increase in the \({\text{O}}_{{3}}\) solubility. The \({\text{O}}_{{2}}\) PAW at the pH value of 5.8 is not very effective in the E. coli inactivation (Fig. 11b). Decreasing T from 30 to 3 °C or increasing T from 30 to 75 °C is helpful for increasing the biological reactivity of \({\text{O}}_{{2}}\) PAW.
However, the \({\text{O}}_{{2}}\) PAW at the pH value of 2.8 is very effective in the E. coli inactivation (Fig. 12). Our study shows that the acidic solution at pH value of 2.8 has no obvious effect on the E. coli inactivation. The synergistic effect of \({\text{O}}_{{3}}\) and acidic solutions on the E. coli inactivation is still unclear. Decreasing T from 30 to 3 °C leads to an increase in the \({\text{O}}_{{3}}\) concentration, thus an improvement in the chemical reactivity of \({\text{O}}_{{2}}\) PAW. Increasing T from 30 to 75 °C leads to an increase in the biological reactivity of \({\text{O}}_{{2}}\) PAW, indicating the synergistic effect of \({\text{O}}_{{3}}\), temperature and a reduction in the pH value on the E. coli inactivation. However, the synergistic effect is not so obvious as the one from the air PAW. This indicates that other RONS, such as \({\text{HOONO}}\) in the air PAW can also contribute to the synergistic effect on the E. coli inactivation.
Discussion
When the plasma-generated RONS flow through the bubbling system, some of them rapidly dissolve in water. The distribution equilibrium of a molecule in water and gas phases can be described by the dimensionless Henry’s law constant H(T) = Ci,g/Ci,w, where Ci,g and Ci,w are the molar concentrations of molecules in the gas and water phases, respectively [39]. The temperature dependence of H(T) can be expressed as lnH = -A/RT + C, where A is equal to ΔHΘ/R, ΔHΘ is the enthalpy change during the transfer of a molecule from the water phase to the gas phase, and C is temperature-independent constant. If the temperature dependence of ΔHΘ is neglected, H(T) is significantly increased with an increase in T, which has been confirmed by experimental measurements [40, 41]. This indicates a decrease in the solubility of RONS in the water phase with an increase in the water temperature. It is previously reported that the concentration of O3 in water decreases by ~ 40% when the water temperature increases from 20 °C to 40 °C [42]. The higher pH value at a relatively higher temperature can be attributed to a decrease in the solubility of RONS in air PAW.
It appears that the chemical reactivity of PAW is controlled by the activated oxygen in PAW. The RONS in PAW, including OH, HOONO, O3 and H2O2 have a relatively high oxidation reduction potential, and they react with various functional groups in biomolecules, and damage the structures of DNA, proteins and lipids [31, 37, 43,44,45,46]. Increasing PAW temperature results in a decrease in the RONS concentration, thus a decrease in the chemical reactivity of PAW. The study by Moldgy et al. indicates that \({\text{HOONO}}\) can be formed via the reaction of \(N_{2} {\text{O}}_{{5}}\) with water (R5) [2]. However, \({\text{HOONO}}\) is unstable, and they can decompose in acidic solutions via isomerization into \({\text{HNO}}_{{3}}\). This reaction rate is temperature–dependent, and the stability of \({\text{HOONO}}\) in PAW decreases at a relatively high temperature. \({\text{O}}_{{3}}\) can be released from PAW due to an increase in the temperature, which decreases the chemical reactivity of PAW.
Our study indicates that the biological reactivity of PAW can be controlled by the synergistic effect of RONS, mild heat, and acidic solutions. The thermal stress can bring about many changes leading to the loss of viability, including enzyme inactivation, membrane damage, and nucleic acid breakdown. Heat can induce a breakage of hydrogen bonds and unfolding of the polypeptide chain, thus a collapse of the native protein structure [47]. Chick et al. [48] pointed to an analogy between disinfection by hot water and the coagulation of proteins as early as 1910. It is considered that moist heat kills bacterial cells by causing an intracellular coagulation of proteins. It has been proposed that an increase in the thermal sensitivity of E. coli is due to the low-temperature inactivation of an enzyme controlling an energy-yielding reaction [49]. The membrane damage has been caused by exposure to the high temperature [50]. The optical analysis of heated suspensions of E. coli indicates that the death of E. coli is due to the breakdown of intracellular RNA [51].
