Abstract
Steam oxidation experiments were conducted at 800–1400 °C with boiling water reactor alloy Zircaloy-2 strip specimens. Sample weight gain measurements were performed on the oxidized specimens before and after the test and were compared to oxygen pickup calculations using the Cathcart–Pawel correlation. The results showed that Zircaloy-2 follows the parabolic law at temperatures above 1000 °C. At or below 1000 °C, the oxidation rate was very low when compared to Cathcart–Pawel correlation and can be represented by a cubic expression. Arrhenius expressions are given to describe the parabolic rate constants at temperatures above 1000 °C and cubic rate constants are provided for temperatures at or below 1000 °C. The weight gains calculated by our Arrhenius correlations are in excellent agreement with the measured sample weight gains at all test temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zircaloy-2 alloy has been used in boiling water reactors (BWRs) for decades. Compared to Zircaloy-4 alloy, it has a relatively thin corrosion layer and absorbs less hydrogen from its interaction with water during periods of normal operation. One major shortcoming of zirconium alloys is that under the conditions of a loss-of-coolant accident (LOCA), they can oxidize rapidly. This reduces the cladding ductility and leads to the excessive hydrogen production, such as during the Fukushima Daiichi nuclear disaster in 2011. Because cladding performance under LOCA conditions is critical in reactor safety analyses, the steam oxidation behavior of zirconium alloys has attracted more and more attention [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Cathcart and Pawel (CP) observed quadratic behavior in an oxidation kinetics study of Zircaloy-4 alloy and established the Cathcart and Pawel (CP) correlation at temperatures from 1000 to 1500 °C [2]. The CP equations were adopted by the US nuclear regulatory commission (NRC) in the Draft Regulatory Guide DG-1262 [3] and widely used in high-temperature steam oxidation modeling and analyses. However, the CP correlation was developed based on an oxidation kinetics study of Zircaloy-4 alloy produced decades ago. Recent studies show that the LOCA oxidation behavior of the Zr–Nb alloy, such as the Russian E110 and E110G [14,15,16,17,18,19], is different from Zircaloy-4 and other PWR alloys. The oxidation rate constant of the E110 and E110G could be much lower than the CP predictions at temperature near 1000 °C [17,18,19]. Also, although steam oxidation characteristics of the PWR alloys are well understood, a similar understanding of oxidation behavior of the BWR Zircaloy-2 is less advanced.
The high-temperature steam oxidation kinetics study of Zircaloy-2 is being conducted at Oak Ridge National Laboratory (ORNL) to provide data that are relevant to licensing criteria for LOCA analyses. Global Nuclear Fuel (GNF) also plays a major role in the program planning and test conduct. The current LOCA licensing criteria limit peak cladding temperature to 1204 °C and maximum Equivalent Cladding Reacted (ECR) to 17% during high-temperature steam oxidation [3]. In this study, high-temperature steam oxidation testing was conducted to determine the weight gain vs. time trends in broad ranges of test conditions from 800 to 1400 °C for test times up to 7000 s. The objective of this work was to experimentally evaluate the kinetics of high-temperature oxidation of Zircaloy-2 under LOCA conditions and to provide new correlations of Zircaloy-2 for comparison with CP predictions.
Experimental Procedures
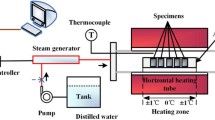
Strip Zircaloy-2 specimens were cut to 19.05 × 11.43 × 3.18 mm, with a hanger hole (diameter = 2.54 mm) centered near one end. The nominal chemical composition of Zircaloy-2 used in the ORNL test program is listed in Table 1. Inductively coupled plasma mass spectrometry (ICP-MS) analysis confirmed that Sn, Fe, Cr, and Ni were within the limits in Table 1. A unique ID number was assigned for each specimen for traceability material control. Weight gain of the specimens was determined by direct measurement of the sample mass before and after the test using a calibrated balance with an accuracy of ± 0.1 mg. The balance was verified with a standard test weight prior to measuring specimen weight, which was divided by the specimen outer surface area to give the normalized weight gain. Specimen dimensions were measured using a caliper with accuracy of ± 0.01 mm, which was calibrated using a calibration rod. High-temperature steam oxidation tests in this work were performed with a tube furnace in the atmosphere. The steam was supplied by a steam generator that converted distilled water to steam at 400C. The oxidation apparatus and method were initially validated using well-known Zircaloy-4 cladding by comparing the sample weight gains to CP-calculated values. The oxidation testing procedure was detailed by Yan et al. [18].
