Abstract
The study investigated NO reduction by N radicals produced by barrier discharge in nitrogen and the possible effect of TiO2 catalyst on this reaction. The experiments for NO reduction were carried out at temperatures ranging from room temperature to 140 °C and at NO concentrations ranging from 200 to 800 ppm. The experimental results were described by a model which was based on six main reactions for the calculation of the NO and NO2 concentrations. The analysis of model suggested two simple methods for the estimation of nitrogen radical production efficiency which in present study was 2 ppm/(J/L). The model also confirmed that N radicals are used very efficiently in the reduction of NO and this process can’t be improved by the catalyst. Nonetheless, experiments showed that the presence of TiO2 catalyst improved the removal of NO. The experimental results can be explained by surface processes taking place with NO2 with the help of other plasma produced nitrogen species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen oxides (NO and NO2) are an important class of pollutants which are inevitably present in flue gases. The main nitrogen oxide constituent in the flue gas is NO which is hard to remove by absorption or adsorption based methods [1]. One possible route for the improvement of the NO removal is the non-thermal plasma oxidation of NO–NO2 which can be removed more easily by the conventional nitrogen oxide removal methods [2,3,4]. The plasma oxidation works when the oxygen content of flue gas during the plasma treatment is above 3% while at lower oxygen content the reductive pathway dominates and direct decomposition of NO can be achieved by plasma [5, 6]. The reduction by N2 plasma is also an interesting alternative to reduction by NH3 or CxHy in the NOx storage and reduction process [7, 8]. However, the efficiency of plasma reduction of NO in nitrogen remains smaller than the efficiency of plasma oxidation of NO in oxygen and nitrogen mixture due to smaller efficiency of N radical production in non-thermal plasmas [9, 10].
It has been shown that in the case of plasma oxidation the efficiency can be improved by the use of catalyst [11, 12]. It has been proposed, that the plasma may activate the catalyst by UV radiation, local temperature increase, plasma produced radicals or other high-energy plasma species [13,14,15]. Among these activation routes, the activation of TiO2 and other metal-oxide catalysts by plasma produced oxygen species, e.g. O radicals and O3 has been demonstrated by several experiments [16,17,18]. In the plasma oxidation of volatile organic compounds (VOC), the reactions with surface bound oxygen species are considerably faster than in gas phase and this allows to utilize the plasma produced oxygen species (O, O3) more efficiently [19, 20]. In the plasma oxidation of NO, the oxidation with plasma produced oxygen species is sufficiently fast but the back-reaction of NO2 to NO caused by O radicals starts to limit the oxidation efficiency [21, 22]. In this case the catalyst allows to suppress the gas-phase back-reaction and improve the efficiency due to additional reactions between NO and oxygen species adsorbed on catalyst surface [22,23,24].
Despite the successful use of catalyst in the plasma oxidation, it is not clear whether the catalyst could improve also the NO reduction process in nitrogen plasma and what could be the main activation mechanism in such case. It is well established that the NO removal in nitrogen plasma is caused by NO reduction with N radicals according to the reaction NO + N → N2 + O [5, 25,26,27,28,29,30]. In fact, this reaction can be used to probe the N radical production of the discharge [27, 31]. However, the role of other reaction pathways, as well as the functional dependence between NO reduction and specific plasma input energy, SIE, is not clear. The reduction reaction by N radicals is fast and proceeds within the time-scale of milliseconds. There is also no back-reaction which could decrease the efficiency of NO reduction. When other fast reactions with N radicals are not competing with NO reduction reaction, the removal should be linear function of SIE and the reduction efficiency would be limited by the efficiency of N radical production [32]. However, the reduction of NO is not a linear function of SIE [25, 26, 28, 31]. In several studies [28, 31], the NO reduction was fitted by exponential function which suggest that N radicals are scavenged by other reactions [32, 33]. In this case, the catalyst could improve the NO reduction when the nitrogen radicals activate the catalyst surface and the reduction rate is higher on the catalyst surface. Alternatively, the nonlinear SIE dependence of the removal of NO in nitrogen plasma may be caused by other reasons than the N radical scavenging and in such case the presence of catalyst could not affect the reduction process. Nonetheless, it could still be possible to improve the NO reduction in nitrogen plasma by activating the catalyst surface by other nitrogen species e.g. excited vibrational and electronic states of nitrogen.
