Abstract
The experimental study of the degradation of gaseous dielectrics after processing in the dielectric barrier discharge (DBD) is presented. Two pure gases trans-CF3CH=CHF (HFO-1234ze(E)), perfluoroketone CF3C(O)CF(CF3)2 (C5K), and also the following mixtures 75 %HFO-1234ze(E):25 %N2, 12 %C5K:88 %N2, 18.5 %C5K:81.5 %dry air, 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2, 9 %C5K:56 %HFO-1234ze(E):35 %CO2 have been used as test-gases. A content of the decomposition products of the gases before and after a 5-h workout in the barrier discharge has been determined by means of the chromatography-mass spectrometry and gas chromatography methods. Dilution of C5K with dry air greatly increases the degree of conversion of the source gas in the barrier discharge. Dilution of HFO-1234ze(E) and C5K with nitrogen, and the use of ternary mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 significantly reduces the degree of conversion of the mixture compared with the source gases in the barrier discharge. After the DBD processing of two test-gases a large quantity of toxic C3F6 was found in pure C5K, and also a large number of highly toxic CF3CCH was found in pure HFO-1234ze(E). The least amount of toxic products after the DBD processing was detected in mixtures HFO-1234ze(E):N2 and C5K:HFO-1234ze(E):N2. The mixture C5K:HFO-1234ze(E):N2 has the best features among studied mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently industrial companies in all types of electrical equipment working under high and very high voltage (circuit breakers, transformers, substations, transmission lines, etc.) most frequently use sulfur hexafluoride (SF6) owing to its very good properties of insulation and cutoff. SF6 has unique characteristics such as relatively low toxicity, extreme inertness and high dielectrics [1]. However, the partial discharges (PD) may appear during the work process of high voltage electrical devices. The partial discharge can be treated as some kind of the corona discharge [2]. The presence of partial discharges tends to occur due to increased stressing in an inhomogeneous electric field region around a faulty component or an abnormality in the insulation material [3]. The PD can be the symptom of a fault or a degradation mechanism in itself [4]. The appearance of PD is accompanied by a large number of physico-chemical processes leading to gradual degradation of SF6 and the formation of decomposition products, most of which are toxic (e.g. S2F10, SF4, and HF) [1]. It should be noted, that decomposition products, produced by electrical and thermal decomposition of SF6 in the presence of other molecules (e.g. H2O, N2, O2, air, Ar, silicon) are all toxic and even corrosive [5–14]. In addition sulfur hexafluoride is one of the most potent greenhouse gases that cause significantly global warming, and has been blanketed into the Kyoto Protocol.
Therefore, there is a need for a gaseous candidate, i.e. gas or mixture of gases, that can replace SF6 in high voltage electrical equipment. The gas mixtures must have acceptable combination of low temperature boiling point, chemical stability, low global warming potential (GWP), non-flammability and high dielectric strength. In addition the gas mixtures must have relatively low toxicity. All these characteristics should be comparable or better than those of SF6.
Luly et al. [15] have found that mixtures SF6 and certain hydrofluoroolefins, such as tetrafluoropropene and pentafluoropropene, produce a synergetic effect with respect to the composition’s dielectric strength, are essentially non-flammable, have relatively low GWP, have good chemical stability and have normal boiling points comparable to common refrigerants. This should take into account that some classes of chemicals, such as chlorofluorocarbons, hydrochlorocarbons, hydrochlorofluorocarbons, hydrofluorocarbons, perfluorocarbons destroy the ozone layer of the stratosphere of the Earth, and their use is also regulated by international agreements [16]. Thus, when choosing a perspective gaseous dielectric, one should pay attention to its dielectric properties, chemical toxicity of the source gas and its decomposition products, environmental properties (low values of GWP100 and of ozone depletion potential).
In this work we study recently appeared gases hydrofluoroolefin 1,3,3,3-tetrafluoropropene HFO-1234ze(E) and perfluoro-(3-methylbutan-2-on) CF3C(O)CF(CF3)2 [15, 17, 18], its mixtures with N2, and also mixtures of perfluoro-(3-methylbutan-2-on) with dry air, HFO-1234ze(E):C5K:N2 and HFO-1234ze(E):C5K:CO2 as possible compositions, which are capable to replace SF6 in high voltage electrical equipment. Now HFO-1234ze(E) and C5K are commercially available. However, pure HFO-1234ze(E) and C5K have a high boiling point: −19 and +24 °C, respectively. This is at low ambient temperatures may result in undesirable formation of condensate in the high voltage electrical equipment during its exploitation. The addition of nitrogen, dry air or carbon dioxide to HFO-1234ze(E) and C5K reduces the boiling points of the appropriate mixtures. In addition, we use hypothesis that nitrogen, dry air or CO2 in diluted C5K or HFO-1234ze(E) can inhibit the formation of some decomposition products.
