Abstract
Plasma synthesis of ammonia is a potential and sustainable way of nitrogen fixation. In this work, a series of M/Al2O3 (M =Co, Ni, Co–Ni) catalysts have been investigated to enhance the synthesis of NH3 from N2 and H2 in a dielectric barrier discharge (DBD) plasma reactor. Under the conditions of a volume ratio of N2:H2 of 1:1, a total gas flow rate of 200 mL min−1, and a discharge temperature of 200 °C, the order of the ammonia synthesis rate filled with different catalysts was Co–Ni/Al2O3 > Co/Al2O3 > Ni/Al2O3 > Al2O3 > only plasma. And the highest ammonia synthesis rate could be obtained was 1500 μmol g−1 h−1 by using Co–Ni/Al2O3 as the catalyst. Further research found that compared with single metal catalysis, on the one hand, the presence of Co–Ni bimetal reduced the total amount and strength of acidic sites on the catalyst surface, which is beneficial for the desorption of synthesized ammonia gas. On the other hand, the presence of Co–Ni bimetal enhanced the plasma discharge observed by the ICCD camera. At the same time, the electrical characterization was found changed with different catalysts added in the plasma synthesis process. Adding a Co–Ni bimetal loaded catalyst in the reaction system increased the average electric field (E) and improved the power efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ammonia (NH3), as the world's second most produced chemical, is an important raw material for the production of many organic and inorganic materials (including fertilizers, plastics, resins, synthetic fibers, etc.) [1, 2]. 85% of them are used to make fertilizer. In addition, ammonia has the characteristics of high hydrogen content, high energy density and easy storage. It has great potential in energy storage [3,4,5] and used as hydrogen fuel [6,7,8], and will become an extremely important sustainable energy source in the future.
At present, the industrial synthesis of ammonia adopts the Haber–Bosch method. This traditional method of ammonia synthesis needs to be combined with highly active catalysts to convert nitrogen and hydrogen into ammonia under harsh conditions of 450–600 ℃ and 150–300 bar [9], which emits more than 300 million tons of carbon dioxide per year and high energy consumption. Its main raw material, fossil fuel, is a non-renewable resource, accounting for 1–2% of the world’s total energy supply, ranking first in the chemical industry, which has a huge impact on the environment and economic development, and restricts the development of conventional ammonia synthesis process [10,11,12,13]. Therefore, it is expected to develop a green, effective, and economically sustainable method for small-scale distributed ammonia production to replace the Haber–Bosch process [14, 15]. In recent years, many researchers have devoted themselves to the direct synthesis of NH3 from N2 and H2 through non-thermal plasma (NTP) technology under environmental conditions [16, 17]. NTP discharge generates high-energy electrons and active species (such as free radicals, excited atoms, molecules and ions), can be used to intensify the general chemical reaction which is not easy to happen at room temperature and atmospheric pressure. The application of plasma technology to synthesize ammonia has great potential for development.