Present study was carried out to investigate the effect of TiO2 catalyst on the NO removal in nitrogen plasma. First part of the studies investigated the removal mechanism of NO in the nitrogen plasma to clarify the efficiency of NO reduction by N radicals. These experiments were carried out at various NO inlet concentrations and catalyst temperatures. A model was proposed to numerically fit the NO removal and calculate the N radical production of the discharge. Second part of the study investigated the effect of TiO2 catalyst in the removal of NO by nitrogen plasma and discussed the possible processes taking place on the catalyst surface. TiO2 was used as catalyst because it has been shown earlier that it is activated by plasma produced oxygen species.
Experimental Setup
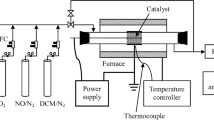
The mixtures of NO and N2 were prepared by Alicat Scientific flow controllers. The removal of NO was carried out by dielectric barrier discharge reactor with coaxial configuration [22, 31]. A stainless-steel tube with 14 mm outer diameter was placed into the quartz tube with 16.3 mm inner diameter. A steel mesh was wrapped around the quartz tube as the second electrode. The length of the active plasma zone was 85 mm. The high voltage was applied to the inner stainless-steel tube. A signal generator was used to provide the sinusoidal voltage with varying frequency (100–2500 Hz). The signal was amplified by Industrial Test Equipment Co power amplifier and a transformer. The power deposited into the plasma was determined by the method of Lissajous figures [34, 35]. The specific input power (SIE) was calculated by dividing the deposited power with the flow rate. The breakdown voltage which was used to calculate the average reduced electric field was also determined from the Lissajous figures. An electrically heated oven was used to change the DBD reactor temperature which was measured by the Osensa Innovations fiber-optical sensor FTX-100-Gen. The effect of catalyst was investigated by pressing the TiO2 nanopowder (Degussa P25) on the inner steel tube of the reactor. The mass of TiO2 powder was 0.45 g.
The concentrations of NO and NO2 were determined by the optical absorption spectroscopy in the UV range. The gas from the outlet of DBD reactor was directed through an absorption cell with 20 cm length having fused quartz windows. The Hamamatsu L2D2 deuterium lamp was used as the UV source and Ocean Optics USB4000 spectrometer was used to collect the absorption spectra. The methodology for the determination of NO and NO2 concentrations from the absorption spectra has been described in our earlier studies [24, 36].
Results and Discussion
NO Reduction Without Catalyst
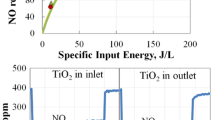
Figure 1 shows the experimentally determined concentrations of NO and NO2 as a function of SIE at various inlet NO concentrations at room temperature. The concentration of NO decreased monotonically with increasing SIE until practically all NO was removed. The SIE values where total NO removal was achieved increased together with the inlet NO concentration. The removal of NO was accompanied with the appearance of NO2 at low SIE values. The NO2 concentration reached maximum values at SIE values where nearly half of NO was removed and started to decrease until diminishing at SIE values where all NO was removed. Figure 2 shows the concentrations of NO and NO2 as a function of SIE at various temperatures. The removal of NO at increasing SIE practically coincided at all investigated temperatures. The production of NO2 was also similar in the whole temperature range.
Model Describing the NO Reduction
The experimental results can be explained by a model which considers the following reactions:
3.1 × 10−11 cm3/s (20 °C and 140 °C) [37]
7.5 × 10−32 cm6/s (20 °C) 6 × 10−32 cm3/s (140 °C) [38]
1.1 × 10−11 cm3/s (20 °C) 8 × 10−32 cm3/s (140 °C) [39]
1.3 × 10−31 cm3/s (20 °C) 8.1 × 10−32 cm3/s (140 °C) [40]
1.7 × 10−11 cm3/s (20 °C and 140 °C) [41]
1.2 × 10−11 cm3/s (20 °C and 140 °C) [42].