Reduction in the pH value of PAW results in a higher concentration of protonated acid, thus decreasing the polarity of the molecule and increasing diffusion of protonated acid across the membrane and into the cytoplasm. The reduction is proposed to affect the microbial activity by cytoplasmic acidification with subsequent uncoupling of energy production and regulation and by accumulation of the dissociated acid anion to toxic level [52]. The organic acid treatments of E. coli increase SYTOX orange permeation to ~ 30% of the maximum level [53]. The reduction in the pH value has been proposed to cause damage to the cellular membrane of E. coli, which contributes to an increase in permeabilization. When the intracellular pH value is decreased, the biological stress response system is induced, which can change the metabolic activity of cells. The leakage of intracellular constituents is generated by the increase in membrane permeability [54].
The synergistic combination of organic acids and ultraviolet (UV) has been previously reported to inactivate the bacteria cells [53, 55]. Analysis indicates that both damages to the bacterial cell membrane and intracellular esterase are closely related to the synergistic lethal effect. Higher water temperature (43 °C) is expected to increase the disinfection efficacy of chlorine by affecting the stability of the chemical disinfectant [56]. The enhanced efficacy of chlorine in the inactivation of bacterial cells could be a result of accelerated binding of chlorine to the cell surface. The synergistic inactivation of S. cerevisiae has been performed by the combined use of PAW and mild heat (40 – 50 °C) [18, 19]. The combined treatment of PAW and mild heat causes significant increases both in membrane permeability and the intracellular levels of reactive oxygen species, and the disruption of mitochondrial membrane potential. These RONS in air PAW including O3, HOONO, H2O2 can also induce peroxidation of membrane lipids. This process can increase its permeability to RONS and protonated acids, thus cause the damage of RNA and proteins in cytoplasm. An obvious increase in the biological reactivity of low-temperature (≤ 15 °C) air PAW is due to the synergistic effect of acidic solutions and a high concentration of RONS. The low biological reactivity of air PAW at 30–45 °C is attributed to the weak thermal stress and low RONS concentration of PAW. The biological reactivity of PAW at a higher water temperature (> 45 °C) can be controlled by the complex synergistic effects of RONS, higher water temperature, and a reduction in the pH value. It appears that the death of E. coli induced by PAW is due, not only to the oxidation process by RONS, but also to the subtle changes in organized systems, which the bacteria cells are difficult to overcome.
Conclusion
In summary, we have prepared the air PAW at the water temperature of 3 – 90 °C, and evaluated the effect of temperature on the biological and chemical reactivities of air PAW. We have found that both biological and chemical reactivities of air PAW are strongly dependent on the PAW temperature. The chemical reactivity of PAW is significantly increased when the PAW temperature decreases from 90 to 3 °C. Decreasing the temperature from 30 to 3 °C or increasing the temperature from 45 to 90 °C significantly contributes to an increase in the biological reactivity of air PAW. This is consistent with previous research that sequential treatment of washing with PAW and mild heating at 60 °C can improve the microbiological quality of salted Chinese cabbage. Our analysis shows that an obvious increase in the biological reactivity of low-temperature (≤ 15 °C) air PAW is due to the synergistic effect of acidic solutions and a high concentration of RONS. The high biological reactivity of air PAW at the temperature of ≥ 60 °C can be attributed to the synergistic effect of acidic solutions, heat and RONS, such as \({\text{O}}_{{3}}\) and \({\text{HOONO}}\).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhou R, Zhou R, Wang P, Xian Y, Mai-Prochnow A, Lu X, Cullen PJ, Ostrikov KK, Bazaka K (2020) Plasma-activated water: generation, origin of reactive species and biological applications. J Phys D Appl Phys 53:303001. https://doi.org/10.1088/1361-6463/ab81cf
