Abstract
A mathematical model has been proposed and verified, based on the combination of thermodynamic calculations of equilibrium plasma and CFD-modeling of the plasma-chemical reactor for hydrogen recovery of volatile halides. An experimental study of the hydrogen reduction processes of BCl3 and SiCl4 was carried out under conditions of RF-IC (RF inductively coupled) discharge at atmospheric pressure. Based on the experimental results, the developed mathematical model was validated. A series of numerical experiments on parametric study of gas dynamics of high-frequency plasmatron with vortex stabilization of flow for various hydrogen concentrations in argon-hydrogen plasma-forming mixture was carried out. Thermodynamic calculations and CFD modeling were made for input into the reaction chamber of chemically active gas mixtures (BCl3 + H2), (BF3 + H2), (SiCl4 + H2), (SiF4 + H2), (MoF6 + H2), (WF6 + H2), (BCl3 + CH4 + H2), (BF3 + CH4 + H2), (SiF4 + CH4 + H2), (MoF6 + CH4 + H2) and (WF6 + CH4 + H2). The values for the predictive efficiency and the degree of conversion of target products were obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The release of stable isotopes of silicon and boron as well as of their carbides from isotopically enriched halides (SiCl4, SiF4, BCl3 and BF3) with high chemical and isotopic purity is of great interest for microelectronics and nuclear power [1, 2].
It is planned to develop a completely silicon quantum computer on the basis of 28Si. The experimental foundations for the development of this system were shown in [3,4,5,6]. In [3, 4], the realization of a quantum computer, completely consisting of silicon, is proposed. Initialization is carried out by optical pumping methods, and for reading information from qubits–the nuclear spins of 29Si located in the form of chains in 28Si matrix, the magnetic resonance force microscopy is used. 28Si and 28SiHCl3, obtained from isotopically enriched 28SiF4 and 28SiCl4, with well developed technologies of isotope enrichment can be the initial substances for a quantum computer [7,8,9].
The main properties of boron, allowing to expect the effect from the use of enriched 10B, is its high value of the cross section of the capture of thermal neutrons (3·10–25 m2) and high thermal conductivity of 19 W/(m °C) [10, 11]. The largest share of boron consumption falls on radiation protection materials. In these materials boron is in the form of carbide. The boron carbide of different enrichment is actually the only absorbing material for the control elements of nuclear reactors on fast neutrons. Since the isotope enrichment technologies are also well developed for BCl3 and BF3 [12, 13], they are the starting materials to obtain various isotopic modifications of boron and boron-containing substances.
The volatile halides of silicon and boron have high chemical and thermal resistance [14, 15]. Therefore, the chemical methods for producing Si or B from them as well as SiC or B4C are characterized by multi-stage nature which leads to large losses of isotopically enriched substance and its chemical purity. An alternative is the plasma-chemical methods where the process of dissociation of halides and the release of elementary substances and compounds proceeds under the action of different discharges as a result of interaction with high-energy plasma electrons. The development and use of plasma-chemical methods will provide a one-stage process for production of isotopically enriched silicon, boron as well as carbides from their halides.
The development of plasma chemical processes based on high-power RF-IC plasmatrons is an acute problem since the use of these plasmatrons enables to implement high-enthalpy processes, provides the chemical purity of high-temperature region formed by plasma and high productivity [16,17,18,19].
For realization of the above-mentioned processes, the high-performance technologies are required with the use of high-power RF-IC—the plasmatrons operating at atmospheric pressure and reaction chambers in which the processes of separation of target products take place. While designing RF-IC-plasmatron and reactor providing the implementation of optimum thermogasdynamic parameters, it is rational to use the virtual experiments based on computational fluid dynamics (CFD).
We have developed the technique for modeling thermogasdynamic processes based on the combination of thermodynamic analysis of thermal, quasi-equilibrium plasma and CFD-modeling of RF-IC plasmatron and plasma-chemical reactor. Thermodynamic calculations make it possible to obtain the temperature dependence of the concentration of substances formed in the plasma. CFD-simulation gives an idea on the distribution of temperature fields and movement in the current RF-IC-plasmatron and plasma-chemical reactor both of the initial components of the gas mixture and of the reaction products. Joint use of computational hydrodynamics and techniques of thermodynamic analysis makes it possible to find the optimum design of the RF-IC-plasmatron and the reactor as well as modes of operation before they will be manufactured and investigated experimentally.
The developed methodology showed good convergence of the results in the theoretical study of the hydrogen reduction processes of SiCl4 and BCl3 in RF-IC plasma with experimental data while studying special features of separation of Si and B.
Using the developed methodology, an analysis was carried out on the possibility of using thermal quasi-equilibrated argon-hydrogen plasma for obtaining and synthesizing the carbides of boron and silicon from their halides as well as molybdenum and tungsten and their carbides from volatile fluorides.
Experimental
An experimental study of the processes of hydrogen reduction of BCl3 and SiCl4 under conditions of RF-IC discharge at atmospheric pressure was carried out on the set-up which schematic diagram is described in detail in [20]. The frequency of HF generator oscillations was 5.28 MHz. RF-IC-plasmatron is a silica tube with over mounted inductor. In the upper part of the silica tube, the head of vortex generator was located providing a tangential supply of plasma-forming gas into the discharge zone. RF-IC plasmatron was started at atmospheric pressure using the spark ignition device “Tesla transformer”. Both pure argon and the mixture of argon with hydrogen were used as a plasma-forming gas. The flow rate of the plasma-forming gas Ar + H2 at the same time was 100–200 l/minin. The power consumed by the HF generator was 85 kW. Accounting for the efficiency of the generator lamp and losses in the oscillatory circuit, the power released in plasma (Wd) was 40 ± 7 kW. The pressure was 1 atm. The specific energy supply was calculated as the ratio of power values and the flow of plasma-forming gas (Q[Mol/s]) and was maintained at 350 ± 25 kJ/mol.