To accelerate tests we use the dielectric barrier discharge instead of the corona discharge. Barrier discharges as well as corona discharges, belongs to streamer discharges [19–25]. Chemical processes accompanying propagation of a streamer through the gas are the same for the corona and barrier discharges [26–29]. The number of streamers incident on the unit area of the electrode surface grows with increase of a voltage [22], so the voltage applied to the electrodes has been varied during the tests. At atmospheric pressure the DBDs in test-gases have filamentary form. Current filaments represent the individual microdischarges chaotically occurring over all volume of the gas gap only during certain phases of a voltage period. The breakdown of a gas gap in microdischarges is carried out due to the streamer mechanism. A typical streamer has a lateral spatial extension of about 0.1 mm and duration of the order of nanoseconds depending on the device configuration and type of gas [19–22]. The charge transported by a single streamer is of the order of 0.1 nC for a 1 mm air gap, increasing with gap spacing and specific capacitance of the dielectric barrier and, does not seem to depend on the applied voltage. Increase in power leads to generation of greater number of microdischarges per unit of a time and/or per unit of an electrode surface area. The minute energy dissipation limits the rise of the gas temperature due to a single microdischarge to a few Kelvin, while the electrons in the microdischarge channel have mean energies of a few eV. In the microdischarge channel, electron densities of 1014 cm−3 and current densities of j = 1000 A cm−2 are reached [19–22]. Plasma properties in a head of streamer result in formation a large number of active species: ions, radicals, excited atoms and molecules, which have high chemical reactivity. So, the dielectric barrier discharges are used for various chemical processes such as decomposition of gaseous pollutants, water treatment, ozone production, surface treatment, plasma-assisted ignition and combustion, biological application and medicine [37, 38].
In this work we determined a relative conversion degree of tested gases and mixtures. We discovered also some toxic compounds among the decomposition products. Among decomposition products of the HFO-1234ze(E) and C5K the carbon and fluorine containing gases can be formed, which may lead to deposition of films at the internal surfaces of the high voltage equipment. A CHNS analysis on the content of films deposited at the inner surface of the discharge tube during test has been carried out.
Experimental Technique
The working chamber for ignition and maintenance of the barrier discharge is arranged as follows. A solid cylindrical electrode made of stainless steel with a diameter of D el = 13.5 mm and length L = 350 mm is placed inside a cylindrical glass tube with an inner diameter d g = 15.9 mm and an external diameter D g = 19 mm. The outer electrode is made of fine brass mesh. The thickness of the discharge gap between the inner electrode and the walls of the glass tube was 1.2 mm. There were special pipe-bends, mounted at the ends of the glass tube, which connected to the pump, gas inlet and pressure sensor.
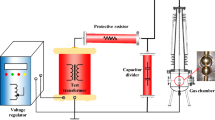
Connection of the DBD chamber to the electrical circuit is shown in Fig. 1. The discharge tube is connected in series with a measuring capacity C I . Capacity C I (180 nF) is chosen much larger than the full capacity of the electrode system of the discharge chamber (96 pF), so the voltage U I on the capacitor C I is negligibly small compared with the voltage drop across the discharge tube [30]. The high voltage electrode is switched to a high voltage winding of the power transformer during the whole processing of the test-gas. The voltage on the capacitor C I is proportional to the electric charge and is recorded on the input 1 of the oscilloscope GW Instek GDS-71102. The DBD voltage is measured using a capacitive voltage divider C1–C2 (33 nF–33 pF). The signal is taken from the midpoint of the divider and fed to the input 2 of the oscilloscope. The area enclosed by the charge–voltage Lissajous figure obtained in each of the tests is proportional to the energy dissipated in the DBD-reactor per cycle of the AC-voltage applied [30, 31]. The product of energy per cycle with the frequency of AC-voltage results in the active power supplied to the DBD-reactor.
Processing each of the tested gases was carried out for 5 h at three input voltages: 2, 6 and 10 kV. The frequency of the applied voltage was 50 Hz. During the tests the DBD set-up was at room temperature, no heating of the working chamber was observed in the process.
The volume between the inner electrode and the walls of the glass tube was pumped out to a pressure of ~10−3 bar and then filled with the test gas up to the atmospheric pressure. DBD processing of the test gas was carried out in a closed volume. The volume of gas in the discharge chamber was ~20 cm3. A sampling of the processed gas was taken from the working chamber after each DBD processing. Before taking a sample of a test gas the working chamber was filled with helium up to 1 bar of excess pressure. The mixture was left for 10 min and then sampled through the membrane into a special package for sampling or in the syringe. Tedlar bags and gas-tight cyringes Hamilton have been used in this procedure.
Two pure gases HFO-1234ze(E), perfluoroketone C5K, and the mixtures 75 %HFO-1234ze(E):25 %N2, 12 %C5K:88 %N2, 18.5 %C5K:81.5 %dry air, 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 have been used as test-gases. The gas mixture composition is specified in volume percents. The relative shares of all components in each of the gas mixtures were chosen experimentally so that the boiling point of the mixture was obviously lower than −25 °C at a pressure of mixture of 1 bar.
The average values of the power absorbed in the barrier discharge in the studied gases and gas mixtures are shown in Table 1. The power absorbed in DBD increases proportionally to the amplitude of the applied voltage.
Chemical Analysis Methods
The gas chromatographic (GC) analysis has been carried out by means of the gas chromatograph Chrompack CP9000 (Chrompack B.V., Middelburg, The Netherlands), equipped by flame-ionizing detector (FID), and thermal conductivity detector (TCD). Separation of gas mixtures was made by means of bonded PLOT column PoraBOND Q 25 m × 0.53 mm × 10μm (with FID detector) and packed column HayeSep Q 80/100 3 m × 2 mm (with TCD detector). Volumes of the inserted sample were 5 μl for the PLOT column PoraBOND Q and 500 μl for packed column HayeSep Q. Component content of a source gases and mixtures have been made using the column HayeSep Q with TCD detector. For CO detection the column CarboSieve S-II 80/100 3 m × 2 mm was used instead of the HayeSep Q column.
