Abstract
New efficient depollution techniques for water decontamination, purification and disinfection are being sought to replace those classic methods (chemical, filtration, ozonisation, photochemical reactions) that have deficiency for some substances. The use of plasma technologies, discharges in, or in contact with, wastewater are promising approaches for the decomposition of pollutants by highly oxidative radicals, charged particles, UV radiation, etc. produced by plasma. In the present study we report on the potential of radiofrequency single and multiple filamentary Dielectric Barrier Discharge (DBD) jets for the decolorization of methylene blue (MB) dye in water solutions. Optical emission spectroscopy (OES) investigations were performed for the characterization of plasma evolving in air, and in liquid. The decolorization process was monitored by absorption spectroscopy. We determined the decolorization time, according to a variety of external parameters. The key parameters for obtaining the maximum decolorization rate were identified as being the discharge tube diameter, tube nature (glass/ceramic), the injected power in the discharge, the type of reactive gas and the number of filamentary plasma jets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, one of the main environmental problems is water pollution [1]. This widespread problem is jeopardizing our health, mainly because the wastewater volume has increased due to the global industrial developments. This wastewater contains harmful organic and inorganic impurities, and these may be responsible for various illnesses. In order to solve this issue, it is necessary to deepen our understanding of the physical–chemical aspects of water-decontamination, in particular those related to treatments for wastewater reprocessing.
There are well-known classical methods for water depollution such as chemical methods [2] and filtration [1]. Chemical decontamination involves major disadvantages due to the use of strong chemicals. These chemicals may remain in the water after treatments and these impurities affect the water quality. Consequently, the use of these substances has a major impact on the environment and the human health. Filtration may have the disadvantage of having low efficiency in the removal of specific pollutants and microorganisms. One of the novel approaches is represented by plasma discharges in contact or immersed in liquids. Electrical discharges in water or in contact with water are very promising because they generate in liquid highly oxidative species, able to decompose organic substances present in solutions. Active species such as ozone (O3), hydroxyl radicals (OH), atomic oxygen (O), excited nitrogen (N2*), hydrogen peroxide (H2O2), peroxynitrite (NO-3 ) etc. are produced during the plasma treatment [3]. Moreover, at the plasma/water interface, UV radiation, strong electric fields, particle collisions, shock waves may appear which contribute to the efficient decontamination [4].
Therefore, atmospheric pressure plasma treatments devoted to water decontamination [5,6,7] are considered an innovative method, characterized by a short decontamination time, with low environment effects, and low costs based on increased efficiency [8]. A list mentioning various reactors and their relative energy efficiencies of decomposing toxic organic compounds in water (including dyes) is provided in the study [9].
In the literature, there are many papers dealing with plasma treatment of dyes. Depending on the treatment condition and the used investigation methods, the used terms are decomposition [13, 14], degradation [14, 16, 21], removal [21, 26] and decolorization [10, 21].
There are several factors that can influence the dye decolorization rate. Complete decolorization may be obtained from a few seconds to tens/hundreds of minutes (e.g. 2 min for the removal of methylene blue hydrate (MBH) in a concentration of 5 mg/L and a volume of 50 mL, by using pin-cylinder configuration with tungsten electrodes at 800 V [10], or a long time of 120 min using a low power cold atmospheric pressure radiofrequency (13.56 MHz, 15 W, 25 mg/L, 50 mL) in argon and oxygen gas mixture [7]).
The most significant factors in obtaining quick decolorization of dyes are first of all the choice of the discharge mode, reactor type, configuration/geometry [9]. The optimization of working parameters and treatments conditions is essential in increasing the decolorization rate. There are reports that present decolorization rate dependencies upon the initial concentration, injected power, gas flow rate, working gas composition (Ar, He, O2, N2 and Air) [11,12,13], voltage amplitude, duration of the voltage pulses (negative/positive polarity [14] distance from electrodes, gap distance [15], permittivity of the reactor tube, channel depth and barrier thickness [12] and types of the electrodes materials (W, Al, Fe, Pt and NiCr) [10], etc. The changes in the working parameters induce many changes in the characteristics of the solution. In many reports we can observe the influence of the electrical conductivity (influenced by ions) [16], temperature of the solution [17], the pH [18,19,20], and the concentration of species H2O2, O3 NO2, NO3 etc. [19,20,21]. Other important factors in the enhancement of degradation are the introduction of catalytic particles and ions (FeCl2, Fe3O4, TiO2, WO3, Ru, Pt, NiO, Cu etc.) [3, 22, 23] that lead to chemical and photocatalytic processes in plasma and more efficient dyes decolorization. With respect to efficiency, there are several papers that reported MB plasma treatments with high energetic efficiency as: 4.6 g/kWh (Tatarova et al. reported in their review a decolorization time of 15–20 min for 90% conversion by pulsed corona discharge [24]); 42 g/kWh (reported for 50% decolorization of 30 mg/L MB solution, which dropped to 14 g/kWh at 97% decolorization by using wire-plate air bubbling plasma in dry air (2 L/min) [25]); 57 g/kWh at 95% conversion of MB (300 mL, concentration of 50 mg/L) obtained in Magureanu’s experiments (where they used DBD in pulsed regime, in negative polarity, for 30 min of treatment after introducing of 300 sccm of O2) [13]. Also, Magureanu et al. presented the optimization of the operating parameters in order to increase the efficiency. Recently, we can mention the study of Hamdan [15, 26], in which it is presented among others a list with various plasma reactors used for MB removal and their semi conversion yields.