The processes of hydrogen reduction are always carried out under conditions of excessive hydrogen concentration [21]. The hydrogen reduction of BCl3 was performed under the following optimal parameters: the ratio of H2/BCl3 = 10, the consumption of Ar-150 l/min, the flow rate of hydrogen in the plasma-forming mixture -30 l/min. When (H2 + BCl3) mixture is supplied, the consumption of hydrogen 33 l/min, BCl3–3.3 l/min (in the gas phase). In order to determine the degree of conversion of the boron trichloride, the comparative studies of the chemical composition of the original mixture, supplied to the plasmatron and gas-phase reaction products by the IR spectroscopy method, were carried out. The total degree of conversion of the boron trichloride is 75%, of which 10% goes into a by-product-dichlorborane, and 65% into the target product-boron. Boron samples are represented by polycrystalline nanodispersed powder with a characteristic size of 200 nm. The productivity of boron in this mode is 0.1 kg/h.
The hydrogen reduction of SiCl4 was carried out with similar parameters to the hydrogen reduction of BCl3: the ratio H2/SiCl4 = 10, the consumption of Ar-150 l/min, hydrogen consumption in the plasma-forming mixture–30 l/min. When the mixture of (H2 + SiCl4) is applied, the flow rate of hydrogen was 33 l/min, SiCl4–3.3 l/min (in the gas phase). Gas chromatographic analysis shows that the conversion of silicon tetrachloride is more than 95%. The main gas-phase products are chlorosilanes (SiHCl3, SiH2Cl2 and SiH3Cl) and hydrogen chloride. Gas chromatographic analysis of chlorosilanes was carried out on the gas chromatograph «CVET-500». A catarometer was used as a detector. The length of partition column was l = 3 m, the inner diameter Ø = 3 mm with HROMATON carrier and 15% of silicone oil E-301. The accuracy of the determination of chlorosilanes was 2%. The degree of conversion of silicon tetrachloride into silicon is 25%. In the realized experimental conditions, the samples of nanodispersed polycrystalline silicon were obtained with a capacity of 0.07 kg/h.
Powdered B and Si were collected on special filter from acid-resistant tissue which was located at the outlet of the plasma chemical reactor. Before the experiment and after unloading the powdered samples, the filter was weighed with an accuracy of 0.1 g.
In practice, the simulated thermal plasma is considered as a system of particles, the interaction between which defines its main characteristics Te and Ne. Each generation process in plasma can be compared with its inverse process–the recombination.
According to the principle of detailed equilibrium, the distribution of electrons is established at energy levels which could be observed at total thermodynamic equilibrium and the number of excited molecules is determined by Boltzmann law. Te is the parameter of such distribution which can be determined by measuring the number of excited atoms. The number of excited atoms can be considered by the intensity of the spectral bands of the emission spectrum of plasma [22].
The emission spectra of the plasma of pure argon and its mixture with hydrogen were recorded in the range of 300–900 nm using the HR4000CJ-UV-NIR emission spectrometer with a resolution of 0.3 nm, optical fiber and collimation lens COL-UV/VIS.
To determine the temperature of the electrons, the atomic lines of argon were chosen with the greatest radiative transition probability 434.51, 750.38 and 811.38 nm [22, 23]. To increase the accuracy of measurements, the calculation was made for three lines, and the result was averaged. To evaluate the concentration of electrons, the line of singly charged ion of argon 406.511 nm was chosen. The assessment methodology is described in detail in [20]. In view of the fact that RF-IC plasma of atmospheric pressure, which is realized in our conditions, represents the thermal equilibrium plasma, it was accepted that the order of magnitude of Tg coincides with that of Te and is in the range of 3500–6200К.
Thermodynamic Prediction of Conversion Products in Quasi-Equilibrium Plasma Accounting for Ionic Forms and Electron Gas
Thermodynamic modeling of a multi-component plasma chemical system is made according to the methodology that combines the computer realization of the Gibbs method with its expansion to the analysis of the so-called conditional equilibrium states together with the bank of thermodynamic functions. Note that high temperatures of plasma chemical synthesis considerably remove the kinetic restrictions on the attainment of equilibrium state, bringing the process to the quasi-equilibrium state. The paper considers the cases of ejecting into the studied reactor of the following chemical-active mixtures (BCl3 + H2), (BF3 + H2), (SiCl4 + H2), (SiF4 + H2), (MoF6 + H2), (WF6 + H2), (SiF4 + CH4 + H2), (BF3 + CH4 + H2), (MoF6 + CH4 + H2) and (WF6 + CH4 + H2).
Thermodynamic modeling of multi-component plasma chemical system is made according to the method of minimization of Gibbs energy. In this case, formulas (4), obtained in the procedure for conditional minimization of Gibbs energy (1), express the concentrations of components through uncertain Lagrange multipliers \({\uplambda }_{j}\).
The solution of the balance Eqs. (2) and (3) by phases \(f_{0}\) and \(j_{0}\) elements relative to {\({\uplambda }_{j}\)} after substitution of expressions (4) in (2) and (3) determines the equilibrium composition of the heterophase system. The values \(g_{i}\), that include standard enthalpy of formation of \(\Delta_{f} H^{^\circ } (298)\) and Planck function \(\Phi_{298}^{^\circ } (T)\), contain the required initial information for each i-th component. The total pressure \(\tilde{P}\) of the mixture of plasma components in Eq. (5) is given in atmospheres. The matrix \(A_{ij} = \{ a_{ij} ,\;a_{if} \}\) apart from the stoichiometric coefficients of the participation of the j-th element in the chemical formula of the i-th substance \(a_{ij}\), includes the coefficients of belonging of the i-th component to phase f \(a_{if} = \{ 1,\;0\}\). According to the initial chemical composition of the reagents, the elemental composition \(b_{j}\) of the system in the balance Eq. (3) is calculated.
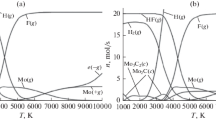
Figures 1, 2, 3, 4, 5, and 6 give the calculated dependences of the number of moles n(T) of basic reaction products on temperature for the conversion of chloride and fluoride of boron and silicon correspondingly into boron and silicon as well as the fluorides of molybdenum and tungsten into molybdenum and tungsten. For better visualization on the diagrams with designation (a) the ordinate scale was constructed within the boundaries of 0–45 mol, and on the graphs with designation (b) the ordinate scale is given magnified in the region close to zero (0–2.5 mol). The volumetric consumption of gases corresponded to experimental ones and was equal to 150 l/min for argon, 63 l/min for hydrogen, 3.3 l/min for gas-reagent (depending on the studied variant: BCl3, BF3, SiCl4, SiF4, MoF6, WF6).