Dielectric barrier discharge (DBD) technology is the most widely used technology in the plasma synthesis of NH3 [18,19,20]. It has been widely applied to the generation of ozone [21], removal of gas pollutants [22,23,24], CH4 activation [25, 26], CO2 hydrogenation [27, 28] and the conversion of water gas and other reactions [29,30,31,32]. In recent years, there have been more and more researches on DBD plasma synthesis of ammonia. For example, Hong et al. [13] synthesized ammonia in a DBD reactor filled with MgO, and the H2 conversion rate reached 4.2%. Patil et al. [33] compared the effect of a series of carriers (including γ-Al2O3, α-Al2O3, MgO, CaO, etc.) on DBD ammonia synthesis. Yin et al. [34] studied the influence of different electrode materials Zn, Al, Cu, Fe, etc. on ammonia synthesis, and found that the order of the influence of electrode materials on ammonia synthesis was Pt > SS > Ag > Fe > Cu > Al > Zn. The output range of ammonia was 0.31–1.46 mmol h−1. Mizushima et al. [35] studied the effect of catalysts loaded with transition metals (Ru, Ni, Pt and Fe) on the DBD plasma synthesis of ammonia. Further researches on plasma ammonia synthesis catalysts revealed that Ru-based, Co-based or Ni-based catalysts have attracted more and more attention. For example, Li et al. [36] developed an efficient route for the preparation of activated carbon supported Ru catalysts with high performance for ammonia synthesis. Kim et al. [37] investigated atmospheric-pressure nonthermal plasma synthesis of ammonia over Ru catalysts. They found that the addition of O2 during the reaction helped the in-situ regeneration of the deactivated catalyst and was beneficial to the synthesis of NH3. Wang et al. [31] investigated the importance of the Ni/Al2O3 catalysts surface on the reaction mechanism. They found that metal sites and weak acidic sites could enhance the production of NH3 via formation of NH2 intermediates on the surface. Shah et al. [16] found the Ni-MOF-74 catalysts has a rich pore structure, which improved mass transfer of active particle and product molecules during reaction. And the presence of unsaturated Ni metal sites caused the increase of ammonia production. Hu et al. [38] compared the M/AC (M=Ru, Co, Ni, and Fe) catalysts. The doping of metal on active carbon (AC) improved the reaction performance by up to 37.3%. Compared with Ru-based catalyst, Co- or Ni-based catalyst is relatively common and cheap. Their synergistic effect with plasma is worthy of systematic study.
In this study, ammonia synthesis was carried out in DBD reactors packed with different catalysts, and the effects of N2/H2 volume ratio, total gas flow rate, discharge power and discharge temperature on NH3 yield have been studied. And online plasma diagnosis combined with N2 physical adsorption measurement, X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), NH3 temperature programmed desorption (NH3-TPD) and other technologies were used for the characterization and analysis of plasma synergistic catalysis.
Experimental
Experimental Setup
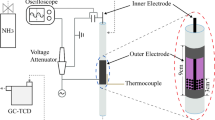
The schematic of the experimental set-up is shown in Fig. 1. A packed-bed DBD reactor was designed for the experiments. The stainless steel rod was used as the high-voltage electrode, connected to the power supply (CTP-2000 K, 40 kHz, Nanjing Suman Electronics Co Ltd, Nanjing, China), and the stainless steel mesh was used as the ground electrode which was wound on the outer wall of the quartz. The length of the discharge quartz tube was 80 mm, and the discharge gap was 2 mm. 2 g of catalyst was filled between the quartz tube and the stainless steel rod, and the DBD reactor was heated by a tube furnace. A mixture of N2 and H2 was introduced into the packed-bed DBD plasma reactor, with their volume ratio regulated through the flow meters. By applying a high AC voltage, intense discharge plasma could be generated around the contact points of the catalyst particles. Thus, the NH3 synthesis reactions would take place under atmospheric pressure.
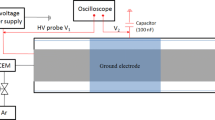
The charge–voltage (Q–U) Lissajous figure method was used to calculate the discharge power of the packed-bed DBD reactor [36]. The voltage (U) was measured by a high voltage probe (Tektronix, P5100A), the current was measured by a current monitor (Tektronix, ZP1025S), and the electrical signal was collected by a digital oscilloscope (Tektronix, DPO 4104). The capacitance C of the capacitor was 0.47 μF, which can be used to determine the amount of charge accumulated in the DBD. The time average optical imaging was measured by an ICCD camera (Princeton Instruments MAX2, 1024 × 1024 pixel), and the exposure time was set to 100 ms to observe the plasma discharge image.
Catalyst Preparation and Characterization
Catalyst prepared by impregnation method is often used in the reaction of plasma synergistic catalysis [31, 36, 38]. 5 wt.% M/Al2O3 (M=Ni, Co, Co–Ni) catalysts were prepared by incipient wetness impregnation using nitrate salts (J&K Scientific, 99%) as metal precursor. Al2O3 catalyst support (6 g) was added to the solution of nitrate salts. The slurry was continuously stirred at 50 °C for 2 h and aged overnight at room temperature. The samples were then dried at 110 °C for 56 h and calcined at 500 °C for 5 h. And then these sample were reduced at 500 °C under Ar/H2 mixed atmosphere (100 mL min−1, Ar/H2 = 7:3) for 5 h. These reduced catalysts were called "fresh catalysts".