In the literature, there is a series of reports presenting the mechanisms of MB decomposition by plasma [11, 18, 27], or by ozone [28]. A lot of other reports present the possible reactions induced in water by plasma [29, 30] that lead to various radicals/nonradicals [31] and reactive species (reactive oxygen species -ROS [19] such as OH, O*, H2O2, O3 etc. and reactive nitrogen species-RNS as NO, NO2, NO3, HONO, HNO2, NH, etc.) that are involved in the degradation process of various contaminants [30, 32]. An overview of the detection methods used for the identification and quantitative measurement of various active species generated by plasma in liquid and the degradation [33] product could be found in [11, 27, 34], but it is difficult to find a model that can be applied to particular discharge.
The present work is based on recent trends that use cold atmospheric pressure plasmas totally immersed in liquids. In this contribution we report the development of dielectric barrier discharge (DBD) filamentary plasma jet sources for water cleaning, intentionally contaminated with Methylene Blue dye (MB, C16H18ClN3S), in concentration of 25 mg/L. These DBD plasma sources that operate at atmospheric pressure with annular electrodes were studied previously [5] and were tested for surfaces treatment in open air [35]. A similar configuration was used for the functionalization of nanocellulose and graphene in water suspension [36]. Herewith, we compare various configurations of the filamentary plasma sources; all of them work with argon as the main gas, and are sustained by radiofrequency power (RF/13.56 MHz). These sources are very versatile, because they can operate in open air within a large domain of parameters (with various admixed gases types, flow rates, and power), but also, they work immersed in liquids. Besides the simplicity in achievement and configuration, these filamentary DBD sources also have the great advantage of not implying additional cooling systems.
In order to identify the optimal dye decolorization conditions several experimental parameters were varied: the gas flow rate (Ar 1000–20,000 sccm), the operating power value (50–200 W), the discharge geometry configuration, the tube diameter (6 and 10 mm) and electrodes position. Moreover, the influence of additional reactive gases (e.g. nitrogen, oxygen and compressed air) was evaluated by adaptation of the discharge geometry in order to work in gas mixtures. Multi-jet configuration arrays with 3 tubes were also tested. For plasma analysis, optical emission spectroscopy (OES) investigations were performed, both for air and in-liquid discharges. The decolorization process was monitored by absorption spectroscopy.
The time for the total decolorization of MB by DBD (50 mL, conc. 25 mg/L) varied from 30 to 13 min after increasing the Ar flow, reaching the shortest decolorization time of 6 min after adding nitrogen (10%) (Ar/N2).
Experimental Techniques
Plasma Sources Description
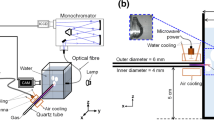
The experiments of MB decolorization were first performed using a single filamentary plasma jet source [5], operating at atmospheric pressure, and immersed in water/dye solutions. The filamentary plasma jet source and two of its configurations are shown in Fig. 1. These sources are made of glass or ceramic tubes fed with Ar through the upper end. The tubes are capacitively coupled with a radiofrequency power supply (RF, 13.56 MHz) and the ignited discharge inside of tubes expands as a plasma jet on the opposite end of the tubes. Most experiments were performed in a single-electrode configuration geometry, using an annular ring as active RF electrode, while the other electrode (grounded electrode) is absent [5].
From our previous experience, with the same plasma source, we observed as major drawback the discharge quenching when adding reactive gases directly in the main Ar flow. Indeed, when an additional reactive gas (O2, N2 or NH3 at rate values above 10 sccm) is simultaneously introduced from the same side with the Ar main flow (e.g. at 3000 sccm), the discharge tends to extinguish during operation in open air, and especially in the case of immersion in liquid (e.g. in nanocellulose suspension [36]). In the present experiments, we succeeded to introduce large amounts of reactive gases by modifying the discharge tube configuration. Thus, we found that the most convenient approach to introduce those gases, is from the lateral side of the discharge tube, in the downstream proximity of the powered RF electrode by interconnecting two tubes. Thus, we developed a new type of source, which is named below in the paper as YDBD, due to its geometry. The YDBD schematic configurations are shown in Fig. 1a, c.
Compared to the previous experiments [5], the discharges were performed with a new RF generator, allowing to explore new areas of operation of the sources and new mass flow controllers, which allowed us to large gas flow rates (up to 20,000 sccm) [5]. The RF- Generator Cito Plus 1310-ACNA-N37A-FP (13.56 MHz, 1000 W (purchased from COMET, Plasma Control Technologies www.comet-pct.com) has been of great help for an easy ignition of these discharges at atmospheric pressure and obtaining a stable plasma filament in open air or submerged in liquid.
We established a standard condition, as a benchmark point for comparison with the previous experiments [4, 5, 7]. This standard condition is: 3000 sccm Ar flow, 100 W RF power, tube with external diameter 6 mm (4 mm inner), one electrode configuration with the electrode placed at 5 cm from the jet exit.
Experiments Description. Plasma treatments of MB solution
After ignition of plasma in open air, the filamentary DBD jet is submerged in water or water/methylene blue solution. The decolorization experiments were performed with the plasma jets immersed in the MB solutions added in quartz vessels. The treatments were performed around the standard conditions, but also varying other parameters as power, additional flows, plasma configurations and tube diameter. The experimental conditions of the most significant treatments of MB decolorization are presented in Tables 1 and 2 in Section: Treatment efficiency.
Additional Plasma Treatment Variants
In addition to the configurations described before, other tested experimental set-up configurations were: two electrodes configuration with grounded electrode at the tube exit and, respectively, RF electrode at the tube exit, 3Y-shaped tubes connected to the same RF electrode, ungrounded or grounded vessel with MB solution. In addition, another experimental setup includes a filamentary plasma jet ignited in ambiental air, but which evolved in a quartz tube closed at bottom and immersed in MB solution. This last configuration was chosen to estimate separately the effect of UV, excluding the chemical effect of plasma reactive species. The experiment proceeded by cumulating several short-time experiments thus avoiding heating the tube and MB solution.