While obtaining the boron from its chloride (Fig. 1), the temperature regions of its formation in the condensed phase (from 1000 to 2500 K) and in gaseous forms should be taken into account: B(g) above 2500 K and ion B(+ g) together with electronic gas above 8000 K. In the case of conversion from fluorides (Fig. 2), the absence of condensed boron at a temperature of from 1000 to 2500 K is due to the existence of a more favorable BF3(g) and BHF2(g). In the temperature range from 2500 to 10,000 K, the boron is contained in forms: B(g) and BF(g) in the ratio of 1: 1000, respectively, and ionic form B(+ g).
In the case of silicon chloride (Fig. 3), the condensed phase up to temperatures of 2000 K is formed by silicon. At temperatures above 2000 K, silicon is in the form Si(g) and with increase in temperature the ionic form Si(+ g) appears.
The separation of silicon from fluoride (Fig. 4), as in the case of boron, takes place without formation of the condensed phase. Up to temperature of 3000, the silicon is in the form of SiF4(g), at higher temperatures Si(g) and Si(+ g) are accordingly formed.
Molybdenum is formed from the gas mixture MoF6 + H2 (Fig. 5) in the condensed state up to temperature ≈ 3000 K. At temperatures higher than 3000 K, molybdenum converts from Mo(g) into ionic form Mo(+ g). The condensed and gaseous phases in the case of formation of tungsten from gas mixtures WF6 + H2 (Fig. 6) are formed similarly.
Figures 7, 8, 9, 10, and 11 show the calculated dependences of changes in the number of basic reaction products on the temperature n(T) in the presence of methane for the following mixtures: (BCl3 + CH4 + H2), (BF3 + CH4 + H2), (SiF4 + CH4 + H2), (MoF6 + CH4 + H2), (WF6 + CH4 + H2). The consumption of gases, reagents, hydrogen and plasma-forming mixture were set as equal to the corresponding consumptions for the variants with the absence of methane. CH4 consumption corresponded to the minimum required quantity for synthesis of carbides. The ordinate scale in Figs.7, 8, 9, 10, and 11 was built similarly to Figs.1, 2, 3, 4, 5, and 6.
According to the results of the thermodynamic calculation of conversions in the gas system BCl3 + CH4 + H2, the condensed phase up to temperature of 3000 K is formed from the boron carbide and carbon (Fig. 7). The gas phase is presented mainly by boron (B(g), B(+ g)) and carbon (C(g)) as well as by HCl(g), Cl(g) and hydrogen (H2(g), H2(+ g), H(g), H(+ g)). While calculating the interaction of BF3 with CH4 + H2 gas mixture (Fig. 8), the carbon is formed in the condensed phase in the temperature range from 1000 to 4000 K. In the gas phase, the boron enters BHF2(g) and B(g). Calculation of thermodynamically determined conversion products of silicon fluoride in the presence of methane and hydrogen (Fig. 9) shows the formation of silicon carbide in the condensed phase up to temperatures of 2000 K. Silicon in the gaseous form is contained in: Si(g), Si(+ g), SiF(g), SiF2(g) at temperatures above 2000 K. In the process of conversion in the gas system MoF6 + CH4 + H2, the mixture of molybdenum carbides (Mo2C(c) and Mo3C2(c)) is formed. At temperatures higher than 3000 K, molybdenum coverts from the form Mo(g) into the ionic form Mo(+ g) (Fig. 10). In the case of tungsten formation in the presence of methane, the condensed phase of tungsten is formed together with carbides (Fig. 11).
CFD-Modeling of the Hydrogen Reduction Process in RF-IC Plasma Chemical Reactor
The modeling was carried out for RF-IC plasmatron used in an experiment with plasma power of 40 ± 7 kW and with vortex supply of plasma-forming gas presented in Fig. 12. The model includes plasmatron I in which the formation and inductive heating of argon–hydrogen plasma are realized; the reaction chamber II where the gas-carrier of hydrogen, gas-reagents are supplied and the reactions of hydrogen recovery and the synthesis of carbides occur as well as a cooling channel III where the target products of conversion condense. The numerical realization of the task was carried out using the Ansys CFX software. The numerical solution of the system of equations of fluid and gas dynamics is carried out using the element-based finite volume method. The structured HEXA mesh is built using the ANSYS ICEM CFD. To determine the convective derivatives, the second order upwind difference scheme is used. Derivatives in diffusion members of the equations are calculated in integrated ratios for the control volume by determining derivatives using the shape functions. Time integration is carried out according to the implicit the second order Euler method.
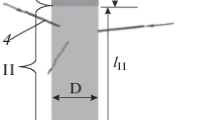
Diagram of RF-IC plasma-chemical reactor: 1- inlet tubes for supplying Ar:H2(8 tubes) mixture; 2-output; 3-four-coil inductor; 4-input tubes for supplying the gas carrier and gas-reagents (3 tubes); I–zone of inductive heating of plasma; II–reaction zone; III–cooled channel; D = 0.1 m, lI = 0.3 m, lII = 0.4 m, lIII = 4 m
It is expedient to divide the modeling gas dynamics of the plasma-chemical reactor into two stages. At the first stage, the problem of the flow of argon-hydrogen inductively coupled in plasmatron zone plasma I is being solved. The turbulent movement of viscous compressible gas in non-uniform temperature field is considered taking into account the induction heating of the gas and the effect of the power of the electromagnetic field on the plasma movement. It is assumed that heat exchange is carried out by means of thermal conductivity, convection and radiation. Mathematical setting of the task is given in the appendix.
At the second stage, the task of the flow of argon–hydrogen plasma-forming mixture and of the gas carrier and gas-reagents incoming from the inlet tubes 4 (Fig. 12) in zones II and III (Fig. 12) is solved. The formation of reaction products in the hydrodynamic model is found by the thermodynamically determined concentration in the cell of the computational domain of gas-reagents. Mathematical setting of the task is given in the appendix.