Thermogravimetry analysis (TG) was used to determine the optimal calcination temperature of precursors (Ni(NO3)2·6H2O, Co(NO3)2·6H2O). The thermogravimetric analysis was performed using a thermogravimetric analyzer (Etelux, Lab2000). Nitrogen with a flow rate of 30 mL min−1 was used as the carrier gas. The measurements were carried out from room temperature to 750 °C at a heating rate of 3 °C min−1. The TG curve was given in Fig. S1. The X-ray diffraction (XRD) was used to analyze the crystal structures of the catalysts. The XRD analysis of the catalysts was performed by using a Cu-Kα radiation. The scanning was conducted in the 2θ range of 30°–90° at 6° min−1 with a step size of 0.02°. A transmission electron microscope (TEM, Japan, JEM1200EX) was used to perform high-resolution transmission electron microscopy (HRTEM) analysis on the fresh catalyst at an accelerating voltage of 300 kV. Samples were ultrasonic treatment before measurement.
N2 adsorption–desorption analysis can be used to characterize the physicochemical properties and structural characteristics of the catalysts. Micromeritics ASAP 2460 physical adsorption instrument was used to test the N2 adsorption–desorption characterization. The specific surface area of the catalyst was calculated according to the Brunauere–Emmette–Teller (BET) equation. The pore size distribution of the catalyst was obtained by the Barrette–Joynere–Halenda (BJH) method. All the catalysts were pretreated at 300 ℃ under vacuum to remove any impurities from the surface.
The surface acidity of the catalysts was evaluated by temperature programmed desorption of ammonia (NH3-TPD) using a DAS-7000 instrument equipped with a mass analyzer. The fresh catalysts (100 mg) was pretreated and flushed in pure He at 500 °C for 1 h and then cooled down to 50 °C. The adsorption of NH3 was conducted at 50 °C in a NH3/He (5 vol.%) gas stream (30 ml min−1) until reaching the adsorption equilibrium. The physical adsorbed NH3 was removed by pure He (50 ml min−1) at 100 °C. The non-isothermal desorption of NH3 was performed in pure He at a heating rate of 10 °C min−1 in the temperature range of 50–700 °C.
Calculation of Parameters
The chemical titration method was used to quantify the concentration of synthetic ammonia which was introduced into a 10 mL, 0.005 mol L−1 dilute H2SO4 solution with methyl red indicator to monitor the reaction endpoint. When the color of the solution changed from red to yellow, the H2SO4 in the solution was completely neutralized by synthetic NH3. Thus, the concentration of synthetic ammonia can be calculated based on the amount of the H2SO4. The ammonia mass yield (g s−1) could be calculated from Eq. 1:
According to the chemical reaction formula: 2NH3·H2O + H2SO4 = (NH4)2SO4 + 2H2O, the time (t) of indicator changed color meant the 10 mL 0.005 mol L−1 H2SO4 had been consumed by 100 μmol NH3·H2O. And the ammonia synthesis rate (μmol g−1 h−1) could be calculated from Eq. 2:
The energy efficiency (g kWh−1) was determined by Eq. 3:
Results and Discussion
Catalyst Characterization
XRD patterns of the fresh catalysts and spent catalysts are shown in Fig. S2. Three major peaks centered at 2θ = 37.6º, 45.9º and 67.0º were identified in the diffraction pattern of pure Al2O3, corresponding to the cubic structure of crystalline Al2O3 (JCPDS 06-0696). These peaks were also found in the diffraction pattern of the M/Al2O3 catalysts. In addition, the peaks of metallic Ni (JCPDS 45-1027) and Co (JCPDS 89-7094) also appeared in the diffraction pattern of the M/Al2O3 catalysts. This indicates that the active component has been successfully loaded on Al2O3. It is mainly in a metallic state after thermal reduction. Comparing the XRD curves of the catalyst before and after the reaction, it can be seen that the composition of the catalyst has not changed after plasma treatment.