Plasma Investigation Methods
The length of the jet was evaluated visually, and measured (with a ruler) as the distance from the tube exit to the end of the filament. The Optical Emission Spectroscopy (OES) measurements were performed for the simple DBD in Ar and for YDBD in Ar/N2 or Ar/Compressed Air at 3000/300 sccm. The experimental setup for spectra acquisition is shown in Fig. 2. As shown in Fig. 2a, the filamentary jet was placed in the center of a plastic lid and the optical fiber support was fixed in this ring. The spectra were acquired using a Jobin–Yvon Horiba FHR1000 spectrometer (1.0 m focal length, 1200 grooves/mm grating, Andor IStar 720 ICCD camera). The spectra were recorded through air, and through the quartz vessel, filled or not with double distilled water. In order to obtain the rotational temperatures, the spectra were processed using the Boltzmann plot method and LIFBASE simulation software. The acquisition geometry was maintained identical for all the recorded spectra, and the acquisition parameters were kept constant in all experiments. Thus, the integration time was 0.5 s with 10 accumulations; the slit was 15 µm, the accepting angle of the optical fiber was 30 degrees and the measurement spectral range 200–1000 nm.
Investigation of Plasma Treated MB Solutions
The methylene blue solution was prepared with a concentration of 25 mg/L and a volume of 50 mL. The pH and temperature measurements were performed with a HI-2550 Benchtop Meter (able to measure pH, T °C, etc.). The parameter of the measuring device were pH range between − 2.00 and 16.00 with a resolution of 0.01 pH and accuracy of ± 0.01 pH. The temperature could be measured from − 10 to 120.0 °C with a resolution of 0.1 °C and accuracy of ± 0.4 °C.
The absorption setup, shown in Fig. 3, consists of a light source (stabilized tungsten and deuterium lamp), connecting optical fibers, the absorption cell, and the Ocean Optics 4000 spectrometer. Optical Multichannel Analyzer -OMA. The absorption cell contains the cuvette holder and the fiber adapter. The holder is designed to collimate the output from the fiber with a fixed, non-removable, uncoated collimating optic (f = 8 mm, NA = 0.547) and to hold 12.5 mm × 12.5 mm × 45 mm cuvettes. All optical components of the absorption spectra acquisition are purchased from Thorlabs.
After plasma treatment, H2O2 concentration in the double distilled water was evaluated using the titanium sulphate method Ti(SO4)2 [37]. This method consists of a spectrophotometric measurement of the color intensity obtained at 405 nm after the interaction between plasma treated solution, which contains hydrogen peroxide and titanium sulphate reagent. This colour intensity is proportional with the H2O2 sample concentration and is determined from a calibration plot. The titanium sulphate method is based on a stock solution of Ti(SO4)2 reagent which was prepared using 1 g of TiO2 powder and 100 mL of sulfuric acid (98%). After cooling, the solution was diluted with 4 parts of distillate water. For the calibration curve, a series of 8 solutions contained concentrations from 1 to 100 mg of hydrogen peroxide per liter were prepared by adding into each tube a quantity of 1 mL of regent Ti(SO4)2. The final volume for calibration solutions was 6 mL. After 2 min interaction, these yellow solutions were transferred into the absorbance cell and the absorption properties were evaluated.
Determination of Decolorization Percentage and Energetic Yield
In the present study, where the main investigations were based on optical spectroscopy, we will refer to discoloration or decolorization, this being the most obvious effect observed on treated solution.
The methylene blue decolorization process was characterized in the terms of conversion (Decolorization Percentage %) in time and efficiency (Energetic Yield, g/kWh) for various conditions (different geometrical configurations, mixture of gases and flow rates, applied power etc.). The concentration of treated MB solutions was determined from absorption spectra (peak maximum at 661 nm) using the calibration curve for this solution. The curve (not presented here) represents the absorbance as function of concentration, and it was obtained using MB solutions with known concentrations, specially prepared for the calibration of the absorption setup.
The dependence of the decolorization percentage of the methylene blue solutions upon time was calculated using the initial and intermediate concentrations of the solutions at each time point. Complete decolorization is considered when the concentration of MB decreases toward zero (absorption disappears from spectra). This period of treatment could be defined as the complete decolorization time.
The decolorization efficiency, representing the amount of MB destroyed by energy unit, was calculated as the ratio between the product of volume, initial concentration and conversion percentage and the product between the discharge power and treatment time. These equations, were described and used in [7, 15, 17, 26]:
where V[l] = initial solution’s volume, Cin = initial concentration, Cout = solution’s concentration after plasma treatment, P = forwarded RF power and Ttr = treatment time, Y-Energy Yield.
As observed, the yield can be calculated for different Cout. In some articles, for easy comparison, the authors report semi-conversion efficiency calculated in terms of Y50 which is the yield value corresponding to 50% conversion of the pollutant [9].
Results and Discussion
Identification of the Operating Domain of Plasma Sources in Open Air and Submerged in Water
In complement to the previous results [5] describing the filamentary jet in Ar, we have established new operating parameter value ranges for operation in air and submerged in liquid. The experiments were performed around standard conditions in order to relate to them. Further on, we present results concerning the influence of the tube material (glass/ceramic, poly-ethylene), tube diameter, gas flow rate and RF power on the plasma source operation.
Moreover, the discharge configurations were built with one or two electrodes at different distances from the tube exit. Thus, we varied several parameters (distance between electrode and tube output, tube diameter, gas flow rate and power) aiming to obtain the maximum length of the plasma jet, useful condition for a maximal contact with the liquid in the case of immersion. Some examples of the jet images for various discharge parameters are presented in Fig. 4, while in Fig. 5 one can find the graphical dependence of jet length with the power, tube diameters, argon and compressed air mixture flow rates.