Results and Discussion
While solving the problem in the region of plasmatron I (Fig. 12), three-dimensional computational experiments were carried out on the study of the affect of the additive of molecular hydrogen required for the realization of plasma-chemical reactions of chemical conversion of fluorides into the plasma-forming gas—argon. Thermal and kinetic conditions of inductive heating of plasma are determined. In the near-wall region there is a vortex movement of the plasma-forming gas (Fig. 13a) which ensures the cooling of the reactor walls. The optimum values of the power of the generator and energy release in the plasma were found at various ratios of the volumetric consumption of argon and hydrogen in the plasma-forming mixture of QAr:H2 gases. The optimum energy release in the plasma region is 38 kW and is achieved at the following inductor current values: 160 A for QAr:H2 = 150 l/min: 18 l/min; 170 A for QAr:H2 = 150 l/min: 24 l/min; 180 A for QAr:H2 = 150 l/min: 30 l/min; 190 A for QAr:H2 = 150 l/min: 40 l/min. The temperature fields, obtained as a result of the calculations for optimum modes of temperature fields in the zone of plasmatron, are shown in Fig. 13b). The average temperature in plasmatron zone, obtained according to the results of calculations for all studied plasma-forming mixtures, changes in the range of 5700 K for mixture with the maximum hydrogen content (40 l/min) up to 5900 K for mixture with the minimum hydrogen content (18 l/min). The obtained values are consistent with the plasma temperature measured in the experiments.
Modeling of the flow of the gas mixture in zones II and III (Fig. 12) was carried out for the values of volumetric consumption, when the chemically active gas mixtures are supplied to the reactor, equal to the corresponding experimental values and the parameters of thermodynamic calculations. The ratio of the gas-reagent to hydrogen during supply to inlet tubes 4 (Fig. 12) was 3.3 l/min: 33 l/min. When methane is supplied to the reactor, its consumption, as in the corresponding thermodynamic calculations, corresponded to the minimum required quantity for the synthesis of carbides. The consumption of the plasma-forming mixture Ar: H2 was 150 l/min: 30 l/min. The energy release of plasma was set to 38 kW which is the optimum value according to the results of the solution of the first stage of the task.
While developing the CFD model, we carried out a number of test calculations on the effect of thermodynamic and transport properties of the studied chlorides, fluorides and target products of reaction on the gasdynamics of the process. The gasdynamics of the reactor was calculated with the input of only hydrogen and optionally of methane (without gas reagent) into the inlet tubes 4 (Fig. 12). Further, a similar calculation was carried out with the addition of several types of reagent gas into the tubes 4 (Fig. 12). The results of calculations show that due to low concentration of gas reagent and products of reaction in the reactor, their contribution to the general gasdynamics of the process and to the additive properties of the environment is low compared to the plasma-forming gas and hydrogen.
Thus, for example, when boron chloride is injected into the reactor in the regions with the maximum volume fraction of BCl3 on the average in the calculated region the density of the medium changes by 6%, the specific heat capacity by 0.3%, viscosity by 11%, and thermal conductivity by 8%. At the same time, for example, the use of boron fluoride as a gas reagent instead of boron chloride leads to increase in the average temperature of gas mixture in the reaction chamber II (Fig. 12) by 3% (by 110 K), and in the cooled channel III (Fig. 12) by 4% (by 50 K). The average axial rate of the flow changes by less than 6% (0.1 m/s).
In general, the gasdynamics and thermal conditions in the plasma chemical reactor are similar during conversion of all studied substances.
The results of modeling the flow of the gas mixture in zones II and III (Fig. 12) with the conversion of BCl3 into B in view of thermal and gas-dynamic conditions in the reactor are shown in Fig. 14. This picture is typical during supply to the reactor of all considered gas-reagents.
The main numerical parameters in the study of the efficiency of the synthesis of products in RF-IC plasmatron were the productivity of QM TP (mass yield of the target product) and the degree of conversion θ characterizing the part of the gas-reagent conversed during the chemical reaction into the target product: \(\theta = {\raise0.7ex\hbox{${Q_{M\;TP - 2} \cdot M_{3} }$} \!\mathord{\left/ {\vphantom {{Q_{M\;TP - 2} \cdot M_{3} } {Q_{{M_{GR - 4} }} \cdot M*}}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${Q_{{M_{GR - 4} }} \cdot M*}$}} \cdot 100\% ,\) where QMTP-2 and \(Q_{{M_{GR - 4} }}\) are the mass consumption of target product, respectively, through the output boundary 2 (Fig. 12) and gas-reagent through the inlet boundary 4 (Fig. 12).
The validation of the methodology for modeling the conversion of target products was carried out on the example of conversion of BCl3 into B and of SiCl4 into Si in the studied reactor. The deviation of the estimated mass yield B (70 g/h) from the experimental value (60 g/h) does not exceed 15% with the degree of conversion of BCl3 of 70%. In the case of conversion of SiCl4 into Si, the calculated mass yield of Si was 50 g/h with a degree of conversion of 18% which is by 30% less than experimental data (70 g/h and 25%). This result is explained by the fact that the thermodynamic calculation for SiCl4 + 19H2 + 45Ar mixture (Fig. 3) indicates the formation in the operating temperature range of the reactor, along with Si, a significant amount of SiCl and SiCl2. After entering the lower temperature zone, these radicals react with atomic hydrogen which leads to the formation of chemically stable compounds–chlorosilanes. Their formation is proved by gas chromatographic analysis. The obtained accuracy of calculations is sufficient for using the developed technique as a tool for analyzing the main mechanisms of reactions and gasdynamics of the reactor.
Table 1 shows the degree of conversion when the chemically active mixtures are supplied to the reactor without methane obtained from the results of cfd-calculations. Similar results of calculations for the cases of methane supply to the reactor are shown in Table 2. The designation in the tables θ corresponds to the degree of conversion of the target product (B, Si, Mo or W depending on the variant of supply of chemically active mixture into the reactor). θCARB symbol in Table 2 denotes the degree of conversion of the carbide of boron, silicon, molybdenum and tungsten. To compare the temperature modes of the formation of the studied substances, in Tables 1 and 2 the threshold values of Tmin temperatures are given above which, according to thermodynamic calculations, the target product is formed: boron, silicon, molybdenum, tungsten in Table 1 and their carbides in Table 2.