The physical properties of the fresh catalysts measured by N2 physical adsorption are listed in Table 1. The BET specific surface area of Al2O3 was 146 m2 g−1. The specific surface area of the fresh Al2O3-supported metal catalysts increased slightly to between 155 and 164 m2 g−1. Fresh Al2O3 also had the most substantial total pore volume of 0.26 cm3 g−1, compared to 0.24 cm3 g−1 for the M/Al2O3 catalysts. In addition, the average pore size of Al2O3 was 7.2 nm, and the average pore diameters of M/A12O3 were reduced to 5.8–5.9 nm. The increase in the specific surface area of the catalyst may be due to the better dispersion of the supported metal on the support, while the slight decrease of the pore volume and pore diameter of the catalyst may be due to the small amount of supported metal filling the pores of the support. Similar results have been reported in previous studies [39, 40].
The nitrogen adsorption–desorption isotherm of the fresh catalysts are shown in Fig. S3. All samples exhibited type-IV isotherms with H1 hysteresis loops and steep increases in the relative pressure of 0.5 < P/Po < 0.9, which indicated that all samples had a mesoporous structure. In addition, when the relative pressure (P/Po) was 0, as shown in the Fig. S3a–d, it had a higher N2 adsorption capacity (about 20 cm3 g−1), which indicated that the catalyst also had a microporous structure [31, 41, 42].
Figure 2 shows the surface morphology and particle size distribution of the fresh catalysts observed by using HRTEM. The particle sizes of most metals on the surface of the catalyst were in the range of 2–16 nm, while the surfaces of Ni/Al2O3 and Co/Al2O3 catalysts had larger particles with a size of more than 26 nm. Compared with Ni/Al2O3 and Co/Al2O3, the metal particles loaded on the surface of double metals catalyst Co–Ni/Al2O3 were more evenly distributed on the surface of the carrier.
The surface acidity of the fresh catalysts was determined by NH3-TPD. Figure 3 shows the chemical desorption peaks of NH3 at weak acidic sites (180–240 °C), medium acidic sites (260–320 °C) and strong acidic sites (450–600 °C) [43]. For each catalyst, the amounts of ammonia adsorbed on these locations are listed in Table 2. Obviously, loading the A12O3 carrier with metal will reduce the total number of acidic sites (Atotal), and the order of acidic sites was: Al2O3 (0.85 mmol g−1) > Co/Al2O3 (0.79 mmol g−1) > Co–Ni/Al2O3 (0.77 mmol g−1) > Ni/Al2O3 (0.71 mmol g−1). Moreover, the concentrations of medium acidic sites and strong acidic sites were significantly reduced after the metal was loaded, which reduces the adsorption energy of the catalyst surface and facilitates the desorption of the product NH3 from the catalyst surface. This is consistent with the ammonia synthesis rate [31].
Parameters Optimization for DBD Plasma Synthesis of Ammonia
The Effect of N2/H2 Volume Ratio on the Ammonia Synthesis Rate
As shown in Fig. 4, the volume ratio of N2/H2 under different catalytic conditions had a significant effect on the ammonia synthesis rate. The best VN2/H2 was 1:1 in all of the different reaction systems. Compared with the plasma synthesis of ammonia without catalyst, the ammonia synthesis rate was obviously increased after catalyst was filled. The results showed that a sufficient N2 atmosphere was beneficial to increase the ammonia synthesis rate, because the N–N bond energy in the N2 molecule is much higher than the H–H bond energy in the H2 molecule, so more energy is required to break the N–N bond. It is worth noting that different researchers have observed different optimal N2/H2 ratios. Hong et al. [20] proposed that this is because the optimal ratio is sensitive to the reduced electric field and the electron density in the reactor.
The Effect of Total Gas Flow Rate on the Ammonia Synthesis Rate
The ammonia production rate is also related to the reaction gas flow rate. As shown in Fig. 5, the ammonia synthesis rate increased with the increase of total gas flow rate. However, when the total gas flow rate exceeded 120 mL min−1, the growth rate of the ammonia synthesis rate slowed down. The production rate of ammonia can be determined by the balance difference between ammonia synthesis and loss reactions in the DBD plasma catalytic reaction system. On the one hand, when the pressure is constant, the increase in gas flow rate will provide more raw reactant gas at the same time, increasing the collision probability between reactive particles in the system, which is beneficial to the production of ammonia. However, on the other hand, the increase of the gas flow rate will reduce the residence time of the reactive particles in the reaction system, and some of the active particles will be blown out of the reaction system before they can react. The loss of active particles is not conducive to the production of ammonia. Bai et al. had similar reports [44].