In the case of standard condition, we observed an increase of filament length by increasing the tube external diameters from 6 to 15 mm (inner 4 to 13 mm); from hereafter we will refer only to the outer diameter. If the tube’s outer diameter is increased up to 15 mm, the filamentary discharge is not stable (filaments rotate on the inner wall of the dielectric tube). In contrast, in the case of a small tube outer diameter (6 and 10 mm), the filamentary plasma jets are stable also in liquids. So, for this reason the further tests were done only with ϕ6 mm and ϕ10 mm external-diameter. The filament length increasing with the applied power for both tubes of ϕ6 mm and ϕ10 mm can be seen in the graph from Fig. 5a. Similar dependencies, upon the Ar flow rate and flow rate of admixing compressed air, are presented in Fig. 5b, c. It is important to mention that the length of the plasma jet reaches a maximum length for 3000 sccm (tube ϕ6 mm) and 6000 sccm (tube ϕ10 mm) after which the length decreases until discharge extinguishes at high Ar flow rates. In terms of flow rate, the operating regimes of filamentary DBD source working only in Ar flow is different for the tubes with various diameters as follows: is larger (up to 20,000 sccm) for the tube ϕ10 mm compared to source made with a tube of ϕ6 mm (where the maximum operating Ar gas flow was 11,000 sccm), at the same power (100 W) and the same position of electrode (-50 mm), see Fig. 5b. In the case of YDBD we observed a decrease of jet length with the increase of the additional reactive gas flow. Thus, after the introduction of compressed air up to 6000 sccm, the discharge jet decreases gradually, and remains inside of the tube at about 3000/6000 sccm of Ar/compressed air mixture (see images of jets in Fig. 4 and their dependence in Fig. 5c).
With respect to the submerged regime, we performed the tests accounting for the operating range of the jet evolving in open atmosphere. We noticed that the operating ranges of source immersed in liquid are approximately the same with that of the sources working in open atmosphere, except the marginal values (of flow, power) delimiting the ranges, where the jet is very short.
Characterization of Plasma Jet Evolving in Air and Water by Optical Emission Spectroscopy, and the Effect of Treatment on Distilled Water
Figure 6 shows the spectra acquired from YDBD plasma source in open atmosphere through quartz for three gases: Ar, Ar/N2, Ar/Compressed Air) used in the experiments of MB decolorization. Argon lines in the ranges (400–440 nm) and (690–900 nm) [38], and oxygen lines (777.4 nm and 844.6 nm) are identified. Another prominent spectral signature is the second positive system (SPS) of nitrogen (C3Πu → B3Πg), especially the bands corresponding to the vibrational transitions 0-0 (band head at 337.1 nm), 1-0 (315.9 nm), 0-1 (357.7 nm). In its vicinity is the first negative system (FNS) of nitrogen ion (B2Σ+u → X2 Σ+g ) at 391.4 nm. We also notice the OH A2 Ʃ + (hydroxyl, 309 nm) and NO (A–X) (200–300 nm) bands.
Figure 7 shows the spectra acquired through the quartz vessel with the jet immersed in double distilled water. In comparison with the spectra acquired through quartz in open air (Fig. 6), there are several differences: decrease of the intensities of the Ar lines, increasing the intensity of the OH molecular band, occurrence of the NH (A3П-X3Ʃ) radical overlapping over the N2 (C3Пu - B3Пg) SPS molecular bands. The spectra are dominated by OH system, by argon atomic lines and by strong emissions from NH system (A3Π+ → X3Σ, 336 nm. Weak bands of second order of OH molecular band (610–630 nm) and Hα line (656 nm) are also observable.
For comparison, in Fig. 8 are superposed the spectra for standard conditions acquired through air and through the quartz vessel filled with double distilled water. In the spectra through water we can observe OH system, argon atomic lines, strong emissions from NH system (A3Π + → X3Σ, 336 nm) and weak bands of second order of OH molecular band (610–630 nm) and Hα line (656 nm), while the OH and NH bands are missing from the spectrum through air.
The gas temperature was estimated from the rotational temperature of OH molecular species present in the discharge [39]. The Trot was calculated using the intensities, wavelengths, Honl-London factors and energies of excited states of particular selection of OH lines. All the information was taken from the database specifically addressing the molecular spectra [40]. Typical Boltzmann [41] plots used for the calculation are shown in Fig. 9. The calculated rotational temperature of OH in standard condition in open air is about 600 ± 50 K, while in same condition trough water is 640 ± 50 K. This might be due to the fact that in presence of water vapors the populating of rotational levels with high J number is favoured [42, 43].
Effect of Adding a Ground Electrode in the Discharge Configuration
The DBD plasma source has one fixed electrode and another one mobile. By placing the bottom of the quartz vessel to a grounded metallic disk connected to the circuit, it becomes the second fixed electrode. In this configuration (grounded), it is possible the enhancement of electrons acceleration and the plasma filament becomes stronger. The most important observation is the following: when the vessel is grounded, the filament appears to be much longer compared to the discharge in an insulated vessel. The changes could be also seen in the OES spectra, where increases of the atomic lines’ intensities were compared for different configurations (grounded/ungrounded) (Fig. 10). The calculated OH rotational temperature is 640 ± 50 K for grounded source, compared to 550 ± 50 K for unground DBD (Fig. 10). Given these differences, the larger plasma volume and the increased lines intensities prove for a higher number of reactive species, and the increased gas temperature indicate they have larger kinetic energies. Therefore, the grounded DBD source may be associated with an intensification of the reactions in the solution and the source should be more efficient for our final application, water decontamination.
Characterization of the Plasma Effects on Distilled Water
For the standard treatment condition of the double distilled water, we investigated the treated water (plasma activated water) in order to observe the changes in pH, temperature, absorption spectra, and some water species concentration after various times of treatment.
We observed a decreasing of pH and a slight increasing of temperature after 15 min of treatment. The behavior could be seen in Fig. 11a, b.