The results of the calculations indicate that when the volatile fluorides are used as gas-reagents, the gasdynamics conditions in the studied plasma chemical reactor are the most efficient for the synthesis of molybdenum, tungsten and their carbides. This result is justified by the fact that the temperature mode in the cooled channel of the reactor 1000–3000 K (Fig. 15) is optimal for the formation of the condensed phase of Mo, W and their carbides on the basis of thermodynamic calculations (Figs. 4 and 5). On the contrary, the considered design of the reactor is unsuitable for the synthesis from volatile boron fluoride and its carbide. The supply of boron fluoride and its movement in the reactor is carried out in the temperature zone T < 3500 K. To realize the process of reduction of the boron from its volatile fluoride, the reactor should be upgraded in order to deliver gas-reagents to the high-temperature zone (T > 4000 K) where, according to the thermodynamic calculation, the formation of the gaseous boron occurs (Fig. 2). Further, while entering the cooled channel of reactor, the formed gaseous boron will be condensed naturally or the additional annealing will be required. In the case of supplying the mixture of (BF3 + CH4 + H2) to the reactor, according to the thermodynamic calculation, the formation of B4C in the studied temperature range (1000–10,000 K) was thermodynamically advantageous (Fig. 8). The intermediate position between the maximum possible productivity of the studied structure for molybdenum, tungsten and their carbides and zero mass yield for boron and its carbide is occupied by the variant of synthesis of silicon and its carbide. In this case, the threshold temperature of the formation of target products is somewhat low as compared to the boron. It makes it possible to produce silicon and its carbide in the studied conditions, however, with a low degree of conversion in contrast to the synthesis of molybdenum and its carbides.
Conclusion
A technique for modeling gasdynamics conditions for obtaining materials in RF-IC plasma-chemical reactor with vortex gas supply was developed based on the combination of thermodynamic calculations of quasi-equilibrium plasma with the modeling of the reactor design by methods of computing hydrodynamics.
Based on the proposed model, a series of numerical experiments were carried out on parametric study of gasdynamics of RF-IC-plasmatron with vortex stabilization of flow. Thermal and kinetic conditions of inductive heating of plasma are determined with a variety of process parameters. The optimum values for the power of generator and energy release in the plasma are found at various ratios of argon and hydrogen in the plasma-forming mixture of gases.
An experimental study of the processes of hydrogen reduction of BCl3 and SiCl4 in the studied RF-IC plasma chemical reactor was carried out.
Thermodynamic calculations and computational experiments are carried out for the process of hydrogen reduction of BCl3, BF3, SiCl4, SiF4, MoF6, WF6, and synthesis of B4C, SiC, Mo2C, Mo3C2 and WC for the studied reactor design. Basing on the calculations, the temperature fields and flow rates are defined during supply of chemically active gas mixtures (BCl3 + H2), (BF3 + H2), (SiCl4 + H2), (SiF4 + H2), (MoF6 + H2), (WF6 + H2), (BCl3 + CH4 + H2), (BF3 + CH4 + H2), (SiF4 + CH4 + H2), (MoF6 + CH4 + H2) and (WF6 + CH4 + H2) into the jet of argon-hydrogen plasma. The values for the predictive productivity and the degree of conversion are obtained.
According to the results of calculations, it was established that the design of the reactor under study is optimal for production of molybdenum, tungsten and their carbides as well as of boron and its carbide from the volatile chloride. At the same time, the synthesis of boron and its carbide from the volatile fluoride in the same conditions cannot be realized, and the synthesis of silicon and its carbide occurs with a low degree of conversion. This is due to the fact that the temperature mode in the zone of movement of gas-reagents does not correspond to the thermodynamically calculated temperatures of the formation of boron, silicon and their carbides from volatile fluorides.
Abbreviations
- G :
-
Gibbs energy
- Δ f H (298):
-
Standard enthalpy of formation
- Φ (T) :
-
Planck function
- \(\tilde{P}\) :
-
Total pressure of the mixture of plasma components
- n :
-
Number of moles of reaction product
- Q Ar:H2 :
-
Volume flow of argon and hydrogen in the plasma gas mixture
- Q M TP :
-
Mass flow of the target product through the output boundary
- θ :
-
Degree of conversion
- Q M GR-4 :
-
Mass flow rate of the reagent gas through the inlet boundary
- θ TP :
-
Degree of conversion of the target product
- Q M CARB :
-
Mass flow rate of the carbide (depending on the type of mixture of boron, silicon, molybdenum or tungsten)
- θ CARB :
-
Degree of conversion of the carbide (depending on the type of mixture of boron, silicon, molybdenum or tungsten)
- T min :
-
Threshold temperature above which the target product is formed
- T :
-
Temperature
- ρ :
-
Density
- V :
-
Velocity
- p :
-
Pressure
- C p :
-
Specific heat capacity
- μ e :
-
Effective viscosit
- λ e :
-
Effective thermal conductivity
- μ :
-
Laminar viscosity
- λ :
-
Laminar thermal conductivity
- μ t :
-
Turbulent viscosity
- λ t :
-
Turbulent thermal conductivity
- F e-m :
-
Lorentz force
- k b :
-
Boltzmann constant
- e :
-
Elementary electronic charge
- S J :
-
Joule heating power
- u rad :
-
Radiation energy lost
- σ :
-
Electrical conductivity of plasma
- \(\overrightarrow {E}\) :
-
Electric field strength
- \(\overrightarrow {B}\) :
-
Magnetic field strength
- \(\overrightarrow {A}\) :
-