Moldgy A, Nayak G, Aboubakr HA, Goyal SM, Bruggeman PJ (2020) Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J Phys D Appl Phys 53:434004. https://doi.org/10.1088/1361-6463/aba066
Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M, Kikkawa F (2013) Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 8:e81576. https://doi.org/10.1371/journal.pone.0081576
Ma R, Wang G, Tian Y, Wang K, Zhang J, Fang J (2015) Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J Hazard Mater 300:643–651. https://doi.org/10.1016/j.jhazmat.2015.07.061
Zhou R, Zhou R, Zhang X, Zhuang J, Yang S, Bazaka K, Ostrikov K (2016) Effects of atmospheric-pressure N2, He, air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci Rep-Uk. https://doi.org/10.1038/srep32603
Guo L, Yao Z, Yang L, Zhang H, Qi Y, Gou L, Xi W, Liu D, Zhang L, Cheng Y, Wang X, Rong M, Chen H, Kong MG (2021) Plasma-activated water: an alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem Eng J. 421:127742. https://doi.org/10.1016/j.cej.2020.127742
Wang S, Xu D, Qi M, Li B, Peng S, Li Q, Zhang H, Liu D (2021) Plasma-activated water promotes wound healing by regulating inflammatory responses. Biophysica 1:297–310. https://doi.org/10.3390/biophysica1030022
Lamichhane P, Veerana M, Lim JS, Mumtaz S, Shrestha B, Kaushik NK, Park G, Choi EH (2021) Low-temperature plasma-assisted nitrogen fixation for corn plant growth and development. Int J Mol Sci 22:5360. https://doi.org/10.3390/ijms22105360
Goldstein S, Lind J, Merényi G (2005) Chemistry of peroxynitrites as compared to peroxynitrates. Chem Rev 105:2457–2470. https://doi.org/10.1021/cr0307087
Tsoukou E, Bourke P, Boehm D (2020) Temperature stability and effectiveness of plasma-activated liquids over an 18 months period. Water-Sui 12:3021. https://doi.org/10.3390/w12113021
Arda G, Hsu C (2023) Preservation of reactive species in frozen plasma-activated water and enhancement of its bactericidal activity through pH adjustment. Plasma Chem Plasma Process 43:599–618. https://doi.org/10.1088/1361-6463/ac286a
Chen J, Wang Z, Sun J, Zhou R, Guo L, Zhang H, Liu D, Rong M, Ostrikov KK (2023) Plasma-activated hydrogels for microbial disinfection. Adv Sci. https://doi.org/10.1002/advs.202207407
Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, Fang J (2016) Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci Rep 6:28505. https://doi.org/10.1038/srep28505
Subramanian P, Jain A, Shivapuji A, Sundaresan N, Dasappa S, Rao L (2020) Plasma-activated water from a dielectric barrier discharge plasma source for the selective treatment of cancer cells. Plasma Process Polym 17:1900260
Rathore V, Nema S (2021) Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J Appl Phys 129:084901. https://doi.org/10.1063/5.0033848
Pang B, Liu Z, Zhang H, Wang S, Gao Y, Xu D, Liu D, Kong M (2022) Investigation of the chemical characteristics and anticancer effect of plasma-activated water: The effect of liquid temperature. Plasma Process Polym 19:2100079. https://doi.org/10.1002/ppap.202100079
Choi EJ, Park HW, Kim SB, Ryu S, Lim J, Hong EJ, Byeon YS, Chun HH (2019) Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control 98:501–509. https://doi.org/10.1016/j.foodcont.2018.12.007
Xiang Q, Zhang R, Fan L, Ma Y, Wu D, Li K, Bai Y (2020) Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT. 126:109336. https://doi.org/10.1016/j.lwt.2020.109336
Zhang R, Ma Y, Wu DI, Fan L, Bai Y, Xiang Q (2020) Synergistic inactivation mechanism of combined plasma-activated water and mild heat against saccharomyces cerevisiae. J Food Protect 83:1307–1314. https://doi.org/10.4315/JFP-20-065
Tian Y, Ma R, Zhang Q, Feng H, Liang Y, Zhang J, Fang J (2015) Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process Polym 12:439–449. https://doi.org/10.1002/ppap.201400082