It was well known that the Zr sample temperature could be higher than the temperature in the test chamber because of self-heating generated by zirconium alloy oxidation at high temperature [2, 11, 18]. Therefore, thermal benchmark testing was performed by measuring the temperature of a strip specimen with a thermocouple (TC) directly welded to its outer surface. Figure 1 shows the thermal benchmark result with a Zircaloy-2 strip specimen for the 1200 °C steam oxidation tests. Sample TC was directly welded onto the strip specimen outer surface, and Monitor TC was suspended in steam at a position ≤ 1.0 cm away from the welded TC. The actual temperatures of sample surfaces were derived from the thermal benchmark results performed at various temperatures.
Steam Oxidation Tests at 800 to 1400 °C
Figure 2 shows the temperature histories of the oxidation tests with Zircaloy-2 strip specimens at 1200ºC for the hold time t = 180, 660, 1140, 1620, and 2100s. These temperature histories were recorded with the monitor TC, and the sample temperature was derived from the thermal benchmark test shown in Fig. 1.
Additional oxidation tests were conducted with Zircaloy-2 at 800–1400 °C for various times. Monitor TC temperature histories were recorded for all tests, and the actual sample temperature was derived from the thermal benchmark tests performed at each test temperature. The results are summarized in Table 2, which shows that the measured sample weight gains are 11–48% lower than the CP-calculated weight gains.
Correlations of the Oxidation Rate Constant for Zircaloy-2
The oxidation rate is temperature dependent. The rate is not a constant and continues changing as the temperature increases or decreases during the ramp-up and cooling. However, temperature profiles from the tests reported in this work indicate that the ramp-up and cooling times are nearly identical for each temperature (see Fig. 2). From these results, the isothermal oxidation rate constants at target test temperatures can be derived.
The Cathcart–Pawel (CP) equations have been used for Zircaloys for decades. In their report [2], four related rate equations were given for Zircaloy-4: (a) the oxide layer thickness; (b) the alpha layer thickness; (c) the sum of the oxide-plus-alpha layer thickness; and (d) the total oxygen consumed, which was given as weight gain in grams per square centimeter of surface area. All of these equations obeyed parabolic kinetics under the assumption of no loss of oxide during or after high-temperature exposure. The rate equation for weight gain, Wg is:
where δW is a temperature-dependent coefficient. Under isothermal oxidation conditions, the integration of Eq. 1 is simply
Therefore, the oxidation pickup of Zircaloy-4 is characterized by a parabolic rate law by the CP equations as explained by Eq. 2. Under our test conditions, the integrated Eq. 1 becomes:
where Wtotal is the total weight gain, ttotal is the total time of an oxidation test, tR is the ramp-up time during heating, tH is the time at the target temperature, tC is the cooling time from target temperature to quench temperature, and \(\delta _{W}\) is the time-dependent temperature coefficient.
Because the oxidation at target hold temperatures can be considered an isothermal process, \(\delta _{W}\) is a constant at the target temperature. Therefore, Eq. 3 can be simplified as
where Wtotal is the total weight gain, tH is the test time of the isothermal oxidation at a target temperature, δW is the weight gain coefficient at the isothermal temperature, and CW is the weight gain during the ramp-up and cooling phases. For the oxidation tests at each target temperature, the ramp-up and cooling durations were the same (see Fig. 2) and can be considered a constant for the multi-oxidation tests at a target temperature. CW is test system dependent, can be derived experimentally, and will be different when the ramp-up or cooling rates change. However, the change in the constant CW has no impact on determination of the isothermal oxidation rate δW. This approach can be applied under certain test conditions, such as in the case described herein.
Figure 3 shows oxidation parabolic curves of Zircaloy-2 strip specimens oxidized at temperature T > 1000 °C. The test data points vs. the square root times of isothermal oxidation are in excellent agreement with a linear fit to the data for each test temperature, which indicates good control of isothermal oxidation temperature. It also confirms the applicability of Eq. 4. In addition, the observation that the lines do not pass through the origin (nonzero Cw) is consistent with the correlation based on Eq. 4. For isothermal oxidation at target temperatures, the weight gain coefficient, δW, can be determined by the slope of the fits in Fig. 3. The intersection of the fit with the y-axis gives the weight gain value formed during the ramp-up and cooling phases shown as Cw in Eq. 4.