The Effect of Discharge Power on the Ammonia Synthesis Rate
The discharge power is an important parameter for plasma intensified chemical reaction process. The effect of the discharge power on the ammonia synthesis rate under different catalytic conditions is shown in Fig. 6. Obviously, whether or not the catalyst was filled, the ammonia synthesis rate of plasma increased with the increase of power. As we all know, increasing the discharge power will strengthen the excitation and ionization of the reactants, which is beneficial to the production of more high-energy electrons and active species [29]. It can increase the number density of reactive species such as N, H and NHx radicals, thereby improving the reaction performance of plasma catalytic synthesis of NH3 [44, 45].
The Effect of Discharge Temperature on the Ammonia Synthesis Rate
The ammonia synthesis reaction is an exothermic process, but due to the high stability of N2 molecule, the industrial ammonia synthesis is mainly carried out at high temperatures. Compared with the conventional Haber–Bosch process, the application of plasma technology can significantly reduce the ammonia synthesis temperature and make the reaction under milder conditions. The effect of discharge temperature on the ammonia synthesis rate under different catalytic conditions is shown in Fig. 7. The discharge temperature had little effect on the ammonia synthesis rate without catalyst. The synthesis rate of NH3 increased slightly with the increase of temperature. However, after catalysts were filled, the discharge temperature had a significant effect on the NH3 synthesis rate which increased significantly with the increase of the discharge temperature. N2 and H2 molecules in the gas phase collide with electrons to generate nitrogen and hydrogen free radicals, metastable N2, N2+ and H2+ particles, which collide with each other to form the intermediate NHX (X = 1, 2) for ammonia synthesis and the final product NH3 [46]. However, if the synthesized ammonia is not desorbed from the surface of the catalyst in time, it will be decomposed, thereby reducing the rate of ammonia synthesis. It is difficult to get desorbed at lower temperature, especially for that adsorbed on the pore channel of the catalyst. Increasing the temperature is beneficial to the desorption of the generated ammonia gas from the surface of the catalyst. At the same time, when the catalysts with less acidic sites and strong desorption capacity for NH3 are applied, this phenomenon are more obvious. There have similar conclusions with previous studies [24].
The stability of catalyst was assessed by continuous reaction for 6 h with different catalysts under same conditions (N2/H2 volume ratio was 1:1, total gas flow rate was 200 mL min−1, input power was 30.81 W, temperature was 200 °C). The ammonia synthesis rates were recorded per 1 h. The catalysts stability curves are shown in Fig. 8. It was found that all catalysts showed stable performance in whole the 6 h reaction process without deactivation.
The balance between the energy efficiency of the plasma catalysis reaction and the ammonia synthesis rate is of guiding significance for the further development of this technology. Table 3 compares the ammonia synthesis rate and energy efficiency of plasma synthesis of ammonia under different catalytic conditions based on the references and present work. It can be seen that different reaction systems, catalytic systems and process parameters had significant effects on the ammonia synthesis rate. Compared with other plasma-catalyst synergistic synthesis systems, alumina-supported double metals catalyst Co–Ni/A12O3 in present work had the best catalytic performance, 3000 μmol h−1 of the ammonia yield with 0.83 g kWh−1 of energy efficiency. Among the previous work, Peng et al. [24] used Ru-MCM-41 as catalyst for plasma catalytic synthesis of ammonia. Its energy efficiency was 1.7 g kWh−1. The total gas flow rate during their reaction was as high as 4000 mL min−1. However, it was only 200 mL min−1 in present work. Shah et al. [16] reported the maximum ammonia yield was 1270 μmol h−1 with a very low energy efficiency of only 0.23 g kWh−1. Therefore, higher ammonia yield and energy efficiency can be achieved under milder conditions by packing Co–Ni/A12O3 catalyst into DBD plasma reactor in present experimental system.