Wang B, Wang W, Xiang Q, Bai Y (2023) Effects of heating on the antibacterial efficacy and physicochemical properties of plasma-activated water. Qual Assur Saf Crop Foods 15:100–108
Okyere A, Boakye P, Bertoft E, Annor G (2022) Temperature of plasma-activated water and its effect on the thermal and chemical surface properties of cereal and tuber starches. Curr Res Food Sci 5:1668–1675. https://doi.org/10.1016/j.crfs.2022.09.020
Man C, Zhang C, Fang H, Zhou R, Huang B, Xu Y, Zhang X, Shao T (2022) Nanosecond-pulsed microbubble plasma reactor for plasma-activated water generation and bacterial inactivation. Plasma Process Polym. 19:e2200004. https://doi.org/10.1002/ppap.202200004
Rothwell J, Alam D, Carter D, Soltani B, Mcconchie R, Zhou R, Cullen P, Mai-Prochnow A (2022) The antimicrobial efficacy of plasma-activated water against Listeria and E. coli is modulated by reactor design and water composition. J Appl Microbiol 132:2490–2500. https://doi.org/10.1111/jam.15429
Wang Q, Salvi D (2021) Evaluation of plasma-activated water (PAW) as a novel disinfectant: Effectiveness on Escherichia coli and Listeria innocua, physicochemical properties, and storage stability. LWT 149:111847. https://doi.org/10.1016/j.lwt.2021.111847
Falkenstein Z, Coogan JJ (1997) Microdischarge behaviour in the silent discharge of nitrogen - oxygen and water - air mixtures. J Phys D Appl Phys 30:817–825. https://doi.org/10.1088/0022-3727/30/5/015
Qi Z, Tian E, Song Y, Sosnin EA, Skakun VS, Li T, Xia Y, Zhao Y, Lin X, Liu D (2018) Inactivation of Shewanella putrefaciens by plasma activated water. Plasma Chem Plasma 38:1035–1050. https://doi.org/10.1007/s11090-018-9911-5
Chasanah U, Yulianto E, Zain AZ, Sasmita E, Restiwijaya M, Kinandana AW, Arianto F, Nur M (2019) Evaluation of titration method on determination of ozone concentration produced by dielectric barrier discharge plasma (DBDP) Technology. J Phys: Conf Ser 1153:12086. https://doi.org/10.1088/1742-6596/1153/1/012086
Marotta E, Ceriani E, Schiorlin M, Ceretta C, Paradisi C (2012) Comparison of the rates of phenol advanced oxidation in deionized and tap water within a dielectric barrier discharge reactor. Water Res 46:6239–6246. https://doi.org/10.1016/j.watres.2012.08.022
Kanazawa S, Furuki T, Nakaji T, Akamine S, Ichiki R (2013) Application of chemical dosimetry to hydroxyl radical measurement during underwater discharge. J Phys Conf Ser 418:12102–12107. https://doi.org/10.1088/1742-6596/418/1/012102
Liu X, Wang Z, Li J, Wang Y, Sun Y, Dou D, Liang X, Wu J, Wang L, Xu Y, Liu D (2022) Inactivation of E. coli, S. aureus, and Bacteriophages in biofilms by humidified air plasma. Int J Mol Sci. 23:4856. https://doi.org/10.3390/ijms23094856
Xi W, Wang W, Liu Z, Wang Z, Guo L, Wang X, Rong M, Liu D (2020) Mode transition of air surface micro-discharge and its effect on the water activation and antibacterial activity. Plasma Sources Sci T 29:95013. https://doi.org/10.1088/1361-6595/aba7ef
Zhou S, Su L, Shi T, Zheng T, Tong Y, Nie W, Che X, Zhao J (2019) Experimental study on the diffusive flame stabilization mechanism of plasma injector driven by AC dielectric barrier discharge. J Phys D Appl Phys 52:265202. https://doi.org/10.1088/1361-6463/ab15cd]
Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, Fang J (2016) Bactericidal Effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci Rep. https://doi.org/10.1038/srep28505
Machala Z, Tarabova B, Hensel K, Spetlikova E, Sikurova L, Lukes P (2013) Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process Polym 10:649–659. https://doi.org/10.1002/ppap.201200113
NDRL/NIST Solution Kinetics Database US. http://kinetics.nist.gov/solution/. Accessed 03 March 2022
Cataldo F (2006) Ozone degradation of biological macromolecules: Proteins, haemoglobin, RNA, and DNA. Ozone Sci Eng. 28:317–328. https://doi.org/10.1080/01919510600900290
Lukes P, Dolezalova E, Sisrova I, Clupek M (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. https://doi.org/10.1088/0963-0252/23/1/015019
Görgényi M, Dewulf J, Langenhove HV (2002) Temperature dependence of Henry’s law constant in an extended temperature range. Chemosphere (Oxford) 48:757–762. https://doi.org/10.1016/S0045-6535(02)00131-5