Recent studies showed that the oxidation pickup of a PWR alloy E110 does not obey the CP correlations. It has different oxidation rate constants [17,18,19], and particularly, its weight gain can be characterized by a cubic rate law at ≤ 1000 °C. E110 is a Zr–Nb alloy. The nominal composition of E110 is given by Shebaldov et al. [13]. Our study indicates that the weight gains of the Zircaloy-2 specimens at 1000 °C or below are 24–48% lower than CP-predicted values (see Table 2). Clearly, the CP correlation is not valid for the steam oxidation kinetics of the Zircaloy-2 strip samples. Above 1000 °C, the rate constants predicted by CP are different for Zircaloy-2, but for both cases parabolic oxidation kinetics prevail. At 1000 °C and below, the kinetics are different. To develop a precise Arrhenius expression for the oxidation rate constant of the Zircaloy-2 strip samples at 1000 °C or below, additional oxidation tests were conducted at 800 °C and 900 °C (see Table 2). The Zircaloy-2 sample weight gains at 800 °C to 1000°C are more closely represented by a cubic expression, as shown Fig. 4. Data points for weight gain vs. cubic root time are in good agreement with a linear fit to the data at 1000 °C or below. For isothermal oxidation at target temperatures of 800 °C, 900 °C, and 1000 °C, the weight gain coefficient ΔW can be determined by the slope of the fits in Fig. 4, and the weight gain at this temperature range can be expressed as:
where Wtotal is the total weight gain, tH is the test time of the isothermal oxidation at a target temperature, ΔW is the temperature-dependent weight gain coefficient, and CW is the weight gain during the ramp-up and cooling phases.
Table 3 summarizes the parabolic and cubic rate constants at each test temperature for isothermal steam oxidation of the Zircaloy-2. Correlation of the rate constant with temperature can be explained in a simple Arrhenius expression:
where R is the gas constant, T is temperature in K, A is the pre-exponential factor, Q is the activation energy, and k is the temperature-dependent rate constant; the pre-exponential factor A and the activation energy are different, depending on the test temperatures. Specifically, it can be shown in Eq. 7 for Zircaloy-2 oxidation following parabolic plots,
where δW2 is the parabolic rate constant for the total weight gain in (g/cm2)2/s, and T is temperature in degrees kelvin. Using the best curve fitting, the weight gain rate is plotted in Arrhenius fashion in Fig. 5 (left-hand side).
As mentioned before, the weight gain rate of Zircaloy-2 strip samples at temperature ≤ 1000 °C is non-parabolic and more closely represented by a cubic expression (see Fig. 4). Therefore, there is a transition region of the oxidation rate constants from the parabolic to cubic laws near 1000 °C. This feature was observed by Kiraly et al. [17] and Yan et al. [18] for the PWR alloy E110. A correlation of the cubic rate constant was given in a simple Arrhenius expression for the E110 at 1000 °C or below by Yan et al. [18]. In this work, the oxidation of Zircaloy-2 strip specimens following the cubic plots at lower temperatures can be shown as:
where ΔW3 is the cubic rate constant for the total weight gain in (g/cm2)3/s and T is temperature in degrees kelvin. Using the best curve fitting, the weight gain rate is plotted in Arrhenius fashion in Fig. 5 (right-hand side).
Figure 6 shows a comparison of experimentally measured weight gains and weight gains calculated with the CP correlation (Fig. 6a) and with our Eqs. 7 and 8 (Fig. 6b) in this work for the Zircaloy-2. The measured sample weight gain is in good agreement with our calculated weight gains at all testing temperatures: The differences between them are less than 10%. The data scattering in Fig. 6b are small, which is an indication of the quality of the experimental data.
Regarding the mechanism of the observed parabolic and cubic oxidation kinetics for Zircaloy-2 in this work, the parabolic kinetics at temperatures above 1000 °C is consistent with rate control by mass transport in the alloy or oxide. It is also consistent with Cathcart–Pawel correlation in Zircaloy-4. The cubic kinetics of Zircaloy-2 was observed at 1000 °C or below. Similar feature was observed for the Zr based alloy E110 [17, 18] at relatively low oxidation temperature (< 1000 °C). The cubic kinetics observed here might exist for other Zr-based alloys at 1000 °C or below.
The Arrhenius equation can be improved if more tests can be conducted at various temperatures. In comparison, Arrhenius expressions of the weight gain correlations for Zircaloy-4 by Cathcart–Pawel [2], E110 by Yan et al. [18], and Zircaloy-2 in this work are given explicitly in “Appendix A”.