As we all know, the traditional ammonia synthesis process, Haber–Bosch process, has a conversion rate of 10–15% [47] and an energy efficiency of 100 g kWh−1 [48]. Some researchers have conducted a full life cycle assessment for comprehensively compared the conventional NH3 synthesis process and DBD plasma NH3 conversion process. It can be seen that current plasma catalytic ammonia synthesis in the conversion rate, energy efficiency and full life cycle assessment also shows the problem of low ammonia synthesis rate and high energy consumption [49, 50]. In order to improve the ammonia production rate and energy efficiency, there are currently many key technical problems need to be solved, such as screening and preparing a stable, efficient, long-life, and low-cost catalyst, designing and optimizing plasma source, using renewable wind and solar energy and in-depth exploring the mechanism of plasma technology for ammonia synthesis. And previous studies have pointed out that the energy efficiency of ammonia synthesis can be significantly improved by expanding the plasma catalytic system [46, 51]. Therefore, in the future, it is recommended to continue to strengthen the systematic research of important parameters, such as plasma source, reactor, catalyst, and comprehensive consideration of process parameters and energy consumption optimization in the industrialization process.
Characterization of Catalysts Packed Plasma Discharge
Figure 9 shows the ICCD images of plasma discharge under different reaction conditions. Figure 9a shows that the plasma discharge was weak in reaction area without any catalyst. When Al2O3 was packed into the reaction area, the plasma discharge was enhanced obviously, as shown in Fig. 9b. The Al2O3 supported metal catalysts packed into the discharge gap made this tendency more obviously. The plasma discharge were further enhanced as the Al2O3 supported metal catalysts (M/Al2O3, M=Co, Ni, Co–Ni) were packed into the discharge gap, as shown in the Fig. 9c–e. The filamentary discharge in the reactor will be transformed into a combination of filamentary discharge and surface discharge in the presence of catalyst [39, 55]. The active particles formed by plasma discharge can interact with the surface of the catalyst. An enhanced electric field was generated near the contact point of the catalyst, which helps the formation of ammonia intermediates, and the surface reaction of the catalyst was increased. which had been well demonstrated in both experimental and modeling studies of packed bed DBD reactors [56, 57]. This conclusion was coincident with the order of the NH3 synthesis rate with different catalysts: Co–Ni/Al2O3 > Co/Al2O3 > Ni/Al2O3 > Al2O3 > only plasma.
In order to further understand the discharge characteristics under different catalytic conditions, plasma electrical diagnosis was carried out. Figure S4 shows the U–Q Lissajous diagram under different catalytic conditions measured by an oscilloscope. And the detailed discharge characteristics has been calculated and listed in Table 4 (The calculation method can be found in section II of the Supporting Information). The average electric field (E) increased after filling the catalysts into the discharge area. This may be due to the increase of the dielectric constant of the filling material, the polarization of the contact point, and the deposition of charge [58, 59]. The improvement order of power efficiency after packing catalyst was: Co–Ni/Al2O3 > Co/Al2O3 > Ni/Al2O3 > Al2O3 > plasma only, which was coincident with the change of the electric field. At the same time, it was also agreed with the order of the NH3 synthesis rate with different catalysts.
Conclusions
In this paper, a coaxial packed-bed DBD reactor has been developed for plasma catalytic synthesis of NH3. The Al2O3 supported transition metals M/Al2O3 (M = Co, Ni, Co–Ni) were used as catalyst in the plasma synthesis reaction. The influences of reaction conditions on the NH3 synthesis rate were explored. Compared with only plasma discharge to synthesize ammonia, plasma assisted catalysis significantly improved the ammonia synthesis rate and energy efficiency. The order of ammonia synthesis rate and energy efficiency was: Co–Ni/Al2O3 > Co/Al2O3 > Ni/Al2O3 > Al2O3 > plasma only. The catalysts can maintain stability for at least 6 h. The investigation of process parameters found that the best N2/H2 volume ratio under different catalytic conditions was 1:1. In addition, increasing the total gas flow rate, discharge power or reaction temperature was beneficial to improve the NH3 synthesis rate. The highest NH3 synthesis rate was 1500 μmol g−1 h−1 by using Al2O3 supported double metal catalyst Co–Ni/Al2O3. Co–Ni/Al2O3 bimetal catalyst not only has the advantage of lower price, but also plays a role in reducing the acidity of the catalyst surface and enhancing the plasma discharge, which is beneficial to the synthesis of ammonia.