Nirmalakhandan N, Brennan RA, Speece RE (1997) Predicting Henry’s Law constant and the effect of temperature on Henry’s Law constant. Water Res 31:1471–1481. https://doi.org/10.1016/S0043-1354(96)00395-8
Dewulf J, Drijvers D, Langenhove HV (1995) Measurement of Henry’s law constant as function of temperature and salinity for the low temperature range. Armos Environ 29:323–331. https://doi.org/10.1016/1352-2310(94)00256-K
Levanov AV, Isaikina OY, Lunin VV (2019) Thermodynamic and kinetic parameters of the solubility of ozone in water. Russ J Phys Chem A. 93:1230–1234. https://doi.org/10.1134/S0036024419070148
Wright A, Bubb WA, Hawkins CL, Davies MJ (2002) Singlet oxygen-mediated protein oxidation: evidence for the formation of reactive side chain peroxides on tyrosine residues. Photochem Photobiol 76:35–46. https://doi.org/10.1562/0031-8655(2002)076%3c0035:sompoe%3e2.0.co;2
Spanggord RJ, Yao CD, Mill T (2000) Oxidation of Aminodinitrotoluenes with ozone: Products and pathways. Environ Sci Technol 34:497–504. https://doi.org/10.1021/es990190h
Radil R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite oxidation of sulfhydryls: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266:4244–4250. https://doi.org/10.1016/S0021-9258(20)64313-7
Uppu RM, Pryor WA (1994) The reactions of ozone with proteins and unsaturated fatty acids in reverse micelles. Chem Res Toxicol 7:47–55. https://doi.org/10.1021/tx00037a007
Allwood MC, Russell AD (1970) Mechanisms of thermal injury in nonsporulating bacteria. Adv Appl Microbiol 12:89–119. https://doi.org/10.1016/S0065-2164(08)70583-5
Chick H (1910) The process of disinfection by chemical agencies and hot water. J Hyg 10:238–286. https://doi.org/10.1017/S0022172400042959
Cousin D (1967) Thermosensitive mutants of Escherichia coli k12.2. studies on a lethal mutation controlling an energy yielding reaction. Ann Inst Pasteur 113:309–325
Russell AD, Harries D (1967) Some aspects of thermal injury in Escherichia coli. Appl Microbiol 15:407–410. https://doi.org/10.1128/am.15.2.407-410.1967
Califano L (1952) Libération d’acide nucléique par les cellules bactériennes sous l’action de la chaleur. Bull World Health Organ 6:19–34
Mani-López E, García HS, López-Malo A (2012) Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int 45:713–721. https://doi.org/10.1016/j.foodres.2011.04.043
De Oliveira EF, Cossu A, Tikekar RV, Nitin N (2017) Enhanced antimicrobial activity based on a synergistic combination of sublethal levels of stresses induced by UVa light and organic acids. Appl Environ Microbiol 83:e00383-e417. https://doi.org/10.1128/AEM.00383-17
Hao J, Lei Y, Gan Z, Zhao W, Shi J, Jia C, Sun A (2021) Synergetic inactivation mechanism of protocatechuic acid and high hydrostatic pressure against escherichia coli O157:H7. Foods 10:3053. https://doi.org/10.3390/foods10123053
Jeong Y, Ha J (2019) Combined treatment of UV-A radiation and acetic acid to control foodborne pathogens on spinach and characterization of their synergistic bactericidal mechanisms. Food Control. 106:106698. https://doi.org/10.1016/j.foodcont.2019.06.024
Muraca P, Stout JE, Yu VL (1987) Comparative assessment of chlorine, heat, ozone, and UV light for killing Legionella pneumophila within a model plumbing system. Appl Environ Microbiol 53:447–453. https://doi.org/10.1128/aem.53.2.447-453.1987
Funding
This work was supported by Natural Science Foundation of China (12275042) and Natural Science Foundation of Liaoning Province of China (2023-BS-148).
Author information
Authors and Affiliations
Contributions
ZZ and DL performed the conceptualization and wrote the main manuscript text. HL performed the validation. ZQ performed the formal analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Li, H., Qi, Z. et al. Biological and Chemical Reactivities of Plasma-Activated Water Prepared at Different Temperatures. Plasma Chem Plasma Process 44, 393–410 (2024). https://doi.org/10.1007/s11090-023-10379-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10379-y