The identification of the gas components has been carried out by the chromatography-mass spectrometry by means of multi-dimensional GC/GC/MS system (Shimadzu QP2010 Ultra Shimadzu, Tokyo, Japan). The GC/GC/MS system contains two thermostats with analytical columns of different polarities. The system employs a pressure switching heart-cut device (Multi-Deans Switch) to direct the effluent of the first column on to a second column. In the first thermostat there were the column PoraPLOT Q 30 m × 0.32 mm (or non-polar column WCOT SBP-1, 60 m × 0.25 mm × 0.25 μm for the separation source mixtures) and FID; in the second one there was an analytical polar column Supercowax-10 30 m × 0.25 mm × 0.5μm with access to mass spectrometric detector with a quadrupole mass filter. The sample is sequentially divided in columns PoraPLOT Q and Supercowax-10 and detected by the mass spectrometer. In the mass spectrometer a beam of electrons with energies ε = 70 eV was used for ionization of the sample. In determining the composition of the source samples before the DBD processing the volume of injected sample was 5 μl, split mode 1:60. In determining the composition of the gas samples after the DBD processing the volume of injected sample was 10 μl, split mode 1:6. In identification of products of the analyzed samples by means of the mass spectra we used the spectra comparison with the NIST 08, NIST 08s and Wiley 9 libraries.
The sample is injected into chromatograph using a Hamilton syringe immediately after sampling from the working chamber.
The spectral measurements have been performed by means of vacuum FT-IR spectrometer Vertex 70v with InSb detector and spectral resolution 0.5 cm−1. The spectral interval was 2000–10,000 cm−1. For absorption lines of HF the HITRAN free database has been used (http://hitran.iao.ru/molecule).
Results
Before processing in the barrier discharge each of the source gases was studied by means of gas chromatography-mass spectrometry (GC/MS) analysis. The source trans-CF3CH=CHF had a small portion (~0.04 wt%) of its isomer cis-CF3CH=CHF (HFO-1234ze(Z)), other gases less than 0.002 wt%. The source perfluoroketone CF3C(O)CF(CF3)2 (~98.65 wt%) had a portion of ester CF3COOCH3 (~1.3 wt%) and a small amount of the water vapor H2O (~0.05 wt%).
On the Conversion Degree
Figure 2 shows the concentration of the source gases/mixtures in the mixture of their decomposition products after the DBD processing (in percentage to their initial concentration in the source gases/mixtures, excluding the gas diluent).
The concentration of the source gases in their decomposition products after the 5 h of the DBD processing depending on the applied voltage (in percentage to their initial concentration prior to the DBD processing). 1 C5K in mixture in decomposition products of 18.5 % C5K:81.5 % dry air; 2 HFO-1234ze(E) in decomposition products of pure HFO-1234ze(E); 3 C5K in decomposition products of pure C5K; 4 C5K in decomposition products of mixture 12 %C5K:88 %N2; 5 total concentration of C5K, HFO-1234ze(E) and CO2 in decomposition products of mixture 9 %C5K:56 %HFO-1234ze(E):35 %CO2; 6 total concentration of C5K and HFO-1234ze(E) in decomposition products of mixture 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2; 7 HFO-1234ze(E) in decomposition products of mixture 75 %HFO-1234ze(E):25 %N2. All concentrations are given in volume percents
It is seen from the figure that after the DBD processing of pure HFO-1234ze(E), C5K and of the mixture 18.5 %C5K:81.5 %dry air with values of the applied voltage of 6 and 10 kV the percentages of the source gases in their mixtures of products are markedly reduced in comparison with 2 kV. Fall of the percentage of source gases in decomposition products of mixtures 12 %C5K:88 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 is less. The minimal decrease of the percentage of the source products is observed in mixtures 75 %HFO-1234ze(E):25 %N2 and 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2. As can be seen, the dilution of HFO-1234ze(E) and C5K by nitrogen, as well as the use of the ternary mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 significantly reduces the degree of conversion of the source gas in the barrier discharge (during propagation of streamers in the gas). Pure gases HFO-1234ze(E) and C5K show relatively low resistance to the electrical discharge. On the contrary, dilution of C5K by the dry air significantly increases the degree of conversion of the source gas.
Note that the number of microdischarges per half cycle of the applied voltage grows with the increase of the discharge power, i.e. the power absorbed in DBD. It seems reasonable to assume a correlation between the discharge power and the dielectric strength of the gas: the higher dielectric strength of the gas, the less power would be absorbed in the gas at a given applied voltage. One would also assume that the degree of conversion increases with increasing the discharge power. For each gas it really is. However, comparing the discharge power in different gases and gas mixtures (Table 1) and the degree of their conversion at given applied voltages (Fig. 2), no clear correlation between the discharge power and the degree of conversion of gas has been found.
