Abstract
A comparative study was carried out of the process of plasma chemical deposition of boron carbide from hydrogen plasma containing the mixtures of BF3 + CH4 and BCl3 + CH4 sustained by RF arc (13.56 MHz) discharge. It was shown that in the case of synthesis of B4C from a mixture of BF3 + CH4, carbon and complex coordination compound [X3B]−H+ (R3B·FH) are formed as the by-products of condensed products. In the case of synthesis of B4C from the BCl3 + CH4 mixture, the only condensed product is carbon. Mechanisms for the formation of boron carbide on the surface of heated electrodes are proposed. The main feature of these mechanisms is the preliminary deposition of a graphite layer from CH4 and then the precipitation of boron with the participation of the radicals BF2, BF and BCl. B4C samples were obtained and the impurity composition, morphology and structure of bulk boron carbide samples obtained using both of its halides were studied. It was found that in both cases a carbon phase is present in boron carbide samples. The main impurities entering the B4C, in the case of using a mixture of BF3 + CH4, is silicon, and in the case of a mixture of BCl3 + CH4, is tungsten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boron carbide is the compound combining such unique properties as high melting temperature, low density, high hardness, high thermal and chemical resistivity [1]. Boron carbide is the most suitable material for coating the walls of Tokamak type reactors [2,3,4]. An interesting aspect is the application of thin layers of boron carbide for fabrication of high-temperature operating diodes and transistors on the basis of «boron carbide/n-Si(111)/boron carbide/n-SiC» and some other structures [5,6,7,8,9].
High values of neutron capture cross-section due to boron-10 isotope about 20% of which is contained in natural boron being equal to 3850 barns for thermal neutrons and several barns for fast neutrons deserve mentioning as a separate point. As a result of this capture with probability of 94% the following reaction takes place
being the basis of application of boron carbide B4C both of natural composition and of 10B enriched for different purposes. First of all these are the systems for regulation of neutron fluxes in nuclear reactors. Recently it became actual to use boron carbide (including enriched 10B) in the form of nano-particles in neutron capture therapy [10] and in the form of films for fabrication of neutron detectors instead of 3He [7, 9, 11].
The methods for preparation of crystalline boron carbide are described in literature in detail [1, 12]. An important group among these methods is the chemical deposition of volatile compounds of boron from the gas phase. In its turn, quite many papers are available where deposition from these compounds is enhanced not by heating the whole reactor (i.e., thermal heating) but by plasma when energy is delivered directly to all participants of reaction in the gas phase (PECVD method). Since the formation of reactive high-energy particles is due to collisions in gas, the substrate can have a relatively low temperature. That is why the films are formed at lower temperatures of substrate as compared with the traditional CVD method and in this sense the PECVD method is of greater virtue. The following initial compounds are used for preparation of boron carbide in the form of films by PECVD method using different types of discharge for sustaining plasma: BCl3 [13,14,15,16,17,18], BBr3 [19], B2H6 [20,21,22,23,24,25], B5H9 [26,27,28], B10H14 [5], B(CH3)3 [20, 29, 30], B(C2H5)3 [20, 29, 30], CH4 [13, 14], C2B10H12 [6,7,8, 29, 31]. Stoichiometry and structure of the formed layers of carbide greatly depend on the type of the used compounds and experimental conditions for deposition [12, 26]. To the best of our knowledge there are no papers devoted to preparation of compact crystalline boron carbide by PECVD method. As it was stated above, in the case of nuclear physical applications it is preferable to use carbide enriched with respect to 10B. It is expedient to use as the initial substances the volatile halogenides of boron—high-boiling chloride 10BCl3 usually enriched by rectification method and low-boiling fluoride 10BF3 enriched by centrifugal or ion-exchange method.
The goal of the present work was to investigate the process of preparation of compact forms of boron carbide of natural isotopic composition from the mixtures of BCl3 + CH4 and BF3 + CH4 in hydrogen plasma of RF arc discharge.