As usual [44, 45], in the case of plasma activated water (PAW), the water pH decreases faster or slower, depending on the plasma condition and acidification [46] of the solution, and play a consistent role in chemical and biological water purification. However, the small temperature variation of the solution after 15 min of treatment does not play a decisive role in the subsequent MB decolorization process.
In order to highlight the importance of introducing additional gases (O2 and N2) in the discharge, in comparison with standard conditions, a method for the quantitative estimation of H2O2 species concentration was used. For this reason, we used a UV marker based on Ti(SO4)2 [13], and make the calibration curve in which we determined the H2O2 species concentration after various plasma treatments of double distilled water.
The concentration of H2O2 after plasma treatment was evaluated using 1 mL of reagent Ti (SO4)2, and 5 mL of liquid after plasma treatment. The absorption properties were evaluated using the same optical absorption setup described above. In order to evaluate the hydrogen peroxide concentration in solution, the absorption values were related to the calibration curve. The UV–Vis absorption results showed that the H2O2 concentration decreased after adding active gases and the highest concentration was obtained only with pure argon gas added in discharge (589.2 mg/L). An important amount of hydrogen peroxide of 139 mg/L was obtained using Ar/N2 gas mixtures, but in contrast with these concentrations, a low amount of H2O2 was obtained using oxygen in the Ar plasma, of 79 mg/L. At first sight, these results may appear surprising: firstly the amount of hydrogen peroxide for every sample is very high in contrast with the using of other plasma sources [19], and secondly, since one expects that the presence of oxygen in plasma would increase the hydrogen peroxide concentration. Similar with us, the concentration of H2O2 was found lower in the case of air (oxygen and nitrogen) micro-jet discharge than in the case Ar micro-jet discharge in water in Ref. [20].
Characterization of Filamentary Plasma Effects on MB Solutions
In the last ten years in our group we have developed many plasma sources that operate at atmospheric pressure in open air [5,6,7, 47], and we worked as well under water or in various liquids and suspensions (graphene and nanocellulose) [4, 36, 48]. In the case of the decolorization of methylene blue, we started with a low power plasma source [7], then we noticed a drastic decrease of the decolorization time during the experiments performed with the filamentary plasma jet. The total decolorization times (for 50 mL of MB solution, 25 mg/L) obtained by using three of our plasma sources are presented in Table 1.
Improvement of Decolorization Rate and Efficiency by Testing Various DBD Configurations
In order to obtain the shortest time as possible for the MB decolorization, we did a comparison between various conditions. These conditions are reported to the absorption spectra, the values of the pH, the conversion, and last but not the least, the energetic yield. The decolorization kinetics could be easily evaluated from visual observation of the treated MB from the vessel. These aspects regarding the colour modification were highlighted in Fig. 12, in which examples of MB solution at various time of treatment are presented.
In Fig. 13a, b, and c we present a few absorption spectra, periodically recorded at 5 min of treatment, until the complete decolorization. In Fig. 13d the sole effect of radiation emitted for plasma is presented. This graph corresponds to the experiments with the jet evolving in a quartz tube immersed in the MB solution.
Treatment Efficiency. The Identification of Experimental Parameters and Configurations for the Shortest Time for Decolorization
In our research, we are looking for a recipe of treatment leading to short decolorization time and high energetic efficiency. We made several experiments (see Table 2) and we observed how the efficiency depends on the experimental conditions. We are reporting the results in comparison with the standard conditions, and made variations around this treatment by changing parameters such as tube diameter, materials of tubes (glass/ceramic), power, additional gas flow etc. Such curves representing the decolorization percentages are presented in Fig. 14, and in Fig. 15 are presented the curves describing the energetic yields, as determined at increasing times of treatment.
Decolorization curves for various treatment conditions: a Ar jets in tube with various diameters (6 and 10 mm) and materials (glass/ceramic); b YDBD jets glass tubes with different diameters and various gases Ar/N2 and Ar/Compressed Air, and c comparison between DBD in Ar and YDBD in Ar/N2 (tube 6 mm) and power
We observed that the efficiency increases with the increase of the Ar flow, and this could be easily explained if we take into account that the number of species carried out by the jet is larger at higher Ar flow. Also, this explanation is sustained by the jet length obtained in open air when a higher Ar gas flow was used. The decrease of decolorization time was obtained when using a grounded recipient, where we suppose that an enhanced discharge is promoted. We remember that for this case, of grounded recipient, the changes in the decolorization kinetic are accompanied by an increase of Ar lines (from 400-450 nm) intensities (see Fig. 10) and the increase of electron rotation temperature.
The lowest efficiency was obtained for the configuration with two external electrodes. This is explained by the fact that the discharge energy is dissipated mainly in the inter-electrode region, which produces only a short and diffuse jet in open air.
According to Table 2, the most efficient treatment, leading to the shortest decolorization time, was obtained for Ar/N2 plasma; it was followed by Ar/O2 plasma treatment and at the end by Ar/Compressed Air (preponderant N2 and O2) plasma treatment. The maximum decolorization efficiency for our plasma source is about 0.072 g/KWh, comparable with [26] where the Energy Yields to remove 50% of MB (estimated from absorbance spectra after decolorization process) were reported.
The decolorization time required for 50% decolorization of MB is usually much shorter than half of total decolorization (as we can observe in our results or in the graph with conversion efficiency [%], as of function of time). From the Y50 column, we observe a maximum value for DBD in Ar-3000 sccm (glass tube with outer diameter of 10 mm), but in future experiments we considered that YDBD source in Ar/N2 is the most useful in complete MB decolorization (shortest time to complete decolorization time and their energetic yield efficiency). In addition, if we take into account our previous results [5], where we found that active power (PA - calculated form signals–voltage and current waveforms) is about 15% off the forwarded power (Pfwd) in standards condition (3000 sccm Ar, 100 W), the efficiency of our plasma source could be compared with a submerged MWPJ source [16] or even to a pulsed corona discharge [14] where the conversion percentages were estimated also from absorbance.