Decomposition Products of HFO-1234ze(E) and Mixture 75 %HFO-1234ze(E):25 %N2 After the DBD Processing
After the DBD training of HFO-1234ze(E) we found the following degradation products: H2, CO, CO2, CF4, C2F6, C2F4, CF2=CH2, C2HF3, CF3CH2CF3, C3F8, CF3CH3, C2HF5, CF3CH2F, CHF2CHF2, hexafluoropropene C3F6, CF3C≡CH, CF3CF=CH2, cis-CF3CH=CHF. Main gas decomposition products of HFO-1234ze(E) are the isomer of the source olefin cis-CF3CH=CHF, trifluoromethylacetylene CF3C≡CH, as well as perfluoroalkanes CF4 and C2F6. The concentration of the majority of decomposition products of HFO-1234ze(E) in the barrier discharge increases with growth of the applied voltage. H2, CO and CO2 in the products of decomposition appear only at a voltage of 10 kV. The highest share in the products represents cis-CF3CH=CHF (HFO-1234ze(Z)), its concentration after the DBD processing of HFO-1234ze(E) with a voltage of 10 kV is 2.85 wt%. Concentrations of other products are in the range 0.04–0.90 wt%.
As an example Fig. 3 demonstrates chromatograms of decomposition products of HFO-1234ze(E) and mixture 75 %HFO-1234ze(E):25 %N2 trained in DBD at 6 kV with PoraBOND Q/FID detection.
The figure shows that trained mixture of 75 % of HFO-1234ze(E):25 %N2 contains fewer decomposition products than pure HFO-1234ze(E) trained in DBD. Dilution of HFO-1234ze(E) by nitrogen leads to the decrease of the degradation of HFO-1234ze(E) (see Fig. 2), reduction of the number hydrogen-containing products and the appearance of small quantities of new products: C5F12, C6F14.
The GC/TCD analysis has shown that the main gas-phase decomposition products of the mixture 75 %HFO-1234ze(E):25 %N2 were CO and CO2. Among the decomposition products of mixture 75 %HFO-1234ze(E):25 %N2 we also found cis-CHF=CH-CF3 (HFO-1234ze(Z)), CF4, C3F8, CF3CHFCF3, CF3C≡CH, CH2=CF–CF3 (HFO-1234yf), CF2=CF–CHF2.
Main channels of the pure HFO-1234ze(E) conversion in DBD are the isomerization into cis-CF3CH=CHF and 1,2-dehydrofluorination with formation of 3,3,3-trifluoromethylacetylene and HF:
In reaction (2) both 3,3,3-trifluoropropyne CF3C≡CH and HF molecules are generated. But the IR spectrometry has found no traces of HF in the products of decomposition of HFO-1234ze(E) and mixture 75 %HFO-1234ze(E):25 %N2. Most likely HF reacted with the glass walls of the discharge tube in the process of the DBD processing. Direct chromatographic analysis of HF is a difficult task due to the high reactivity of HF.
Decomposition Products of Perfluoro-(3-Methylbutan-2-on) (C5K) and Mixtures C5K:N2, C5K:Dry Air After the DBD Processing
Concentrations of the decomposition products of pure C5K and mixtures 12 %C5K:88 %N2, 18.5 %C5K:81.5 % dry air after the DBD processing at U0 = 10 kV are shown in Table 2. According to results of the analysis the main decomposition products of pure C5K in the barrier discharge are hexafluoropropene C3F6, 1,1,2,3,3-pentafluoropropene-1 CF2=CF–CHF2, perfluorobutane C4F10 and perfluoromethylacetate CF3COOCF3. We identified also perfluoro compounds C1–C6, the yield of which grows considerably with changing of the applied voltage from 6 to 10 kV. At a voltage of 10 kV, the number of products grows substantially, the destruction processes lead to the formation of perfluoroalkanes CF4, C2F6, C3F8, heptofluoropropane CF3CHFCF3, perfluoropentane C5F12 and perfluorohexane C6F14.
As can be seen from Table 2, dilution of C5K both by nitrogen and dry air leads to a drastic reduction in the number of decomposition products of the source gas trained in the barrier discharge. However, great amounts of CO2 (11.48 wt%) and CF2=CF–CHF2 (9.08 wt%) are produced in a mixture C5K:dry air. As a result, the dilution of C5K by dry air strongly rises the conversion degree of the source gas. When C5K diluted by nitrogen, the conversion degree of the source gas is significantly reduced. Products hexafluoropropene C3F6 and perfluorobutane C4F10 are more likely formed in the trained mixture of C5K with dry air, though perfluoropentane C5F12 and perfluorohexane C6F14 are formed in the trained mixture of C5K with N2.
Figure 4 shows the effect of voltage on the concentration of decomposition products of a mixture 12 %C5K:88 %N2 after the DBD processing. One sees that concentrations of CO, CO2, C5F12, C6F14, CF2=CF–CHF2 grow, concentrations of C2F4, C4F10 fall with increasing voltage and concentrations of C3F6, CH2=CF–CF3, trans-CHF=CH-CF3 (HFO-1234ze(E)) first grow and then fall to almost zero.
Decomposition Products of Mixtures C5K:HFO-1234ze(E):N2 and C5K:HFO-1234ze(E):CO2 After the DBD Processing
After the DBD processing of mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 it was found a large number of the same products: CO, CO2, CF4, C2F6, C3F8, C3F6, C5F12, C6F14, CF3CCH, CF3CHFCF3, CH2=CF–CF3 (HFO-1234yf), cis-CHF=CH-CF3 (HFO-1234ze(Z)). Among the decomposition products of trained mixture 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 we also found C2F4, which was not detected in the decomposition products of trained mixture 9 %C5K:56 %HFO-1234ze(E):35 %CO2. The concentration CO after the DBD processing of the mixture with CO2 is an order of magnitude higher than in the mixture with N2 after similar processing. The degree of conversion in the barrier discharge of mixture C5K:HFO-1234ze(E):CO2 is higher than in mixture C5K:HFO-1234ze(E):N2 (see Fig. 2). The increase of the applied voltage in the barrier discharge leads to growth of concentrations of some decomposition products and reduction of the concentrations of the others.