Experimental
Boron fluoride and boron chloride of natural isotopic composition with the content of the main substance not less than 99.9% were used in this work. Hydrogen of special purity grade was used as the plasma generating gas: the content of hydrogen—not less than 99.9999%, of oxygen—2·10−5 %, of argon—2·10−5 %, of nitrogen—8·10−5 %, of water vapors—1·10−4 % and methane with purity of 99.999% was a carbon-containing substance.
The used RF arc discharge (RFA) of high-pressure (P > 200 torr) in itself combines the arc discharge generated between two coaxially located electrodes and RF capacitive discharge. Not going into details of investigation of the physics of discharge of this type, we will just state some of its peculiarites.
The classic arc discharge of direct current is characterized by low voltages and high currents. In arc discharge the cathode is heated up to high temperature which leads to thermoelectron emission. High-pressure arc is one of the forms of low-temperature equilibrium plasma and is characterized by temperature equilibrium between electrons and gas [32]. In our case the discharge glows between tungsten electrodes in hydrogen atmosphere at pressure close to atmospheric pressure and represents an electric arc of alternating current with frequency of 13.56 MHz. Its active component is due to thermoelectron emission with subsequent high heating of the end parts of electrodes contacting with plasma. This temperature was calculated by technique [33] modeling thermal processes taking place in reaction chamber accounting for experimentally measured amount of power delivered to discharge area, distribution of temperature of reactor wall along its length, geometry of reactor and individual characteristics of substance. Under these experimental conditions the gas temperature in the central area of plasma and, consequently, at the end parts of electrodes contacting with this area was ~ 3500 К. According to thermodynamic calculations [34] this temperature is sufficient for dissociation of molecules BF3 or BCl3 with predominant formation of the particles of BF2 and BF in the first case and of BCl in the second case.
In our case the application of high-frequency electromagnetic field to electrodes leads to appearance of the reactive component of current or bias current in addition to the active component due to thermoelectron emission. As a result a part of power, delivered to discharge, is consumed on activation of molecules by electrons oscillating due to bias currents; thus, in general the concentration of active particles is increased as compared with the situation when only the process of thermal ionization (discharge of direct current) takes place.
Therefore, the main advantage of the used RF discharge is realization of the experimental conditions under which the chemical activation of gas molecules takes place both thermally and under the effect of oscillations of electrons in alternating electric field.
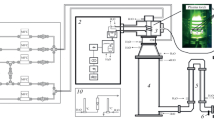
Experiments were conducted on experimental set-up shown in Fig. 1 with the total pressure in reactor of 760 torr. The operating frequency was 13.56 MHz. Power input to the plasma discharge zone was calculated as a difference in the power of respectively reflected and incident waves, measured by directional coupler with directive gain of 30 dB and an oscilloscope, and was equal to 350 ± 30 W. Consumption of plasma forming gas H2 as well as of gas-reagents BF3, BCl3 and CH4 during the experiment was controlled by flow-controllers regulators and was maintained constant with H2—350 ± 5 cm3/min.; BCl3—60 ± 5 cm3/min.; BF3—60 ± 5 cm3/min.; CH4 - 40 ± 5 cm3/min. Mole ratio H2/BCl3/CH4 and H2/BF3/CH4 was kept constant equal to 9/1, 5/1. Plasma chemical reactor consisted of silica glass tube (diameter 60 mm, length 200 mm) inside which the tungsten electrodes (diameter 4 mm, length 50 mm) were located equipped with special nozzles (diameter 10 mm, length 80 mm) for formation of gas flow connected with RF generator via matching device. The inter-electrode gap was 15 mm. Special nozzles are cylindrical hollow tubes from stainless steel with inserted electrodes. Gas mixture was delivered to discharge area via these nozzles. The total degree of conversion of fluorides was determined by analysis of IR spectra of exhaust gases with accuracy of 3 mol% (Bruker Vertex 80v spectrometer). X-ray analysis of the prepared samples was conducted on XRD-7000 diffractometer. Morphological investigation of the prepared samples was carried out by the methods of scanning electron microscopy. To obtain the images, the scanning electron microscopes SUPRA 50VP and NEON 40 (Carl Zeiss, Germany) were used. The diagnostics of elemental composition of samples was conducted by the method of X-ray microanalysis using the energy dispersive spectrometer of Oxford Instruments Company. The impurity composition of samples was determined on laser mass-spectrometer EMAL-2. NMR investigations were carried out on Bruker Avance III. The operating frequency H-1 400 MHz, B-11 128.4 MHz (15% F3B·OEt2 standard), F-19 376.5 MHz (CCl3F standard). NMR spectra B-11 and F-19 were recorded suppressing splitting on protons. The spectra were recorded at room temperature (25 °C).