According to earlier studies [5, 43], the removal of NO in N2 is caused by the reduction with N radicals through the reduction reaction (1) whereas O radicals produced in this reaction oxidize NO by reaction (2). The O radicals also reduce part of the produced NO2 back to NO by the reaction (3). The results are influenced also by NO2 oxidation to NO3 and NO3 reduction to NO2 by O radicals which is taken into account by reactions (4) and (5). Furthermore, considerable amount of NO2 is converted to N2O by reaction (6).
The effect of these reactions was analysed by solving a set of linear differential equations based on the reactions (1)–(6). The model additionally assumes that N radicals are produced uniformly along the discharge tube length with the efficiency GN. Reasonably good fit with the experimental results was obtained by assuming GN value 2 ppm/(J/L) as shown on Figs. 1 and 2. The results are insensitive to the variation of the reaction rate coefficient (1) due to its high value. The reaction rate coefficients (2) and (3) influence mostly the amount of produced NO2. It was possible to get similar result as shown on Figs. 1 and 2 when using 20% lower reaction rate coefficient for reaction (2) and omitting reactions (4)–(6).
The effect of the reactions (1)–(3) on the outlet NO concentration as a function of SIE is shown on Fig. 3. When considering only the reduction with N radicals by reaction (1), the NO removal would be linear function of SIE with the assumption that N radical production along the reactor is proportional to SIE. This reaction is sufficiently fast and consumes practically all N radicals as long as there is still NO available in the gas. It is noteworthy that without NO, the formation of N2 from N radicals would be the main loss channel for N radicals, but its importance diminishes in the presence of NO. At these conditions, the slope of NO concentration as a function of SIE would correspond to the N radical production efficiency GN. However, the produced O radicals also quickly react with NO by reaction (2) resulting in oxidative channel of NO removal. Without the presence of back-reaction (3), the removal efficiency of NO would be two times higher than the production efficiency of N radicals as shown by black line (1)–(2) in Fig. 3. The presence of the back-reaction (3) reduces some of the NO2 back to NO resulting in the loss of O radicals. Consequently, the NO removal deviates from the straight line marked as (1)–(2) on Fig. 4 and at higher SIE values the NO removal approaches the line corresponding to reaction (1). The efficiency GN can be roughly estimated by dividing the inlet NO concentration with the SIE value where NO diminishes to zero. In the present study, this value was approximately 2 ppm/(J/L) which was same as used in the numerical calculations. This value corresponds to approximately 0.8 N atoms per 100 eV which is somewhat larger than the value 0.6 N atoms per 100 eV obtained in our previous study by using the exponential fit [31]. Same procedure can be used to compare our results with the results of other studies [5, 25, 44]. The GN value obtained from these studies was between 0.7 and 2.4 ppm/(J/L) which is comparable with the value obtained in present study. The highest outlet concentration of NO2 was also comparable with the concentrations obtained in the present study.
An important outcome of the analysis with reactions (1)–(3) is the finding that the sum of NO and NO2 (dashed blue line) coincides with NO concentration without oxidative reactions (black line). The inclusion of reactions (4)–(6) results in small deviation from the line (shown by dashed blue line) but the deviation remains in the uncertainty of NO concentration measurements. The slope of outlet NOx (NO + NO2) concentration [NOx]out = [NO]in − GN·SIE gives another possibility to obtain the GN and the value determined from the experiment was again 2 ppm/(J/L).
The Effect of Average Reduced Electric Field on N Radical Production Efficiency
The estimated N radical production efficiencies were practically independent from the temperature within the uncertainty of the measurements. In barrier discharges, the production of N radicals is a monotonically increasing function of the reduced electric field [9, 43] which is the ratio of electric field and the gas number density. The gas number density decreases with the gas temperature and this should result in the increase of reduced electric field unless the electric field adjusts to lower values.
The average electric field in the discharge gap can be calculated by dividing the breakdown voltage Vb with the discharge gap length d. The breakdown voltage could be obtained from Lissajous figures. Our results showed that the average electric field decreased with the increasing temperature but didn’t counterbalance the effect of temperature on reduced electric field as shown by Fig. 4.