Figure 5 illustrates the effect of the applied voltage (contributed power) on the concentration of decomposition products of mixture 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 after the DBD processing.
Toxic Products
Different quantities of the toxic products have been found in decomposition products of all tested gases and gas mixtures. CF4 as well as fluoro-olefins: tetrafluoretylene C2F4 and hexafluoropropylene C3F6 can be regarded as moderate or slightly toxic decomposition products of studied gases and gas mixtures trained in the barrier discharge [33, 34]. These compounds are irritating to the respiratory tract and lungs in lethal concentrations as judged by animal exposures, but in addition they also can cause kidney injury. Carbon monoxide CO relates to highly toxic decomposition products [35].
Figures 6 and 7 show the concentrations of these toxic decomposition products of the tested gases and gas mixtures after the DBD processing depending on the voltage applied to the electrodes of the reactor. Figure 7 also shows the concentration of CO2.
Concentrations of toxic products CF4 (a), C2F4 (b), C3F6 (c), CF3C≡CH (d) found in decomposition products of studied gases and gas mixtures (1–7) after the DBD processing as functions of the applied voltage. 1 HFO-1234ze(E); 2 75 %HFO-1234ze(E):25 %N2; 3 C5K; 4 C5K:88 %N2; 5 18.5 %C5K:81.5 %dry air; 6 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2; 7 9 %C5K:56 %HFO-1234ze(E):35 %CO2
Concentrations of CO2 (a) and CO (b) in decomposition products of studied gases and gas mixtures (1–7) after the DBD processing as functions of the applied voltage. Gases and gas mixtures 1–7 are the same, as in Fig. 6
C3F6 is detected in the decomposition products of all the test gases except for mixture 75 %HFO-1234ze(E):25 %N2. Its concentration grows with the voltage increase in the decomposition products of C5K, HFO-1234ze(E), C5K:dry air, weakly depends on the voltage in the decomposition products of mixtures C5K:N2, C5K:HFO-1234ze(E):CO2 and drops with increasing voltage in the decomposition products of mixture C5K:HFO-1234ze(E):N2. In decomposition products of pure C5K and mixture C5K:dry air, trained in DBD at 10 kV, the concentration of C3F6 reaches a tenth of a percent.
C2F4 is detected in the decomposition products of pure gases C5K, HFO-1234ze(E) and mixtures containing C5K. The most quantity of C2F4 is detected in the decomposition products of pure HFO-1234ze(E). CF4 is detected in the decomposition products of all the test-gases except the mixture 12 %C5K:88 %N2. CF3CCH is found in the decomposition products of the pure HFO-1234ze(E) and mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2, 9 %C5K:56 %HFO-1234ze(E):35 %CO2.
CO is found in varying amounts in decomposition products of all tested gases and gas mixtures. A significant amount of CO and CO2 (>1 %) appear in the decomposition products of mixtures C5K:dry air and C5K:N2 after the DBD processing. These two compounds are contained in smaller amounts in the decomposition products of pure C5K trained in DBD. Even less content of CO and CO2 has been revealed in the mixture of decomposition products of C5K:HFO-1234ze(E):N2. Significantly less CO and CO2 has been detected in the decomposition products of pure HFO-1234ze(E) and its mixtures with nitrogen than in the decomposition products of C5K and C5K:N2.
In the DBD tests with pure HFO-1234ze(E) and its mixtures with other gases the probability of formation of highly toxic HF is quite high [36]. Although HF was not detected in the decomposition products of these test gases trained in the barrier discharge (see Decomposition Products of HFO-1234ze(E) and Mixture 75 %HFO-1234ze(E):25 %N2 After the DBD Processing section), we can assume the initial formation of HF because of presence of 3,3,3-trifluoromethylacetylene, which accompanies HF in the reaction of 1,2-dehydrofluorination (2).
Film Deposition
After the DBD tests with some of the gas mixtures we have discovered segments of films, deposited at the inner surfaces of the glass tube. The film, found at the inner surfaces of the reactor after the DBD tests with a mixture 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2, was analyzed by the gas chromatography with advanced combustion of a sample film in the dynamic flash (CHNS analysis). The film contained ~9.0 % of nitrogen, ~21 % of carbon and ~2.2 % of hydrogen. After the DBD processing of pure HFO-1234ze(E) the composition of the film contained ~39 % of carbon and ~1.6 % of hydrogen. It is likely that fluorine is also a constituent of the films. A film deposition in the work process of the high voltage electrical equipment is undesirable, since films can change the characteristics of the device.
Monitoring the pressure in the working chamber during the DBD processing of the pure gases and gas mixtures showed that in some cases the pressure in the reactor is gradually reduced. As an example Fig. 8 shows a pressure change in the reactor during processing a mixture 75 %HFO-1234ze(E):25 %N2 in the barrier discharge.
The pressure drop in the reactor in the process of the DBD processing is an indirect indication of the film deposition. Since the DBD processing took place in an isolated volume, without forced gas circulation, then at low degree of the gas conversion and in the absence of film deposition the gas pressure should not drop. Over the course of time, with increasing the concentration of decomposition products which contain fewer atoms than the molecules of the source test gas (as shown by GC analysis), the pressure in the reactor should gradually grow. However, if the deposition of films occurs in the discharge at the walls of the reactor, the pressure will gradually decrease due to the transition of a part of the test gas from the gas phase to the solid phase.