Results and Discussion
Main Chemical Reactions Resulting in Preparation of Boron Carbide
Structurally related molecules of boron halogenides are noticeably different from each other with respect to strength of B–X bond. For instance, the enthalpy of abstraction reaction of one atom of halogen from BX3 molecule is equal to 180 kcal/mole for X = F, 129 kcal/mole for X = Cl [13] which should manifest itself in different reaction behavior under the same conditions. Unfortunately, we did not have any methods for diagnostics of the investigated high-reaction plasma sustained by arc RF discharge and thus these distinctions could be found by studying the products of plasma chemical transformations.
In the case of H2 + BF3 + CH4 mixture the boron carbide is the main product deposited on the surface of electrodes in the form of bulk crystals. Besides, a highly dispersed carbon black is deposited on the walls of plasma chemical reactor and oily liquid is condensed.
An attempt was taken to study its composition by the methods of IR and NMR spectroscopy. IR spectrum (Fig. 2) comprises a number of bands in the range of 700–200 cm−1 with the most intensive band at 1000 cm−1 which is referred to B-C vibrations. A number of other weaker bands can be assigned to vibrations of B–F, C–H and B–C bonds.
A broad structureless band with the maximum at 3400–3500 cm−1 probably represents the superposition of oscillation bands of different functional groups. Most likely these are hydroxyl groups which can indicate a partial hydrolysis of this product of plasma chemical reaction. Additional information on its chemical nature is given by NMR spectra recorded on nuclei 1H, 11B, 19F (Fig. 3). In spectra on Fig. 3a, b three lines are registered with two of them being mostly intensive which indicates different chemical environment of the nuclei of boron-11 and fluorine-19. The lines in spectrum of Fig. 5a for chemical shifts 0.33 and -1.33 ppm are close in their position to F3B·OEt2 standard generally accepted while investigating NMR spectra of boron compounds.
To our opinion it indicates that the formed by-pass product probably also refers to coordination compound of boron (of one or several types). In view of IR spectrum it can also be assumed that it comprises alkyl groups and/or hydroxyl groups. A broad band with the maximum at 11 ppm in the spectrum of proton resonance (Fig. 3c) is characteristic of exchange processes of proton with different particles and indicates the acid nature of the latter. Thus, the probable chemical forms of the found liquid product of plasma chemical reaction in BF3 + CH4 + H2 system can be [R3BF]−H+, [(OH)3BF]−H+ or complexes of intermediate composition. On the other hand, the IR spectrum of gaseous products of plasma chemical reaction with participation of boron fluoride (Fig. 4) does not comprise the band of HF at 3900–4000 cm−1 which presence is characteristic of plasma chemical hydrogen reduction of other volatile fluorides. This fact makes it possible to assume that the composition of studied liquid comprises the coordination compounds with participation of HF molecules, e.g., R3B·FH or R2B(HO)·HF. A complicated nature of NMR spectra indicates that there can be several structures of this type and of above-mentioned type.