Species and Reactions Involved in the Decomposition Process
The importance of temperature and pH. In our case, we can eliminate solution temperature as a factor in influencing the time of decolorization, because the temperature is bellow 25 °C after 15 min of treatment, see Fig. 9 b. Also, the pH of the MB solution does not have the dominant effect in establishing the maximum conversion. The pH of the treated methylene blue solutions was changed, but it does not have the same trend with increasing the decolorization (maximum for Ar/N2 plasma). The initial pH of MB solution (before treatment) was alkaline 8.9 and their character was changed after treatment. In standard treatment conditions the pH of Ar treated solution is 4.8 and decreases to 4.1 for Ar/N2 plasma (3000/300 sccm) and even to a value 3.6 for Ar/Compressed Air plasma (3000/300 sccm), values in a medium acidity range. The decrease of pH over time with plasma treatment is well known phenomenon, and also the decreasing of pH (acidification) is accompanied with increasing of solution conductivity [26]. Recent reports in MB degradation showed a maximum in the case of nitrogen containing plasma in a mild acidy and low range of conductivities (about 10 μS/cm), while at higher conductivity (100–10,000 μS/cm) the removing of MB is less efficient [16, 26, 49].
The role of ROS. Regarding the influence of oxidative species (H2O2, OH, O) we can not make a plausible discussion regarding only their quantities, because these are not in trend with the decrease of decolorization time. The H2O2 concentration decreases after the introduction in the Ar plasma of some reactive gases like nitrogen and oxygen. The intensities of OH band at 309 nm also decreases (Fig. 6), from a maximum in Ar discharge, to Ar/N2 plasma and lower in the case of Ar/Compressed Air plasma submerged in water. The lines of Oxygen at 777.4 nm have a maximum for compressed air plasma and are missing for Ar plasma immersed in water.
In our case, besides the presence of oxidative species, we must consider reactive nitrogen species (RNS) and their conversion into the other species, that could explain the best result obtained for Ar/N2 plasma. On the other hand, a larger contribution of UV radiation should be considered for Ar/N2 plasma.
The role of RNS. The importance of nitrogen species generated by plasma in water is also reported by many authors. For examples, species as: nitrites, nitrates, acidified nitrites, nitric oxide, peroxynitrite etc. generated by plasma in water have a bactericidal effect on cultures of Escherichia coli [50]. The major effect of the nitrogen species in the decolorization of MB by a MW plasma jet, were described by the authors [26] who found a significant improvement in the efficiency by adding 1–3% of N2 into the Ar plasma (2 L/min la 200 W). They explain the improvement of MB decolorization by the combined effects of radicals NO, NO2 and NH (having high redox potential). Our results for the case of Ar/N2 plasma point out the same line; moreover, the emitted lines and bands reported in their paper are similar to those identified in our spectra, see Figs. 7 and 8.
Moreover, the rotational temperature in Ar plasma in water (approx. 650 K) is more than 2 times lower than the rotation temperature in Ar/N2 plasma (approx. 1600 K), both calculate from OH band. These temperatures were determined fitting the recorded spectra with the spectra simulated with LIFBASE spectrum simulation program. The experimental spectra were recorded with high resolution, allowing to the exclude in the procedure of the errors caused by the partial pollution of OH rotational lines with N2 (Δv =+1) SPS rotational lines.
Importance of UV radiation. Another important thing that must be considered for the best result regarding MB decolorization using Ar/N2 plasma is the generation of stronger UV radiation in this case. This assumption is sustained by the following results: as we can see in the OES spectra for Ar/N2 plasma the intensity of bands NO (A–X) and N2(C-B)) are stronger than in the OH (A–X) region and NO bands are missing in Ar plasma spectra. In addition, in the region between 50-300 nm, the nitrogen species emission are stronger in the UV [51] while for Ar plasma has just weak lines. The effects due only to UV radiation were proved by the experiment where the MB solutions were exposed to plasma trough the quartz tube (Fig. 13d). After 30 min of MB treatment with YDBD in Ar/N2 plasma trough the quartz tube, the concentration of MB decreased from 10 mg/L to about 8 mg/L.
Thus, from those described above we observed an important effect of UV radiation cumulated with RNS species for Ar/N2 plasma, beside of ROS presence in the increase of efficiency. In the tested condition (mild pH) we obtained the maximum efficiency for decolorization in argon/nitrogen plasma. Similar results in terms of maximum efficiency for nitrogen-containing plasmas have been reported by others [16, 26, 49, 52]. However, the treatment in Ar/N2 plasma in MB solution should be perform in a moderate range of acidity (short treatment times) [49], but especially low conductivity [16, 49].
Conclusions
The plasma working parameters were optimized to reach maximum decolorization rate and increase the efficiency. We started the experiments of MB decolorization using a DBD filamentary plasma source (operated only in Ar) with a complete conversion after 30 min. After optimization of the plasma sources (development of YDBD source) and of the treatment parameters the complete decolorization time decreased to 6 min. We indentified the key parameters leading to the decrease of the conversion time and increase of the efficiency. These parameters are: plasma source design (YDBD), gas composition, Ar gas flow rates, power, position of electrodes, grounded of solution container, tube diameter and tube material. By placing a grounded electrode in/under the solution vessel, we obtained an additional decrease of the decolorization time. After the introduction of reactive gases in the discharge trough the YDBD filamentary plasma jet–Y tube, we observed the increase in the energetic efficiency and further decrease the time for complete decolorization of dye.