The summary data of changes in gas pressure inside the reactor after 5 h of the DBD processing for studied gases and gas mixtures are shown in Table 3. It is clear from the table that the largest pressure drop in the reactor has been observed in pure HFO-1234ze(E), C5K and in mixture HFO-1234ze(E):N2. The smallest change in pressure during the processing is observed in mixtures C5K:N2 and C5K:dry air. No change or a small change of pressure in the reactor during the DBD processing of mixtures C5K:N2 and C5K:dry air confirms that the degree of conversion and the rate of film deposition of these gases are very low.
Discussion
The experimental study of the degradation of gaseous dielectrics after processing in the barrier discharge is presented.
Qualitative and quantitative analysis of decomposition products of the test gases in the barrier discharge showed that pure gases HFO-1234ze(E) and C5K display relatively low resistance to the electrical discharge. After 5 h of the processing in the barrier discharge at 10 kV of the applied voltage approximately 9–12 % of the source gas has been converted into different products.
Dilution of C5K by the dry air increases the conversion degree of the source gas in the barrier discharge. After 5 h of the DBD processing of the mixture C5K:dry air at 10 kV approximately 25 % of C5K has degraded. Addition gases such as O2 molecules (dry air) increase the chemical reaction in the plasma and on the dielectric surface. The effects of oxygen additives on the fluorocarbon decomposition was evaluated by Ogata et al. [39], who found that the enhancement of the decomposition by the additives was due to the fact that they could scavenge the decomposition fragments, preventing the reproduction of stable fluorocarbons. The increase in the decomposition efficiency with amount of oxygen is probably because the gas decomposition products can be rapidly scavenged by oxygen species such as O*, O2, and O3. O atoms are easy to associate with fluorocarbon radicals, and to decrease the density of fluorocarbon radicals in the plasma [40]. The additives also affected the distribution of the decomposition products. A large amount of CO2 and CO has been found in the decomposition products of the mixture C5K:dry air. Earlier such increase in CO2 production was observed by Spiess et al. [41] through the breakdown of clorfluorocarbons with additives of oxygen and water vapor.
Dilution of HFO-1234ze(E) and C5K by nitrogen, as well as the use of ternary mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 significantly reduces the degree of conversion of the source gas in the barrier discharge. After 5 h of the DBD processing of these mixtures at 10 kV 1–5 % of the source gases have degraded. The formation of the byproducts strongly depends on plasma chemistry. The plasma provides radicals, ions, and electrons in the discharge. However, behaviors of fluorocarbon radicals in plasma with N2 or CO2 additives have not been understood enough.
It should be noted that SF6 has a great electric strength and at U = 2 kV of applied voltage the barrier discharge does not exist. On the other hand at higher voltages (U > 3.7 kV) the resistance of SF6 to decomposition in the barrier discharge is lower than resistance of mixtures studied in this work. The total mass concentration of SF6 + SO2F2 in the decomposition products of pure SF6 trained in DBD at 10 kV was found equal to 66.2 % [32].
Toxic products in different quantities have been detected in all tested gases and gas mixtures after the DBD processing. In pure C5K, trained in the barrier discharge, a large quantity of moderately toxic C3F6 have been found. After the DBD processing of pure HFO-1234ze(E) there was a large quantity of highly toxic CF3C≡CH. Large quantities of moderately toxic C3F6 and highly toxic CO have been detected in trained mixture C5K:dry air. Large quantity of CO has been detected after the DBD processing of mixtures C5K:N2, C5K:HFO-1234ze(E):CO2. The least amounts of toxic products after processing in the barrier discharge have been found in mixtures HFO-1234ze(E):N2 and C5K:HFO-1234ze(E):N2.
Interestingly, that in conditions of absence of oxygen atoms, CO and CO2 were detected in pure HFO-1234ze(E) as well as in the HFO-1234ze(E):N2 mixture, suggesting that oxygen can be released from the glass tube (it contains SiO2 and Al2O3). In a previous study Mok et al. [42] detected N2O and CO in dielectric-packed plasma reactor for hydrofluorocarbons decomposition even when oxygen was not used. Also, Ogata et al. [39] show that lattice oxygen atoms can be released from the surface of TiO2 or Al2O3 pellets in the plasma discharge, which agrees with our results. Obviously, the formation of CO and CO2 results from consecutive reactions of the decomposition products.
On the other hand, there were no traces of NO or NO2 or any others nitrogen-containing radicals detected by the FTIR spectrometry in the decomposition products of mixture HFO-1234ze(E):N2 after the DBD test. That indicates that the density of reactive N* radicals as well as the dissociation degree of the N2 molecules are very small in the discharge.
It should be noted that CF4 and C2F6 detected in decomposition products of test-gases are mostly inert and noncorrosive gases that intensely absorb infrared radiation and, therefore, are capable of influencing of greenhouse effect [43].
The film deposition on the inner surface of a discharge tube has been revealed during the DBD tests. If one takes a gradual reduction of pressure inside the working chamber during the processing as a criterion of film deposition, the worst characteristics belong to pure HFO-1234ze(E), C5K gases and the mixture of HFO-1234ze(E):N2. The mixture C5K:N2 has better performance, the pressure in the reactor during the DBD processing weakly varies only at 10 kV of the applied voltage (increased by 2 Torr).