Coming back to IR spectra in Fig. 4, we should note that the gaseous products of plasma chemical reaction include ethylene and acetylene formed as a result of conversion of methane and silicon tetrafluoride as a product of interaction of HF with silica walls of reactor. Thus, the total scheme of plasma chemical transformation in the system on the basis of boron fluoride can be represented to our opinion in the following way:

The plasma chemical reaction in BCl3 + CH4 + H2 system, in view of the composition of its products, occurs in a different way. Apart from deposition of compact boron carbide on electrodes, the residue of powdered carbide and soot is observed on the walls of reactor. None of other condensed products (similar to above-mentioned oily liquid) was detected. It is known that boron chloride is less favorable to the formation of strong coordination compounds as compared with fluoride [35] and, obviously, that is why in the IR spectrum of gaseous exhaust gases (Fig. 4b) the band was found referred to HCl. As in the first case, the bands of ethylene and acetylene are due to plasma chemical conversion of methane. Thus, the scheme of the process looks like the following:
Growth Mechanism of Boron Carbide
The main target product of plasma chemical reactions of boron fluoride and boron chloride with methane is compact polycrystalline boron carbide deposited on electrodes. However, as it follows from the above-mentioned discussion and as it is seen from Fig. 5, the processes of crystal growth in both cases are somewhat different. Carbide is deposited from the mixture based on boron fluoride in the form of polycrystals of cylindrical shape on the ends of electrodes actually following their diameter (Fig. 5a-1, b-1). In the case of boron chloride the formation of carbide takes place on the end part of electrodes in the form of growing disc (Fig. 5a-2, b-2). This difference can be explained in the following way. Rod electrodes, located coaxially and oriented towards each other, provide the distribution of field lines as shown in Fig. 5a-1, a-2.
At the same time a higher density of electric field and, consequently, a larger energy release with the given geometry is provided in the direction of axis along which the electrodes are located. In this direction the channel is formed via which the main part of electric current is passing. Density of field lines, located on the periphery with respect to central axis, and, hence, electric field intensity in this range are less but sufficient for dissociation of BCl3 molecule. That is why the growth of boron carbide in the form of disc is observed in the direction of electric lines of force. Probably, due to this reason the deposition of powdered carbide on the walls of reactor takes place. For chemical activation of BF3 a higher energy is required available only in the local interelectrode space. That is why the growth of polycrystalline boron carbide is observed directed strictly along electrodes.
It should be stated that, in our opinion, the role of methane is not limited only by transport of carbon to the reaction zone since in reaction mixtures H2 + BF3 and H2 + BCl3, i.e., without methane, the deposition of elementary boron under the conditions of our discharge does not take place. Probably, the required condition for starting the growth of boron carbide is the initial deposition on electrodes of graphite layer in the form of dendrites on surfaces heated, as it was indicated previously, approximately up to 3500 K. The intensity of electric field and its gradient on the formed dendrites are sufficiently high for polarization of the molecules of BCl3 or BF3 which facilitate the deposition of boron layer. Then again the deposition of graphite is required. Taking into account the above-mentioned facts on the nature of particles dissociating in the studied plasma, the total process of deposition of bulk boron carbide on electrodes can be represented by the reactions shown on scheme of Fig. 6a, b.
As a result the content of carbide phase in the residue according to X-ray analysis is just about 60% which certainly degrades the quality of material. Perhaps, the situation can be improved be using other carbon-containing compounds instead of methane, e.g., freons less inclined to pyrolysis or by selection of other conditions for deposition.
Investigation of Morphology and Structure of the Samples of Boron Carbide
Figure 7 gives the elementary composition and morphology of B4C samples by X-ray fluorescent spectroscopy. Apart from the main components (boron 54.15 mass% and carbon 44.13 mass%) the sample, prepared from boron trichloride (Fig. 7a), comprises oxygen and iron with concentration of 1.62 and 0.1 mass%, respectively. The content of carbon different from stoichiometry indicates its presence in sample not only in the form of B4C compound but also in the form of free carbon. The presence of oxygen can be explained by adsorption from atmosphere and the entry of iron is possibly due to etching of structural materials by HCl formed during plasma-chemical process.