Complex vector of magnetic potential
- (r θ z):
-
System of cylindrical coordinates
- A R :
-
Real component of the complex vector of the magnetic potential
- A I :
-
Imaginary component of the complex vector of the magnetic potential
- μ 0 :
-
Magnetic permeability
- f :
-
Inductor current frequency
- J к :
-
Current density of the inductor
- a :
-
The radius of the cross section of the inductor
- i :
-
Substance index
- ϕ :
-
Volume fraction
- S i :
-
Mass source term of the corresponding substance
- \(\dot{n}\) :
-
Change in the amount of substance per unit of time
- n 0 :
-
Initial amount of substance
- M :
-
Molar mass
- c :
-
Mass concentration
- D :
-
Diffusion coefficient
- p cr :
-
Critical pressure
- T cr :
-
Critical temperature
References
Zulehner W (2000) Mater Sci Eng B 73:7–15
Risovannyj VD (2012) Pogloshchayushchie materialy sterzhnej upravleniya i zashchity yadernyh reaktorov. Izdatel’skij centr Ul’yanovskogo gosudarstvennogo universiteta, Ul’yanovsk (in Russian)
Ladd TD (2002) Phys Rev Lett 89:017901
Kohei M (2005) Solid State Commun 133:747–752
Morton JJL, Tyryshkin AM, Brown RM, Shankar S, Lovett BW, Ardavan A, Schenkel T, Haller EE, Ager JW, Lyon SA (2008) Nature 455:1085–1088
Yang A, Steger M, Sekiguchi T, Thewalt MLW, Ladd TD, Itoh KM, Riemann H, Abrosimov NV, Becker P, Pohl H-J (2009) Phys Rev Lett 102:257401
Godisov ON, Kaliteevskii AK, Korolev VI, Ber BY, Davydov VY, Kaliteevskii MA, Kop’ev PS (2001) Semiconductors 35:877–879
Babichev AP, Zhernova ZY, Kurochkin AV, Mishachev AA, Popov GE, Rudnev AI, Tikhomirov AV (2002) Inorg Mater 38:425–426
Bulanov AD, Troshin OY, Greben’kov KS, Churbanov MF (2017) Sposob polucheniya izotopno-obogashchennogo tetrahlorida kremniya Patent RF (RU) Registracionnyj nomer 2618265. Zayavka 2016102741 ot 27.01.2016. opubl. 03.05.2017[In Russian]
Anderson UK (1965) Pogloshchayushchie materialy dlya regulirovaniya yadernyh reaktorov. Per. s angl. Atomizdat, Moskva (in Russian)
Gvardciteli IG, Karumidze GS, Shengeliya LA (1993) Atomnaya energiya 74:252–253 (in Russian)
Andriec SP, Gushchin AA, Kalashnikov AL, Kozyrev AS, Mochalov YS, Horoshilov AV (2010) Perspekt Mater 8:193–198 (in Russian)
Polevoj AS (1990) Itogi nauki i tekhniki Seriya «Radiohimiya. YAdernaya tekhnologiya» Tom 2 Razdelenie i ispol’zovanie stabil’nyh izotopov bora. Moskva: VINITI. (in Russian)
Ryss IG (1956) Himiya ftora i ego neorganicheskih soedinenij. Moskva: Goskhimizdat [in Russian]
Furman AA (1980) Neorganicheskie hloridy. Himiya, Moskow (in Russian)
Reed TB (1961) J Appl Phys 32:821–824
Eckert HU (1972) Report SAMSO-TR-72-227, Los Angeles
Frolov V, Matveev I, Ivanov D, Zverev S, Ushin B, Petrov G (2011) Rom J Phys 56:36–40
Matveev I, Matveyeva S, Zverev S (2014) IEEE Trans Plasma Sci 42:3891–3895
Kornev RA, Sennikov PG, Shabarova LV, Shishkin AI, Drozdova TA, Sintsov SV (2019) High Energy Chem 53:246–253
Cvetkov YV, Panfilov SA (1980) Nizkotemperaturnaya plazma v processah vosstanovleniya. Nauka, Moskow (in Russian)
Laux CO, Spence TG, Kruger CH, Zare RN (2003) Plasma Sources Sci Technol 12:125–138
Isola LM, G’omez BJ, Guerra V (2010) J Phys D Appl Phys 43:015202
Menter FR (1994) AIAA J 32:1598–1605
Langtry RB, Menter FR (2009) AIAA J 47:2894–2906
Murphy AB (2000) Plasma Chem Plasma Process 20:279–297
White FM (2006) Viscous fluid flow, 3rd edn. McGraw-Hill, Boston
Bretshnajder S (1966) Svojstva gazov i zhidkostej. Himiya, M-L (in Russian)
Osborn RH (1941) J Opt Soc Am 31:428–432
Grigor’ev IS, Mejlihov EZ (1991) Fizicheskie velichiny. Energoatomizdat, Moskow (in Russian)
Acknowledgements
The authors are very grateful to the RSF Grant No 20-13-00035 basic support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Mathematical Formulation of the Problem in the Zone of Plasmatron I (Fig. 12)
The flow of plasma-forming gas in plasmatron I (Fig. 12) while solving the first stage of the gas-dynamics problem of RF-IC-plasmatron with vortex generator is described by the system of Eqs. (6–10) and an additional relation (11) with the corresponding boundary and initial conditions. Due to the lack of physical symmetry of the flow in plasmatron with vortex generator, the thermogasdynamic component of the problem was solved in a three-dimensional formulation. The axial cross section of the calculated field of the problem is represented in Fig. 15a. Figure 15b shows the photo of the studied RF-IC-plasmatron. The field of solving the problem includes plasmatron (zone A Fig. 15a) with the walls of the final thickness (zone B Fig. 15a), the inductor (zone C Fig. 15a) and the outer part of the air space (zone D Fig. 15a) adjacent to the device. Excluding the bias current, the spiral inductor can be represented as a system of four cylindrically symmetric parallel rings.
In the equation of continuity (6), movement (7) and energy (8) ρ is the density of the gas mixture Ar:H2, V is the velosity, p is the pressure, \(c_{p}\) is the specific heat capacity, T is the temperature, \(\mu_{e} ,\lambda_{e}\) is the effective viscosity and thermal conductivity, \(\mu_{e} = \mu + \mu_{t}\), \(\lambda_{e} = \lambda + \lambda_{t}\), \(\mu ,\lambda\) are the laminar viscosity and thermal conductivity, \(\mu_{t} ,\lambda_{t}\) are the turbulent (vortex) viscosity and thermal conductivity, \(F_{e - m} = 0.5 \cdot \sigma [\vec{E} \times \vec{B}]\) is the Lorentz power, kb is the Boltzmann constant, e is the elementary electric discharge, \(S_{J} = 0.5 \cdot \sigma \vec{E}^{2}\) is the power of joule heating, \(u_{rad}\) is the radiation energy lost, σ is the plasma electrical conductivity, \(\vec{E}\) is the electric field strength, \(\vec{B}\) is the magnetic field strength, the upper index T is the transposition operator of the matrix.