The growth of reduced electric field should result in the increased radical production efficiency, which was not found by experiments. The discrepancy can be explained by dominant production of N radicals in the streamed head where the electric field is considerably higher than the field in the channel [10, 45]. The efficiency of N radical production at SIE values between 100 and 120 J/L should be approximately 0.1 N atoms/100 eV [9, 43] while the experimental efficiency was 0.8 atoms/100 eV. This experimentally found efficiency corresponds to the reduced electric field value of ≈ 300 Td which shows the strong contribution of the N radical production in streamer head. The electric field in the streamer head may not follow the average electric field of the discharge gap.
The Effect of Catalyst
The analysis of the NO removal in nitrogen plasma demonstrated that the produced N radicals are efficiently used for NO reduction and the presence of catalyst should not improve the reduction by N radicals. Figure 5 shows the effect of catalyst on the removal of NO. The total NO removal was achieved at similar SIE values as without catalyst while at lower SIE values the NO removal was increased in the presence of catalyst. The outlet NO2 concentrations on the other hand were comparable with the concentrations obtained without TiO2 catalyst.
Stable outlet concentrations of NO and NO2 as a function of SIE with and without the catalyst. Filled symbols denote NO concentration and empty symbols NO2 concentration. Lines show the results of calculations. Solid blue lines show the calculations results where the reaction rate coefficient for reaction (3) was used while the dashed yellow lines show the calculation results where the reaction rate coefficient for reaction (3) was ten times higher (Color figure online)
Similar SIE values required for the total removal of NO with and without the catalyst show that the reduction by N radicals is not improved by the catalyst. This result complies with the numerical calculations which demonstrated that the NO reduction reaction is the main loss channel of N radicals. Furthermore, these results also demonstrate the absence of other major channels for NO reduction, e.g. the reduction by excited N2 molecules on the catalyst surface. These reactions, when proceeding independently from NO reduction by N radicals, would decrease the SIE values where total NO removal is achieved.
Another possible explanation for the increased NO removal in the presence of catalyst could be the enhancement of NO2 production by reaction (2) in comparison of the back-reaction (3). This effect of catalyst was observed in our earlier studies of NO removal in oxygen containing plasma [23]. It was proposed that NO is then also oxidized by O radicals adsorbed on the surface whereas the back-reaction does not proceed on the surface. The calculated yellow lines on Fig. 6 were obtained by tenfold increase of the reaction rate coefficient for reaction (2). Similar result could be obtained also by decreasing the reaction rate coefficient for reaction (3). According to the calculations, the NO2 concentration should correspondingly increase as shown by yellow dotted line. In experiments however, the NO2 concentration remained practically same. It is possible that this missing NO2 is converted to N2O or reduced to N2 by surface reactions with excited N2 molecules. The reduction of surface adsorbed nitrogen oxides by nitrogen plasma has been observed recently in several studies [7]. However, there is need for additional studies with specific nitrogen sources to clarify this possibility.
Another explanation for the differences in NO removal and NO2 production in the presence of catalyst could be the adsorption of NO and NO2 on the surface. This effect was also observed in the NO oxidation in oxygen containing plasma [23]. Indeed, time-dependent changes occurred in the outlet concentrations as shown on Fig. 6. These changes can be connected with adsorption and desorption effects of nitrogen oxides [23, 46]. It should be noted that these adsorption and desorption effects were less pronounced at 400 ppm when compared to 800 ppm. The outlet concentrations stabilized during the first minutes of plasma treatment when the adsorption and desorption of nitrogen species reached equilibrium. The results in Fig. 5 are shown for these stable concentrations and therefore, the missing amount of NO and NO2 can’t be explained by adsorption.
The time-dependent changes of NOx concentrations during the removal of NO by nitrogen plasma in the presence of TiO2 were similar with the results obtained during the removal of NO by oxygen containing plasma [23]. At the beginning of plasma treatment, both NO and NO2 concentrations decreased below the value which was reached after several minutes of plasma treatment. At the end of plasma treatment, the NO2 concentration decreased slowly at low SIE values whereas at high SIE values the concentration of NO2 even increased abruptly and then started to decrease. The outlet NO concentration remained below the inlet value until NO2 concentration diminished. The results indicate that during the plasma treatment NO reacts with oxygen species on the TiO2 surface and part of the NO2 also adsorbs on the TiO2 surface [23]. After the end of the plasma treatment, the NO reacts with the nitrogen oxides adsorbed in the form of NO3− and produces gas phase NO2 [23, 46]. The amount of adsorbed and desorbed NOx species increased with the SIE values. The desorption of NOx at the end of the plasma treatment was observable even at high SIE values where NO and NO2 concentrations decreased to zero and the amount of desorbed NOx had the highest values. This suggests that O radicals produced by reaction (1) adsorbed on the surface and reacted with NO producing stable NO3− on the surface.