The morphology of this sample (Fig. 7c) proves its compact form despite the fact that a part of carbon, as it has been mentioned, is present in its free form and can be the reason for lamination. In X-ray fluorescence spectrum the band of boron in the sample of boron carbide (Fig. 7b), prepared from boron trifluoride, has substantially less intensity as compared with the carbon band and according to the spectrum the content of B4C and C in this sample is at the same level. The sample of boron carbide (Fig. 7d) has a layered structure. It can be assumed that the growth of B4C is of layer-to-layer nature. As it has been already mentioned, the required condition for growth is the preliminary deposition of graphite layer on electrode surface (Fig. 6). That is why the samples, grown under these conditions, should have a substantial amount of free carbon. The prepared sample is of porous nature which assists the increased adsorption of oxygen from atmosphere (the content of oxygen is 4.13 mass% against 1.62% in the first sample).
X-ray phase analysis of both samples, given in Fig. 8, proves the presence of the carbide phase of B4C. In B4C sample, prepared with the use of boron trichloride (Fig. 8a), apart from the phases of boron carbide and free carbon the phase of W2B5 is also registered. It can be assumed that the entry of W into sample takes place due to etching of tungsten electrode by HCl. B4C sample, prepared with the use of boron trifluoride (Fig. 8b), has intensive bands connected with the phase of free boron and boron carbide which is in agreement with the data of X-ray fluorescence analysis.
Table 1 shows the impurity composition of both B4C samples according to the data of laser mass spectrometry. The increased content of the impurities of oxygen, fluorine, silicon, iron and tungsten should be noted. The presence of iron and tungsten at rather high level in B4C sample, prepared from boron trichloride, is due to erosion of tungsten electrode and nozzle made from stainless steel under the effect of HCl. Since hydrogen chloride does not react with silica walls of reactor, silicon impurities do not enter this sample of boron carbide. On the contrary, in B4C sample, prepared with the use of boron trifluoride, the etching of silica takes place
proved both by appearance of the band of impurity SiF4 in IR spectrum of exhaust gases (Fig. 4a) and by increased content of silicon in corresponding sample of carbide.
Conclusion
Bulk samples of polycrystalline boron carbide were prepared in plasma sustained by arc RF discharge by PECVD method with the use of methane, boron fluoride and boron chloride as the initial compounds. The main gas-phase and solid phase products during synthesis of carbide are found and the chemical reactions, responsible for their formation, are considered. Assumptions are made on the growth mechanism of polycrystalline boron carbide on the surface of electrodes. Morphology and phase composition of carbide, prepared with the use of different initial substances, are investigated. It is found that in both cases the samples of boron carbide comprise the carbon phase. On the basis of analysis of the obtained experimental data the further ways of investigation can be proposed with the goal of their optimization and preparation of B4C of higher quality.