In our experiments, we observed an important effect of UV radiation, cumulated with RNS species for Ar/N2 plasma beside of the ROS presence in the increase of efficiency. The maximum efficiency for decolorization was obtained for argon/nitrogen plasma. One strong advantage of this type of source is the utilization of an assembly built from more YDBD sources, which multiplies the decolorization effect. The developed sources and procedure obtained herein are useful for the decomposition of other pollutants too. For example, we have achieved promising results in the case of pesticides.
References
Voutchkov N (2010) Considerations for selection of seawater filtration pretreatment system. Desalination 261(3):354–364
Ölmez H, Kretzschmar U (2009) Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact LWT. Food Sci Technol. 42:686–693
Jiang B, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, Xue Q (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368
Ionita MD, Vizireanu S, Stoica SD, Ionita M, Pandele AM, Cucu A, Stamatin I, Nistor LC, Dinescu G (2016) Functionalization of carbon nanowalls by plasma jet in liquid treatment. Eur Phys J D 70(2):1
Teodorescu M, Bazavan M, Ionita ER, Dinescu G (2015) Characteristics of a long and stable filamentary argon plasma jet generated in ambient atmosphere. Plasma Sources Sci Technol 24:025033
Peng JW, Lee S (2013) Atmospheric pressure plasma degradation of azo dyes in water: pH and structural effects. Plasma Chem Plasma Process 33:1063
Stancu EC, Piroi D, Magureanu M, Dinescu G (2011) Decomposition of methylene blue by a cold atmospheric pressure plasma jet source. In Proceedings of the 20th international symposium on plasma chemistry, Philadelphia, PA, USA, pp 24–29, 375
Jamróz P, Gręda K, Pohl P, Żyrnicki W (2014) Atmospheric pressure glow discharges generated in contact with flowing liquid cathode: production of active species and application in wastewater purification processes. Plasma Chem Plasma Process 34:25
Malik MA (2010) Water purification by plasmas: which reactors are most energy efficient? Plasma Chem Plasma Process 30:21–31
Son G, Lee H, Gub JE, Lee S (2015) Decoloration of methylene blue hydrate by submerged plasma irradiation process. Desalin Water Treat 54:1445–1451
Takemura Y, Yamaguchi N, Hara T (2013) Decomposition of methylene blue by using an atmospheric plasma jet with Ar, N2, O2, or air. Jpn J Appl Phys 52–5:56102
Patinglag L, Sawtell D, Iles A, Melling LM, Shaw KJ (2019) A microfluidic atmospheric-pressure plasma reactor for water treatment. Plasma Chem Plasma Process 39:561–575
Magureanu M, Piroi D, Mandache NB, Parvulescu V (2008) Decomposition of methylene blue in water using a dielectric barrier discharge: optimization of the operating parameters. J Appl Phys 104:103306
Magureanu M, Bradu C, Piroi D, Mandache NB, Parvulescu V (2013) Pulsed corona discharge for degradation of methylene blue in water. Plasma Chem Plasma Process 33:51–64
Diamond J, Profili J, Hamdan A (2019) Characterization of various air plasma discharge modes in contact with water and their effect on the degradation of reactive dyes. Plasma Chem Plasma Process 39–6:1483–1498
Hamdan A, Profili J, Cha MS (2020) Microwave plasma jet in water: effect of water electrical conductivity on plasma characteristics. Plasma Chem Plasma Process 40–1:169–185
Benetoli LODB, Cadorin BM, Postiglione CDS, Souza IGD, Debacher NA (2011) Effect of temperature on methylene blue decolorization in aqueous medium in electrical discharge plasma reactor. J Braz Chem Soc 22(9):1669–1678
Huang F, Chen L, Wang H, Yan Z (2010) Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem Eng J 162:250–256
Magureanu M, Dobrin D, Bradu C, Gherendi F, Mandache NB, Parvulescu VI (2016) New evidence on the formation of oxidizing species in corona discharge in contact with liquid and their reactions with organic compounds. Chemosphere 165:507–514
Vlad IE, Anghel SD (2017) Time stability of water activated by different on-liquid atmospheric pressure plasmas. J Electrost 87:284–292
Liu X, Zhang H, Qin D, Yang Y, Kang Y, Zou F, Wu Z (2015) Radical-initiated decoloration of methylene blue in a gas-liquid multiphase system via dc corona plasma. Plasma Chem Plasma Process 35–2:321–337
Mihai S, Cursaru DL, Ghita D, Dinescu A (2016) Morpho ierarhic TiO2 with plasmonic gold decoration for highly active photocatalysis properties. Mater Lett 162:222–225
Mihai S, Cursaru DL, Matei D, Manta AM, Somoghi R, Branoiu G (2019) Rutile RuxTi1-xO2 nanobelts to enhance visible light activated photocatalytic activity. Scientific Rep 9:18798
Tatarova E, Bundaleska N, SarrettebJ Ph, Ferreira CM (2014) Plasmas for environmental issues: from hydrogen production to 2D materials assembly. Plasma Sour Sci Technol 23(6):063002
Abdelaziz A, Ishijima T, Tizaoui C (2018) Development and characterization of a wire-plate air bubbling plasma for wastewater treatment using nanosecond pulsed high voltage. J Appl Phys 124:053302
Hamdan A, Liu JL, Cha MS (2018) Microwave plasma jet in water: characterization and feasibility to wastewater treatment. Plasma Chem Plasma Process 38:1003–1020
Shirafuji T, Ishida Y, Nomura A, Hayashi Y, Goto M (2017) Reaction mechanisms of methylene-blue degradation in three-dimensionally integrated micro-solution plasma. Jpn J Appl Phys 56(6S2):06HF02