While modeling the gasdynamics of plasmatrons with straightforward gas supply, as a rule, a laminar model is used which is justified by the results of physical experiments. However, preliminary calculations showed that for adequate description of the movement in the investigated plasmatron with vortex generator, the turbulence should be taken into account both near walls and in the reactor volume. The system of Eqs. (6–8) is closed by a semi-empirical k-ω SST model of turbulence of Menter [24]. The selection of the model is due to its popularity for solving a wide range of engineering problems and the potential possibility of joint use of the model of laminar-turbulent transition of γ-Reθ [25].
The values of density, viscosity, specific heat, thermal conductivity, electrical conductivity and the specific energy of the radiation of the gas mixture in the model are set by the temperature functions [26].
To find the values \(\vec{E}\) и \(\vec{B}\) it is necessary to find the solution to electromagnetic problem. For the process under consideration, the Maxwell equations system can be reduced to the Poisson equation for the complex vector of magnetic potential \(\vec{A}\). In this case, in the cylindrical coordinates (rθ z) only the angular component of the vector \(\vec{A}\) is different from zero, and then the equations for the real \(A_{R\theta }\) and imaginary \(A_{I\theta }\) parts of the magnetic potential are:
where \(\mu_{0}\) is the magnetic constant, \(\omega = 2\pi f\), Jк is the inductor current (different from zero only in zone III and is determined from the conditions of the physical experiment), and a is the radius of the cross section of the inductor. The value of the electrical conductivity of the medium is:
The strength of electrical and magnetic fields, included in Eqs. (7) and (8), have the form:
The system of Eqs. (6–8) is solved in zone A of the computational domain (Fig. 15a) and is complemented by boundary conditions which are set in accordance with experimental data. The plasma-forming gas of room temperature with the given volume consumption and Ar/H2 ratio is supplied to the input boundary of plasmatron, the zero excessive pressure is set on the output boundary, the adhesion condition is set on the solid walls while on the upper wall of plasmatron the temperature is 293 K, on the side wall 1450 K.
The boundary conditions of Eqs. (9) and (10) are: continuity \(A_{\theta }\) on the internal boundaries of the computational domain; equality to zero of the vector of magnetic potential at the outer boundaries of the environment and walls of the channels; and equality to zero of the flow of magnetic potential at the outer boundaries of plasmatron.
It is assumed in the model that zones B, C, D of the computational domain (Fig. 15) are a fictitious solid medium with permanent properties. Due to the fact that the temperature of plasmatron walls is considered to be specified, there is no need to solve the thermodynamic problem in zones B, C and D. Only Eqs. (9) and (10) are solved in these zones. This assumption does not affect the correctness of the solution of the thermogasdynamic problem in zone A, but leads to a significant optimization of computational resources.
Mathematical Formulation of the Problem in Zones II and III of the Plasma Chemical Reactor (Fig. 12)
Consider, first of all, the case of input to the reactor of chemically active mixtures for the synthesis of heavy metals (molybdenum and tungsten) and their carbides. The investigation is carried out on the homogeneous flow of argon-hydrogen mixture, gas-carrier H2, gas-reagent, powdered particles of target products, and, optionally, in the case of the synthesis of carbides, methane and carbon, formed in significant amount that is proved by thermodynamic calculations. Note that test calculations indicate a violation of the homogeneity of the flow under study with radius of the convertible particles more than 10 μm which is by two orders of magnitude larger of the assumed dimensions of fine particles (about 200 nm) obtained in the current RF-IC-plasmatron. The homogeneous turbulent flow of the mixture is described by the system of equations of continuity, momentum and energy. The heat exchange is carried out by convection and thermal conductivity. In the continuity equation for the corresponding components of the gas mixture, the mass sources have been added (for carbon, molybdenum or tungsten, carbides of molybdenum or tungsten) and discharge (for gas-reagent and methane). In this case, the mass source terms in each cell of the computational domain are determined on the basis of the dependence of the concentration of the corresponding substance on temperature obtained as a result of thermodynamic calculations. Based on test calculations, the description of the movement of media mixture in the reactor is carried out with the following assumptions:
-
The mass transfer of molybdenum fluoride or tungsten fluoride, hydrogen and methane (in the case of the synthesis of carbides) into hydrogen fluoride and target reaction products–molybdenum or tungsten and, optionally, their carbides is considered. Note that according to thermodynamic calculations, the formation of other solid fluorides does not occur in the studied temperature range. The formation of atomic hydrogen, fluorine as well as carbon, in the case of the synthesis of carbides, occurs at temperatures above 3000 K (Figs. 5, 6, 10, and 11), and is not taken into account in the gas-dynamic model due to small volume of a part of the computational domain where temperature exceeds 3000 K (< 7% of the total volume);
-
The formation of fine condensed phase of target products in the model of gas-dynamic flow is thermodynamically determined by the concentration in this cell of the computational domain of gas-reagents while the condensation process is not modeled in the problem;
-
Excluding chemical kinetics, it is assumed that the thermodynamically determined composition of the mixtures, represented in Figs. 1, 2, 3, 4, 5, 6, 7,,8, 9, 10, and 11, is formed depending on the temperature in an infinitely small period of time.
The computational domain includes the reaction zone of plasma chemical reactor (Fig. 12, II) and the cooled channel (Fig. 12, III). The turbulent movement of viscous compressible perfect gases and fine particles in inhomogeneous temperature field is considered taking into account heat exchange by thermal conductivity and convection.