References
Thevenot F (1990) J Eur Ceram Soc 6:205–225
Buzhinskij OI, Semenets YM (1999) Fusion Eng Des 45:343–360
Verprek S (1990) Surf Coat Technol 43–44:154–166
Buzhinskij OJ, Optimach IV, Barsuk VA, Otrozhenko VG et al (1995) J Nucl Mater 22–222:922–925
Hwang S, Byun D, Ianno NJ, Dowben PA (1996) Appl Phys Lett 68:1495–1497
Adenwalla S, Welsh P, Harken A, Brand JI, Sezer A, Robertson BW (2001) Appl Phys Lett 79:4357–4359
Robertson BW, Adenwalla S, Harken A, Welsh P, Brand JI, Dowben PA (2002) Appl Phys Lett 80(19):3644–3646
Byun D, Hwang S, Dowben PA, Perkins FK, Filips F, Ianno NJ (1994) Appl Phys Lett 64:1968–1970
Emin D, Aselage TL (2005) J Appl Phys 97:013529–013535
Mortensen MW, Sorensen PG, Bjoerkdahl O, Jensen MR, Gundersen HJG, Bjornholm T (2006) Appl Radiat Isot 64:315–324
Hoeglund C, Birch J, Anderson K, Bigault T et al (2012) J Appl Phys 111:104908-1–104908-8
Sezer AO, Brand JI (2001) Mater Sci Eng B79:191–202
Lee KW, Harris SJ (1998) Diamond Relat Mater 7:1539–1543
Lee S, Dowben PA (1994) Appl Phys A 58:223–227
Mackinnon IM, Reuben BG (1975) J Electrochem Soc 122:806–811
Postel O, Heberlein J (1998) Surf Coat Technol 108–109:247–252
Person JF, Czerwiec T, Belmonte T, Michel H (1997) Surf Coat Technol 97:749–754
Eroglu OD, Sezgi NA, Oezbelge H, Durmazucar HH (2003) Chem Eng Commun 190:360–372
Cholet V, Herbin R, Vandenbulcke L (1990) Thin Solid Films 188:143–155
Winter J, Esser HG, Reimer H, Grobusch L, Von Seggern J, Wienhold P (1990) J Nucl Mater 176–177:486–489
Fugimori N, Imai T, Doi A (1986) Vacuum 36:99–102
Komatsu S, Moriyoshi Y (1989) J Appl Phys 66:1180–1184
Dimmey LJ, Park H, Jones PL, Cocks FH (1981) J Elektron Mater 10:111–117
Cocks FH, Jones PL, Dimmey LJ (1980) Appl Phys Lett 36:970–972
Lin SH, Li D, Feldman J (1995) Mater Res Soc Symp Proc 383:127–132
Lee S, Mazurowski J, Ramseyer G, Dowben PA (1992) J Appl Phys 72:4925–4933
Byun D, Spady BR, Lanno NJ, Dowben PA (1995) Nanostruct Mater 5:465–471
Mazurowski J, Lee S, Ramseyer G, Dowben PA (1992) Mat Res Soc Symp Proc 242:637–642
Alimov VK, Bogomolov DB, Churaeva MN, Gorodetsky AE, Kanashenko SL et al (1992) J Nucl Mater 196–198:670–675
Sharapov VM, Kanaev AI, Zakharov AP, Gorodetsky AE (1992) J Nucl Mater 191–194:508–511
Byun D, Spady BR, Lanno NJ, Dowben PA (1995) Nanostruct Mater 5:465–471
Raizer YuP (1987) Fizika gazovogo razryada. Nauka, Moscow (in Russian
Kornev RA, Shaposhnikov VA, Kuz’min AM (2014) Russ J Appl Chem 87(9):1246–1250
Nester SA, Potapkin BV, Levitskii AA, Rusanov VD, Trusov BG, Fridman AA (1988) Kinetiko-staticheskoe modelirovanie khimicheskikh reaktsii v gazovom razryade. TsNII Atominform, Moscow (in Russian)
Mikailov BM (1967) Khimiya borovodorodov. Nauka, Moscow (in Russian)
Acknowledgements
Authors express their gratitude to Prof. Yu. Kurski (G.A. Rasuvaev Institute of Organometallic Chemistry of RASA) and to Dr. S.A. Gusev and P.A. Yunin (Institute for Physics of Microstructures of RAS) for registration and help in interpretation of NMR and X-ray spectra. This work was financially supported by Russian Scientific Foundation Grant No 17-13-01027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sennikov, P.G., Kornev, R.A. & Shishkin, A.I. Preparation of Boron Carbide from BF3 and BCl3 in Hydrogen Plasma of Arc RF Discharge. Plasma Chem Plasma Process 37, 997–1008 (2017). https://doi.org/10.1007/s11090-017-9821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9821-y