References
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Science 320:889–892
Licht S, Cui B, Wang B, Li FF, Lau J, Liu S (2014) Science 345:637–640
Valera-Medina A, Xiao H, Owen-Jones M, David WIF, Bowen PJ (2018) Prog Energy Combust Sci 69:63–102
Dunn R, Lovegrove K, Burgess G (2012) Proc IEEE 100:391–400
Chen C, Zhao LL, Lavine AS (2018) Sol Energy 176:638–647
Avery WH (1988) Int J Hydrogen Energy 13:761–773
Morgan E, Manwell J, McGowan J (2014) Renewable Energy 72:51–61
Yapicioglu A, Dincer I (2019) Renewable Sustainable Energy Rev 103:96–108
Hargreaves JSJ (2014) Appl Petrochem Res 4:3–10
Aika K-i (1972) Hori H, Ozaki A. J Catal 27:424–431
Tanabe Y, Nishibayashi Y (2013) Coord Chem Rev 257:2551–2564
Marnellos G, Stoukides M (1998) Science 282:98–100
Hong J, Pancheshnyi S, Tam E, Lowke JJ, Prawer S, Murphy AB (2018) J Phys D: Appl Phys 51: 109501
Kitano M, Inoue Y, Yamazaki Y, Hayashi F, Kanbara S, Matsuishi S, Yokoyama T, Kim SW, Hara M, Hosono H (2012) Nat Chem 4:934–940
Mehta P, Barboun P, Herrera FA, Kim J, Rumbach P, Go DB, Hicks JC, Schneider WF (2018) Nat Catal 1:269–275
Shah J, Wu T, Lucero J, Carreon MA, Carreon ML (2018) ACS Sustainable Chem Eng 7:377–383
Xie Q, Zhuge S, Song X, Lu M, Ruan R, Nie Y, Ji J (2018) Int J Hydrogen Energy 43:14885–14891
Tu X, Whitehead JC (2012) Appl Catal B 125:439–448
Xie D, Sun Y, Zhu T, Fan X, Hong X, Yang W (2016) RSC Adv 6:105338–105346
Hong J, Aramesh M, Shimoni O, Seo DH, Yick S, Greig A, Charles C, Prawer S, Murphy AB (2016) Plasma Chem Plasma Process 36:917–940
Yuan D, Ding C, He Y, Wang Z, Kumar S, Zhu Y, Cen K (2017) Plasma Chem Plasma Process 37:1165–1173
Gómez-Ramírez A, Cotrino J, Lambert RM, González-Elipe AR (2015) Plasma Sources Sci Technol 24: 065011
Ma H, Chen P, Zhang M, Lin X, Ruan R (2002) Plasma Chem Plasma Process 22(2):239–254
Peng P, Cheng Y, Hatzenbeller R, Addy M, Zhou N, Schiappacasse C, Chen D, Zhang Y, Anderson E, Liu Y, Chen P, Ruan R (2017) Int J Hydrogen Energy 42:19056–19066
Sheng Z, Watanabe Y, Kim H-H, Yao S, Nozaki T (2020) Chem Eng J 399: 125751
Yi Y, Zhang R, Wang L, Yan J, Zhang J, Guo H (2017) ACS Omega 2:9199–9210
Wang J, AlQahtani MS, Wang X, Knecht SD, Bilén SG, Song C, Chu W (2021) Green Chem 23:1642–1647
Zeng YX, Tu X (2016) IEEE Trans Plasma Sci 44:405–411
Neyts EC, Ostrikov K, Sunkara MK, Bogaerts A (2015) Chem Rev 115:13408–13446
Wang L, Yi YH, Wu CF, Guo HC, Tu X (2017) Angew Chem Int Ed 56:13679–13683
Wang Y, Craven M, Yu X, Ding J, Bryant P, Huang J, Tu X (2019) ACS Catal 9:10780–10793
Stere CE, Anderson JA, Chansai S, Delgado JJ, Goguet A, Graham WG, Hardacre C, Taylor SFR, Tu X, Wang Z, Yang H (2017) Angew Chem Int Ed Engl 56:5579–5583