Summary and Conclusions
Present study investigated the NO reduction by nitrogen plasma and the possible role of catalyst in this process. It was found that in the gas phase the reduction of NO by N radicals by reaction NO + N → N2 + O is sufficiently fast to prevent the losses of radicals by other reactions. This means that the NO reduction efficiency in nitrogen plasma is limited by the production efficiency of N radicals. The oxidation of NO by O radicals formed in the reduction reaction is important in the SIE range where O radicals and unreacted NO are simultaneously present. Based on the analysis of the experimental and calculated results, two simple methods for the estimation of the production efficiency of N radicals of the nitrogen plasma were proposed.
According to present study, the use of exponential fit for NO reduction as a function of SIE is not justified in nitrogen plasma when all reactions take place in gas phase. The deviation from linear dependence comes due to the aforementioned oxidation of some unreacted NO to NO2. The exponential removal is still possible at conditions where the N radical recombination on the surface becomes the dominant scavenger of N radicals. This situation could be most likely encountered in the case of surface barrier discharges where the plasma channels form only near the surface. It should result in the decreased efficiency of NO reduction and apparent decrease of N radical production.
The catalyst can’t improve the NO reduction by N radicals because this reaction is sufficiently efficient. The experiments also pointed out the unlikeliness of NO reduction by other excited nitrogen species on the TiO2 surface. Nonetheless, it is possible that these processes proceed more efficiently on the surfaces of other catalyst materials. The improved NO removal in the SIE range where NO2 forms is most likely caused by surface reactions with NO2 which is either reduced or converted to N2O.
References
Skalska K, Miller JS, Ledakowicz S (2010) Science of the total environment trends in NOx abatement: a review. Sci Total Environ 408:3976–3989. https://doi.org/10.1016/j.scitotenv.2010.06.001
Sun Mok Y, Young Yoon E (2006) Effect of ozone injection on the catalytic reduction of nitrogen oxides. Ozone Sci Eng 28:105–110. https://doi.org/10.1080/01919510600559278
Mok YS, Lee H-J, Dors M, Mizeraczyk J (2005) Improvement in selective catalytic reduction of nitrogen oxides by using dielectric barrier discharge. Chem Eng J 110:79–85. https://doi.org/10.1016/j.cej.2005.02.032
Mok YS, Koh DJ, Kim KT, Nam I-S (2003) Nonthermal plasma-enhanced catalytic removal of nitrogen oxides over V2O5/TiO2 and Cr2O3/TiO2. Ind Eng Chem Res 42:2960–2967. https://doi.org/10.1016/j.cattod.2006.07.032
McLarnon CR, Penetrante BM (1998) Effect of gas composition on the NOx conversion chemistry in a plasma. In: Society of automotive engineers fall fuels and lubricants meeting, San Francisco, CA, 19–22 October 1998
Zhao G-B, Garikipati SVBJ, Hu X et al (2005) Effect of oxygen on nonthermal plasma reactions of nitrogen oxides in nitrogen. AIChE J 51:1800–1812. https://doi.org/10.1002/aic.10452
Yu Q, Wang H, Liu T et al (2012) High-efficiency removal of NOx using a combined adsorption–discharge plasma catalytic process. Environ Sci Technol 46:2337–2344
Okubo M, Arita N, Kuroki T et al (2008) Total diesel emission control technology using ozone injection and plasma desorption. Plasma Chem Plasma Process 28:173–187. https://doi.org/10.1007/s11090-008-9121-7