The flow of gases is described by the system of equations of momentum (12), energy (13) and continuity (14):
In (12–14) index i = 1 corresponds to the argon–hydrogen plasma-forming mixture, i = 2–gas-carrier of hydrogen H2; i = 3–gas-reagent (MoF6 or WF6); i = 4–methane CH4 (in the case of supplying it for hydrogen recovery of carbides), i = 5–hydrogen fluoride HF (g), i = 6–molybdenum Mo or tungsten W, i = 7–molybdenum carbide Mo2C or tungsten carbide WC; i = 8–molybdenum carbide Mo3C2 (when applying gas-reagent MoF6); ϕi, ρi,– volume fraction and density of the corresponding medium, while: \(\sum\nolimits_{i = 1}^{8} {\varphi_{i} } = 1;\rho = \sum\nolimits_{i = 1}^{8} {\varphi_{i} \rho_{i} }.\)
The system of Eqs. (12–14) is closed by the k–ω SST turbulence model which describes the vortex of the flow in the near-wall region and, according to the results of test calculations, provides a high speed of the task convergence.
In Eq. (14) Si are the mass source terms of the corresponding substance in the unit of volume while in the case of supplying chemically active mixtures (MoF6 + H2) and (WF6 + H2) \(S_{4} = S_{7} = S_{8} = 0.\) to the reactor.
As an example, in expressions (15), (16) and (17) the type of source member for the target reaction products is given –Mo and Mo2C, Mo3C2, respectively, (while supplying CH4 to the reactor):
Hereinafter \(\dot{n}(T) = \frac{\partial n(T(t))}{{\partial t}}\) is the change in the amount of substance per unit of time; n0i(T) is the initial amount of the substance given in the thermodynamic calculation; Mi is the molar mass. The index i corresponds to the notations in Eqs. (12–14). For the convenience of perception the substance is added to the mass source index in brackets to which this source refers.
The values of density, viscosity, the specific heat and thermal conductivity of the plasma-forming gas, H2 and CH4 in the model are defined by the temperature functions [26,27,28]. For MoF6, HF(g), as well as for Mo, the specific heat capacity was determined using the NIST database \((c_{p} (T) = \frac{1000}{M}(A + B\frac{T}{1000} + C(\frac{T}{1000})^{2} + D(\frac{T}{1000})^{3} + E(\frac{T}{1000})^{ - 2} )\), where A, B, C, D, E are the coefficients of the polynomial equation cp(T) taken from the NIST database), viscosity and thermal conductivity were calculated by the formulas [28]:
where pcr is critical pressure (atm); Tcr is critical temperature (K); M is the molar mass (g/mol), \(\Pr = \frac{{c_{p} }}{{1.204 \cdot c_{p} (T) + 1.47}}\). The target products of reaction are assumed as incompressible media, their densities are defined equal to the densities of the corresponding materials at room temperature. Temperature dependence of thermal conductivity Mo is taken from [29]. Temperature dependences of the specific heat and thermal conductivity coefficient for Mo2C in the literature are absent; in the model the thermophysical properties of this material are set by constants corresponding to the room conditions [30]. The data on the thermophysical properties of Mo3C2 are not given in open sources, they are considered to be equal to the corresponding characteristics for Mo2C. While developing the model, a parametric study was carried out on the effect of the physical properties of the reaction products on the gas-dynamic pattern in the reactor. The admissibility of applying these assumptions about the thermophysical properties of the fine condensed phase is established.
In the case of modeling the input of the mixtures of (BCl3 + H2), (BF3 + H2), (SiCl4 + H2), (SiF4 + H2), (SiF4 + CH4 + H2), (BF3 + CH4 + H2) into reactor, the simplified model is considered. It is assumed that the powdered particles B, Si, B4C and SiC due to their low concentrations, expected on the basis of the thermodynamic calculations, do not affect the flow gasdynamics in the reactor. A homogeneous turbulent flow of the mixture is considered which includes only argon–hydrogen mixture, gas-carrier, gas reagent (one of the following compounds depending on the type mixture supplied to input 4 (Fig. 12): BCl3, BF3, SiCl4, SiF4) and, optionally, methane and carbon. To describe the distribution of powdered particles of target products in the gas mixture, the equation of diffusion was used with the addition of an appropriate source term that defines the formation of B, Si, B4C and SiC according to thermodynamic calculations. The diffusion coefficients of the particles of boron, silicon and their carbides in the carrying gas stream are estimated by the Stokes–Einstein formula. The radius of particles was assumed equal to 200 nm.
where c и Di are the mass concentration and diffusion coefficient in the corresponding medium of the powder particles of the target product.
Boundary conditions are specified in accordance with the experimental conditions of the conversion process in the plasma-chemical reactor with the vortex stabilization of the flow. In the case of modeling the synthesis of carbides, the chemically active mixture of H2, gas-reagent and CH4 of room temperature is supplied to the inputs of tubes 4 (Fig. 12). While carrying out calculations for the reduction B, Si, Mo and W, the CH4 is not applied to the computational domain. The values for the volumetric flow and the ratio of components at the inlet 4 (Fig. 12) for each studied chemically active mixture are set equal to the corresponding values for which thermodynamic calculations were carried out. The plasma-forming gas with the velocity fields, specified on the boundary, and temperature fields, defined from solving the problem in the plasmatron zone I is supplied to the inflow boundary separating plasmatron zone I and reaction chamber II. The zero excessive pressure is set at the outflow boundary 2 (Fig. 12). On all solid walls, the condition of adhesion is set and the temperatures of walls correspond to the experimental ones. The boundary conditions of diffusion equations for particles B, Si, B4C and SiC are zero concentrations of particles at the inflow 1, 4 (Fig. 12) and the absence of particle flow through the solid walls of the computational domain. The physical properties of the components of the gas mixture are set by the functions of temperature. For BCl3, BF3, SiCl4, SiF4 specific heat capacity was determined using the NIST database, viscosity and thermal conductivity were calculated using formulas (18) and (19).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shabarova, L.V., Kornev, R.A., Ermakov, A.A. et al. Modeling of Thermogasdynamics and Thermal Processes of Volatile Halides of Elements of III, IV and VI Groups in Thermal Plasma of RF-IC Discharge at Atmospheric Pressure. Plasma Chem Plasma Process 42, 1381–1403 (2022). https://doi.org/10.1007/s11090-022-10276-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10276-w