Benitez FJ, Beltran-Heredia J, Gonzalez T, Pascual A (1993) Ozone treatment of methylene blue in aqueous solutions. Chem Eng Commun 119(1):151–165
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42:53001
Locke BR, Shih KY (2011) Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sour Sci Technol 20(3):34006
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Phys D Appl Phys 45–26:263001
Liu Z, Zhou C, Liu D, He T, Guo L, Xu D, Kong MG (2019) Quantifying the concentration and penetration depth of long-lived RONS in plasma-activated water by UV absorption spectroscopy. AIP Adv 9:15014
Shirafuji T, Nomura A, Hayashi Y, Tanaka K, Goto M (2016) Matrix-assisted laser desorption ionization time-of-flight mass spectrometric analysis of degradation products after treatment of methylene blue aqueous solution with three-dimensionally integrated microsolution plasma. Jpn J Appl Phys 55:01AH02
Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG, Graves DB, Hofman-Caris RCHM, Maric D, Reid JP, Ceriani E, Fernandez Rivas D, Foster JE, Garrick SC, Gorbanev Y, Hamaguchi S, Iza F, Jablonowski H, Klimova E, Kolb J, Krcma F, Lukes P, MacHala Z, Marinov I, Mariotti D, Mededovic Thagard S, Minakata D, Neyts EC, Pawlat J, Petrovic ZLJ, Pflieger R, Reuter S, Schram DC, Schröter S, Shiraiwa M, Tarabová B, Tsai PA, Verlet JRR, Von Woedtke T, Wilson KR, Yasui K, Zvereva G (2016) Plasma-liquid interactions: a review and roadmap. Plasma Sour Sci Technol. 25(5):53002
Ionita MD, Teodorescu M, Stancu C, Stancu EC, Ionita ER, Moldovan A, Acsente T, Bazavan M, Dinescu G (2010) Surface modification of polymers at atmospheric pressure in expanding RF plasmas generated by planar dielectric barrier discharges. J Optoelectron Adv Mater 12(3):777–782
Panaitescu DM, Vizireanu S, Nicolae CA, Frone AN, Casarica A, Carpen LG, Dinescu G (2018) Treatment of nanocellulose by submerged liquid plasma for surface functionalization. Nanomaterials 8:467
Eisenberg GM (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal Edition 15–5:327–328
Iordanova S, Koleva I (2007) Mode operation of inductively-coupled argon plasmas studied by optical emission spectroscopy. J Phys: Conf Ser 63:012026. https://doi.org/10.1088/1742-6596/63/1/012026
Bazavan M, Iova I (2008) Temperature determination of a cold N2 discharge plasma by the fit of the experimental spectra with the simulated emission spectra. Rom Rep Phys 60(3):671–678
Kramida A, Ralchenko Y, Reader J, NIST ASD Team (2018) NIST atomic spectra database (version 5.6.1) https://physics.nist.gov/asd
Teodorescu M, Bazavan M, Ionita ER, Dinescu G (2012) Characterization of an argon radiofrequency plasma jet discharge continuously passing from low to atmospheric pressure. Plasma Sour Sci Technol 21:055010
Bruggeman PJ, Schram DC, Gonzalez MA, Rego R, Kong MG, Leys C (2009) Characterization of a direct DC-excited discharge in water by optical emission spectroscopy, plasma sources science and technology. Plasma Sour Sci Technol 18:2
Bruggeman PJ, Sadeghi N, Schram DC, Linss V (2014) Gas temperature determination from rotational lines in non-equilibrium plasmas: a review. Plasma Sour Sci Technol 23:023001
Zhou R, Zhou R, Prasad K, Fang Z, Speight R, Bazaka K, Ostrikov K (2018) Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem 20:5276–5284
Chen Q, Shirai H (2012) Diagnostics of atmospheric pressure microplasma with a liquid electrode. Eur Phys J D 66(6):161
Oehmigen K, Hähnel M, Brandenburg R, Wilke C, Weltmann K-D, von Woedtke T (2010) The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym 7(3–4):250–257
Dinescu G, Ionita ER, Luciu I, Grisolia C (2007) Flexible small size radiofrequency plasma torch for Tokamak wall cleaning. Fusion Eng Des 82:2311–2317
Vizireanu S, Panaitescu DM, Nicolae CA, Frone AN, Chiulan I, Ionita MD, Satulu V, Carpen LG, Petrescu S, Birjega R, Dinescu G (2018) Cellulose defibrillation and functionalization by plasma in liquid treatment. Sci Rep 8:15473
Hamdan A, Gagnon C, Aykul M, Profili J (2020) Characterization of a microwave plasma jet (TIAGO) in-contact with water: application in degradation of methylene blue dye. Plasma Process Polym 17(3):1900157
Lukes P, Dolezalova E, Sisrova I, Clupek M (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sour Sci Technol 23(1):15019
Brandenburg R, Lange H, von Woedtke T, Stieber M, Kindel E, Ehlbeck J, Weltmann KD (2009) Antimicrobial effects of UV and VUV radiation of nonthermal plasma jets. IEEE Trans Plasma Sci 37(6):877–883
Nikiforov AYu, Leys CH, Li L, Nemcova L, Krcma F (2011) Physical properties and chemical efficiency of an underwater dc discharge generated in He, Ar, N2 and air bubbles. Plasma Sour Sci Technol 20(3):34008
Acknowledgements
This work was supported by a grant of the Romanian Ministry of Research and Innovation, CCCDI - UEFISCDI, in the frame of Nucleus Programme INFLPR LAPLASVI- contract n. 16 N/2020 and project number PN-III-P1-1.2-PCCDI-2017-0637/33 PCCDI-MultiMonD2 within PNCDI III. The authors acknowledge Dr. Monica Magureanu for the fruitful discussions and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yehia, S.A., Zarif, M.E., Bita, B.I. et al. Development and Optimization of Single Filament Plasma Jets for Wastewater Decontamination. Plasma Chem Plasma Process 40, 1485–1505 (2020). https://doi.org/10.1007/s11090-020-10111-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10111-0