Penetrante BM, Bardsley N, Hsiao MC (1997) Kinetic analysis of non-thermal plasmas used for pollution control. Jpn J Appl Phys 36:5007–5017
Naidis GV (2012) Efficiency of generation of chemically active species by pulsed corona discharges. Plasma Sources Sci Technol 21:042001. https://doi.org/10.1088/0963-0252/21/4/042001
Van Durme J, Dewulf J, Sysmans W et al (2007) Efficient toluene abatement in indoor air by a plasma catalytic hybrid system. Appl Catal B Environ 74:161–169. https://doi.org/10.1016/j.apcatb.2007.02.006
Kim H-H (2004) Nonthermal plasma processing for air-pollution control: a historical review, current issues, and future prospects. Plasma Process Polym 1:91–110. https://doi.org/10.1002/ppap.200400028
Thevenet F, Sivachandiran L, Guaitella O et al (2014) Plasma catalyst coupling for volatile organic compound removal and indoor air treatment: a review. J Phys D Appl Phys 47:224011. https://doi.org/10.1088/0022-3727/47/22/224011
Neyts EC (2016) Plasma–surface interactions in plasma catalysis. Plasma Chem Plasma Process 36:185–212
Whitehead JC (2016) Plasma-catalysis: the known knowns, the known unknowns and the unknown unknowns. J Phys D Appl Phys 49:243001. https://doi.org/10.1088/0022-3727/49/24/243001
Roland U, Holzer F, Pöppl A, Kopinke F-D (2005) Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds. Appl Catal B Environ 58:227–234. https://doi.org/10.1016/j.apcatb.2004.11.025
Ogata A, Kim H-H, Futamura S et al (2004) Effects of catalysts and additives on fluorocarbon removal with surface discharge plasma. Appl Catal B Environ 53:175–180
Guaitella O, Lazzaroni C, Marinov D, Rousseau A (2010) Evidence of atomic adsorption on TiO2 under plasma exposure and related C2H2 surface reactivity. Appl Phys Lett 97:011502. https://doi.org/10.1063/1.3462295
Kim HH, Ogata A, Futamura S (2005) Atmospheric plasma-driven catalysis for the low temperature decomposition of dilute aromatic compounds. J Phys D Appl Phys 38:1292–1300
Kim HH, Ogata A, Futamura S (2008) Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl Catal B Environ 79:356–367. https://doi.org/10.1016/j.apcatb.2007.10.038
Jõgi I, Levoll E, Raud J (2016) Plasma oxidation of NO in O2:N2 mixtures: the importance of back-reaction. Chem Eng J 301:149–157. https://doi.org/10.1016/j.cej.2016.04.057
Jõgi I, Erme K, Levoll E et al (2018) Plasma and catalyst for the oxidation of NOx. Plasma Sources Sci Technol 27:035001. https://doi.org/10.1088/1361-6595/aaae3c
Jõgi I, Levoll E, Raud J (2017) Effect of catalyst placement on the plasma-catalytic oxidation of NO. Catal Lett 147:566–571. https://doi.org/10.1007/s10562-016-1953-9
Jõgi I, Haljaste A, Laan M (2014) Hybrid TiO2 based plasma-catalytic reactors for the removal of hazardous gasses. Surf Coat Technol 242:195–199. https://doi.org/10.1016/j.surfcoat.2013.10.016
Penetrante B, Hsiao M, Merritt B et al (1996) Pulsed corona and dielectric-barrier discharge processing of NO in N2. Appl Phys Lett 68:3719–3721. https://doi.org/10.1088/0963-0252/2/3/005
Zhao G-B, Hu X, Yeung M-C et al (2004) Nonthermal plasma reactions of dilute nitrogen oxide mixtures: NOx in nitrogen. Ind Eng Chem Res 43:2315–2323
Fresnet F, Baravian G, Pasquiers S et al (2000) Time-resolved laser-induced fluorescence study of NO removal plasma technology in N2/NO mixtures. J Phys D Appl Phys 33:1315–1322
Malik MA, Kolb JF, Sun Y, Schoenbach KH (2011) Comparative study of NO removal in surface-plasma and volume-plasma reactors based on pulsed corona discharges. J Hazard Mater 197:220–228. https://doi.org/10.1016/j.jhazmat.2011.09.079