Conclusions
The experimental study of the degradation of gaseous dielectrics HFO-1234ze(E), C5K and their mixtures 75 %HFO-1234ze(E):25 %N2, 12 %C5K:88 %N2, 18.5 %C5K:81.5 %dry air, 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 has been carried out after the DBD processing at the atmospheric pressure. It is shown that pure HFO-1234ze(E) and C5K show relatively low resistance to influence of the barrier discharge. Dilution of C5K by the dry air greatly increases the conversion degree of the source gas in the barrier discharge. Dilution of HFO-1234ze(E) and C5K by nitrogen, as well as the use of ternary mixtures 9 %C5K:57.5 %HFO-1234ze(E):33.5 %N2 and 9 %C5K:56 %HFO-1234ze(E):35 %CO2 significantly reduces the conversion degree of the source gas in the DBD.
The highest degree of conversion of the test gases after their processing in the barrier discharge relates to pure C5K, HFO-1234ze(E) gases and the mixture C5K:dry air. The worst toxic product yield is revealed in mixtures C5K:N2 and C5K:HFO-1234ze(E):CO2, since large quantities of toxic CO are generated in these mixtures after processing in the DBD. The mixture HFO-1234ze(E):N2 has a sufficiently large pressure drop (up to 8.5 %) during the process of the DBD processing, indicating the film deposition. The mixture C5K:HFO-1234ze(E):N2 can be considered as the best gaseous dielectric among studied mixtures. The degree of conversion of this mixture after a 5-h processing in the barrier discharge at a voltage of 2–10 kV does not exceed 1–2 %. Small quantities of weakly toxic CF4 (<0.07 %), C3F6 (<0.02 %) and a certain amount of CO (<0.3 %) have been found in the decomposition products of this mixture. The pressure drop in the working chamber after a 5-h processing in the barrier discharge at a voltage of 10 kV is less than 3 % of the initial pressure. We note that for this mixture a content of CO in the products exceeds the maximum concentration limit by more than 2 orders of magnitude. However, the results of our studies were obtained for a much more intensive regimes of interaction of the electromagnetic fields with gaseous mixtures. Therefore the usage of the proposed mixture in high voltage electrical equipment requires preliminary long term high voltage experiments in real devices with further animal exposure tests.
References
Tsai W-T (2007) The decomposition products of sulfer hexafluoride (SF6): rewiews of environmental and health risk analysis. J Fluor Chem 128:1345–1352
Reid AJ, Judd MD (2012) Ultra-wide bandwidth measurement of partial discharge current pulses in SF6. J Phys D Appl Phys 45:165203
Krueger FH (1989) Partial discharge detection in high voltage equipment. Butterworth-Heinemann, Oxford
Kuffel E, Zaengl WS, Kuffel J (2000) High voltage engineering: fundamentals, 2d edn. Butterworth-Heinemann, Oxford
Diaz J, Casanovas AM, Casanovas J (2003) Effect of the percentage of SF6 (100–10–5 %) on the decomposition of SF6–N2 mixtures under negative dc coronas in the presence of water vapour or oxygen. J Phys D Appl Phys 36:1558–1564
Casanovas AM, Coll I, Pradayrol C, Casanovas J (1998) Influence of a solid insulator on the spark decomposition of SF6 and 50 % SF6 + 50 % CF4 mixtures. J Phys D Appl Phys 31:2835–2845
Pradayrol C, Casanovas AM, Aventin C, Casanovas J (1997) Production of SO2F2, SOF4, (SOF2 + SF4), S2F10, S2OF10 and S2O2F10 in SF6 and (50–50) SF6–CF4 mixtures exposed to negative coronas. J Phys D Appl Phys 30:1356–1369
Griffin GD, Sauers I, Christophorou LG, Easterly CE, Walsh PJ (1983) On the toxicity of sparked SF6. IEEE Trans Electr Insul EI-18:551–552
Griffin GD, Nolan MG, Easterly CE, Sauers I, Votaw PC (1990) Concerning biological effects of spark-decomposed SF6. IEE Proc (Pt. A) 137:221–227
James DR, Sauers I, Griffin GD, Van Brunt RJ, Olthoff JK, Stricklett KL, Chu FY, Robins JR, Morrison HD (1993) Investigation of S2F10 production and mitigation in compressed SF6 insulated power systems. IEEE Electr Insul Mag 9(3):29–40
Dervos CT, Vassiliou P (2000) Sulfur hexafluoride (SF6): global environmental effects and toxic byproduct formation. J Air Waste Manage Assoc 50:137–141
Shih M, Lee WJ, Tsai CH, Tsai PJ, Chen CY (2002) Decomposition of SF6 in an RF plasma environment. J Air Waste Manage Assoc 52:1274–1280
Lee HM, Chang MB, Wu KY (2004) Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasmas. J Air Waste Manage Assoc 54:960–970
Wang WF, Shih M, Tsai CH, Tsai PJ (2006) Total toxicity equivalents emissions of SF6, CHF3, and CCl2F2 decomposed in a RF plasma environment. Chemosphere 62:1681–1688
Luly MH, Singh RR and Richard RG (2010) Compositions containing sulfur hexafluoride and uses thereof. US Patent. US 2010/0171062 A1