Patil BS, van Kaathoven ASR, Peeters FJJ, Cherkasov N, Lang J, Wang Q, Hessel V (2020) J Phys D: Appl Phys 53:144003
Yin KS, Venugopalan M (1983) Plasma Chem Plasma Process 3:343–350
Mizushima T, Matsumoto K, Ohkita H, Kakuta N (2007) Plasma Chem Plasma Process 27:1–11
Li Y, Pan C, Han W, Chai H, Liu H (2011) Catal Today 174(1):97–105
Kim HH, Teramoto Y, Ogata A, Takagi H, Nanba T (2017) Plasma Processes Polym 14:1600157
Hu X, Zhu X, Wu X, Cai Y, Tu X (2020) Plasma Process Polym 17:2000072
Wu J, Zhao Z, Huang T, Sheng P, Zhang H, Tian H, Zhao X, Zhao L, He P, Ren J, Gao K (2017) Catal commun 93:62
Serrano‐Ruiz JC, Ramos‐Ferna´ndez EV, Silvestre‐Albero J, Sepu´lveda‐Escribano A, Rodrı´guez‐Reinoso F (2008) Mater Res Bull 43:1850
Peng P, Li Y, Cheng Y, Deng S, Chen P, Ruan R (2016) Plasma Chem Plasma Process 36(5):120110.
Kowalczyk Z, Krukowski M, Rarog-Pilecka W, Szmigiel D, Zielinski J (2003) Appl Catal A Gen 248(12):67–73
Wang C, Ma X, Ge Q, Xu H (2015) Catal Sci Technol 5:1847–1853
Bai MD, Zhang ZT, Bai XY, Bai MD, Ning W (2003) IEEE Trans Plasma Sci 31:1285–1291
Uyama H, Matsumoto O (1989) Plasma Chem Plasma Process 9:13
Zhu X, Hu X, Wu X, Cai H, Zhang H, Tu X (2020) J Phys D: Appl Phys 53: 164002
J Humphreys, Lan R, Tao S (2020) Adv Energy Sustainability Res, 2000043
Liu HZ (2011) Chem Ind Eng Pro (China) 30:1147–1157
Anastasopoulou A, Keijzer R, Patil B, Lang J, van Rooij G, Hessel V (2020) J Ind Ecol 24:1171–1185
Riotto T, Cao G, Luyben WL, Baltrusaitis J (2021) ACS Sustain Chem Eng 9:13233–13244
Bai MD, Bai XY, Zhang ZT, Bai MD (2000) Plasma Chem Plasma Process 20:511–520
Akay G, Zhang K (2017) Ind Eng Chem Res 56:457–468
Shah J, Wang WZ, Bogaerts A, Carreon ML (2018) ACS Appl Energy Mater 1:4824–4839
Nakajima J, Sekiguchi H (2008) Thin Solid Films 516:4446–4451
Carrasco E, Jimenez-Redondo M, Tanarro I, Herrero VJ (2011) Phys Chem Chem Phys 13:19561
Tu X, Gallon HJ, Whitehead JC (2011) IEEE Trans Plasma Sci 39:2172–2173
Gao M, Zhang Y, Wang H, Guo B, Zhang Q, Bogaerts A (2018) Catalysts 8:248
Mei DH, Zhu XB, He YL, Yan JD, Tu X (2015) Plasma Sources Sci Technol 24: 015011
Van Laer K, Bogaerts A (2016) Plasma Sources Sci Technol 25: 015002
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 11505013, 11875090), Beijing Municipal Natural Science Foundation (No. 1192008) and Science and Technology Project of Beijing Municipal Education Commission (No. KM202010015003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, CW., Xu, XF. et al. Synergistic Effect of Co–Ni Bimetal on Plasma Catalytic Ammonia Synthesis. Plasma Chem Plasma Process 42, 267–282 (2022). https://doi.org/10.1007/s11090-021-10223-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10223-1