Leipold F, Fateev A, Kusano Y et al (2006) Reduction of NO in the exhaust gas by reaction with N radicals. Fuel 85:1383–1388. https://doi.org/10.1016/j.fuel.2005.12.020
Gallego JL, Minotti F, Grondona D (2014) Experimental and theoretical study of the efficiency of a three-electrode reactor for the removal of NO. J Phys D Appl Phys 47:205202
Jõgi I, Erme K, Levoll E, Stamate E (2017) Radical production efficiency and electrical characteristics of a coplanar barrier discharge built by multilayer ceramic technology. J Phys D Appl Phys 50:465201
Yan K, Van Heesch EJM, Pemen AJM, Huijbrechts PAHJ (2001) From chemical kinetics to streamer corona reactor and voltage pulse generator. Plasma Chem Plasma Process 21:107–137. https://doi.org/10.1023/A:1007045529652
Rosocha LA (2005) Nonthermal plasma applications to the environment: gaseous electronics and power conditioning. IEEE Trans Plasma Sci 33:129–137
Manley TC (1943) The electric characteristics of the ozonator discharge. J Electrochem Soc 84:83–96
Jõgi I, Bichevin V, Laan M et al (2009) NO conversion by dielectric barrier discharge and TiO2 catalyst: effect of oxygen. Plasma Chem Plasma Process 29:205–215. https://doi.org/10.1007/s11090-009-9171-5
Jõgi I, Erme K, Haljaste A, Laan M (2013) Oxidation of nitrogen oxide in hybrid plasma-catalytic reactors based on DBD and Fe2O3. Eur Phys J Appl Phys 61:24305. https://doi.org/10.1051/epjap/2012120421
Atkinson R, Baulch DL, Cox RA et al (1989) Evaluated kinetic and photochemical data for atmospheric chemistry: supplement III. J Phys Chem Ref Data 18:881–1097
Tsang W, Herron JT (1991) Chemical kinetic data base for propellant combustion I. Reactions involving NO, NO2, HNO, HNO2, HCN and N2O. J Phys Chem Ref Data 20:609–663
Estupinan EG, Nicovich JM, Wine PH (2001) A temperature-dependent kinetics study of the important stratospheric reaction O(3P) + NO2 → O2 + NO. J Phys Chem A 105:9697–9703
Hahn J, Luther K, Troe J (2000) Experimental and theoretical study of the temperature and pressure dependences of the recombination reactions O + NO2(+M) → NO3(+M) and NO2 + NO3(+M)→ N2O5(+M). Phys Chem Chem Phys 2:5098–5104
Atkinson R, Baulch DL, Cox RA et al (2004) Evaluated kinetic and photochemical data for atmospheric chemistry: volume I—gas phase reactions of Ox, HOx, NOx and SOx species. Atmos Chem Phys 4:1461–1738
Wennberg PO, Anderson JG, Wesenstein DK (1994) Kinetics of reactions of ground state nitrogen atoms (4S3/2) with NO and NO2. J Geophys Res 99:18839–18846
Penetrante BM, Hsiao MC, Merritt BT, Vogtlin GE (1997) Fundamental limits on NOx reduction by plasma. In: SAE technical papers series, International Spring Fuels & Lubricants Meeting, Deadborn, Michingan, 5–8 May 1997
Hu X, Zhang J-J, Mukhnahallipatna S et al (2003) Transformations and destruction of nitrogen oxides—NO, NO2 and N2O—in a pulsed corona discharge reactor. Fuel 82:1675–1684
Kozlov KV, Wagner HE, Brandenburg R, Michel P (2001) Spatio-temporally resolved spectroscopic diagnostics of the barrier discharge in air at atmospheric pressure. J Phys D Appl Phys 34:3164–3176
Erme K, Raud J, Jõgi I (2018) Adsorption of nitrogen oxides on TiO2 surface as a function of NO2 and N2O5 fraction in the gas phase. Langmuir 34:6338–6345. https://doi.org/10.1021/acs.langmuir.7b03864
Acknowledgements
The study was financed by Estonian Research Council Grant No. 585.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jõgi, I. The Effect of TiO2 Catalyst on the NO Reduction by Barrier Discharge in Nitrogen. Plasma Chem Plasma Process 39, 1191–1202 (2019). https://doi.org/10.1007/s11090-019-10001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10001-0