Hodnebrog ∅, Etminan M, Fuglestvedt JS, Marston G, Myhre G, Nielsen CJ, Shine KP, Wallington TJ (2013) Global warming potentials and radiative efficiencies of halocarbons and related compounds: a comprehensive review. Rev Geophys 51:300–378
Lallier J-P, Rastelletti E Compositions based on fluorinated hydrocarbons and secondary butanol for defluxing electronic boards. EP 1 678 288 B1. Intern Publ number: WO 2005/026310
Taniguchi N, Wallington TJ, Hurley MD, Guschin AG, Molina LT, Molina MJ (2003) Atmospheric chemistry of C2F5C(O)CF(CF3)2: photolysis and reaction with Cl atoms, OH radicals, and ozone. J Phys Chem A 107:2674–2679
Kogelschatz U (2002) Filamentary, patterned and diffuse barrier discharges. IEEE Trans Plasma Sci 30:1400–1408
Kogelschatz U, Eliasson B, Egli W (1997) Dielectric-barrier discharges. Principle and applications. J Phys IV FRANCE 7(C4):47–66
Siliprandi RA, Roman HE, Barni R, Riccardi C (2008) Characterization of the streamer regime in dielectric barrier discharges. J Appl Phys 104:063309
Gibalov VI, Pietsch GJ (2000) The development of dielectric barrier discharges in gas gaps and on surfaces. J Phys D Appl Phys 33:2618–2636
Kaptsov NA (1950) The electric phenomena in gases and vacuum. Gostekhizdat, Moscow (in Russian)
Mraihi A, Merbahi N, Yousfi M, Abahaze’e’m A, Eichwald O (2011) Electrical and spectroscopic analysis of mono- and multi-tip pulsed corona discharges in air at atmospheric pressure. Plasma Sourc Sci Technol 20:065002
Antao DS, Staack DA, Fridman A, Farouk B (2009) Atmospheric pressure dc corona discharges: operating regimes and potential applications. Plasma Sources Sci&Technol 18:035016
Grabowski LR, van Veldhuizeen EM, Rutgers WR (2005) Removal of phenol from water: a comparison of energization methods. J Adv Oxid Technol 8:142–149
Penetrante BM, Hsiao MC, Merritt BT, Vogtlin GE, Wallman PH (1995) Comparison of electrical discharge techniques for nonthermal plasma processing of NO in N2. IEEE Trans Plasma Sci 23:679–687
Rosocha LA, Korzeekwa RA (1999) Advanced oxidation and reduction processes in the gas phase using nonthermal plasmas. J Adv Oxid Technol 4:247–264
Kim Y, Kang WS, Park JM, Hong SH, Song Y-H, Kim SJ (2004) Experimental and numerical analysis of streamers in pulsed corona and dielectric barrier discharges. IEEE Trans Plasma Sci 32:18–24
Pek´arek S (2012) Experimental study of surface dielectric barrier discharge in air and its ozone production. J Phys D Appl Phys 45:075201
Kogelschatz U (2003) Dielectric-barrier discharges. Their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46
Tatarinov AV, Shakhatov VA, Bilera IV, Avtaeva SV (2015) Comparative studies of decomposition of trans-1,3,3,3-tetrafluoropropene, 2,3,3,3-tetrafluoropropene, perfluoro-(3-methylbutan-2-on) and sulfur hexafluoride in the barrier discharge. High Energy Chem (accepted for publishing)
Clayton JW (1967) Fluorocarbon toxity and biological action. Fluorine Chem Rev 1:197–242
Clayton JW (1977) Toxicology of the fluoroalkanes: review and research needs. Env Health Persp 21:255–267
Prockop LD, Chichkova RI (2007) Carbon monoxide intoxication: an updated review. J Neurological Sci 262:122–130
Brock WJ (1999) Hydrogen fluoride: how toxic is toxic? A hazard and risk analysis. In: Halon Options Technical Working Conference pp 559–566
Samoylovich VG, Gibalov VI, Kozlov KV (1989) Physical chemistry of the barrier discharge. MSU Publishing House, Moscow (in Russian)
Avtaeva S (2011) Barrier discharge. Research and application. LAP Lambert Academic Publishing GmbH & Co. KG, Saarbrücken (in Russian)
Ogata A, Kim H-H, Futamura S, Mizuno K (2004) Effects of catalysts and additives on fluorocarbon removal with surface discharge plasma. Appl Catal B Environ 53:175–180
Takahashi K, Hori M, Goto T (1996) Fluorocarbon radicals and surface reactions in fluorocarbon high density etching plasma. I. O2 addition to electron cyclotron resonance plasma employing CHF3. J Vac Sci Technol, A 14:2004–2010
Spiess FJ, Chen X, Brock SL, Suib SL, Hayashi Y, Matsumoto H (2000) Destruction of Freons by the use of high-voltage glow plasmas. J Phys Chem A 104:11111–11120
Mok YS, Demidyuk V, Whitehead JC (2008) Decomposition of hydrofluorocarbons in dielectric-packed plasma reactor. J Phys Chem A 112:6586–6591
van Zant P (2000) Microchip fabrication: a practical guide to semiconductor processing, 4th edn. McGraw Hill Books, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tatarinov, A.V., Bilera, I.V., Avtaeva, S.V. et al. Dielectric Barrier Discharge Processing of trans-CF3CH=CHF and CF3C(O)CF(CF3)2, Their Mixtures with Air, N2, CO2 and Analysis of Their Decomposition Products. Plasma Chem Plasma Process 35, 845–862 (2015). https://doi.org/10.1007/s11090-015-9635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9635-8