Summary
Steam oxidation tests were performed on Zircaloy-2 strip specimens for oxidation kinetics study. The oxidation apparatus and method were initially validated using well-known Zircaloy-4 cladding by comparing the sample weight gains to CP-calculated values. The good agreement between the experimental results and CP predictions indicates that the oxidation system was well calibrated. However, this study shows that the CP correlation is no longer valid for Zircaloy-2 strip samples. For temperatures at 1000 °C or below, oxidation is more appropriately represented by a cubic expression [see Eq. 8]. For oxidation tests performed at temperatures above 1000 °C, the oxidation pickup can be characterized by a parabolic rate law [see Eq. 7] that has different parameters from the CP correlation. Based on our experimental data, we developed Arrhenius expressions to describe the parabolic rate constants for the weight gain at high temperatures (above 1000 °C) and cubic rate constants at low temperatures (1000 °C or below); the measured sample weight gains were in excellent agreement with the weight gains predicted by our equations at all test temperatures.
References
L. Baker and L. C. Just, Studies of Metal-Water Reactions at High Temperatures; III. Experimental and Theoretical Studies of the Zirconium-Water Reaction, ANL-6548, May 1962.
J. V. Cathcart, R. E. Pawel, R. A. McKee, R. E. Druschel, G. J. Yurek, J. J. Cambell, and S. H. Jury, Zirconium Metal-Water Oxidation Kinetics IV. Reaction Rate Studies, ORNL/NUREG-17, Aug. 1977.
U.S. Nuclear Regulatory Commission, Office of Nuclear Regulatory Research, DG-1262 Testing for Postquench Ductility," ML111100368, 2011.
Garde, A. M., Comstock, R. J., Pan, G., Baranwal, R., Hallstadius, L., Cook, T., and Carrera, F., Advanced zirconium alloy for PWR application, J. ASTM Intl., Vol. 7, No. 9, 2010, doi: https://doi.org/10.1520/JAI103030.
S. Leistikow and G. Schanz, Kinetics and morphology of isothermal steam oxidation of Zircaloy-4 at 700–1300C, KFK 2587, March 1978.
H. M. Chung and T. F. Kassner, Journal of Nuclear Materials 84, 1979 (327).
Y. Yan, R. V. Strain, T. S. Bray, and M. C. Billone, High Temperature Oxidation of Irradiated Limerick BWR Cladding, NUREG/CP-0176 (May 2002) pp. 353–372.
F. Nagase, T. Otomo, and H. Uetsuka, Journal of Nuclear Science & Technology 40, 2003 (213).
J.-P. Mardon, D. Charquet, and J. Senevat, Influence of Composition and Fabrication Process on Out-of-Pile and In-Pile Properties of M5 Alloy, Zirconium in the Nuclear Industry: 12th International Symposium, ASTM STP 1354, G. P. Sabol and G. D. Moan, Eds., American Society for Testing and Materials, West Conshohocken, PA, (2000) pp. 505–524.
Y. Yan, T. Burtseva, and M. C. Billone, LOCA Results for Advanced-Alloy and High-Burnup Zircaloy Cladding, NUREG/CP-0185 (June 2004) pp. 97–121.
M. Billone, Y. Yan, T. Burtseva, and R. Daum, Cladding Embrittlement During Postulated Loss-of-Coolant Accidents, NUREG/CR-6967; ANL-07/04 (July 2008).
R. J. Comstock, G. Schoenberger, and G. P. Sabol, Influence of Processing Variables and Alloy Chemistry on the Corrosion Behavior of ZIRLO Nuclear Fuel Cladding, Zirconium in the Nuclear Industry: 11th International Symposium, ASTM STP 1295, E. R. Bradley and G. P. Sabol, Eds., American Society for Testing and Materials (1996) pp. 710–725.
P. V. Shebaldov, M. M. Peregud, A. V. Nikulina, Y. K. Bibilashvilli, A. F. Lositski, N. V. Kuz’menko, V. I. Belov, and A. E. Novoselov, E1110 Alloy Cladding Tube Properties and Their Interrelation with Alloy Structure-Phase Condition and Impurity Content, Zirconium in the Nuclear Industry: 12thInternational Symposium, ASTM STP 1354, G. P. Sabol and G. D. Moan, Eds., American Society for Testing and Materials, West Conshohocken, PA (2000) pp. 545–559.
L. Yegorova, K. Lioutov, N. Jouravkova, A. Konobeev, V. Smirnov, V. Chesanov, and A. Goryachev, Experimental Study of Embrittlement of Zr-1%Nb VVER Cladding under LOCA-Relevant Conditions, NUREG/IA-0211 (Mar. 2005).
Y. Yan, T. Burtseva, and M. Billone, Journal of Nuclear Material 393, 2009 (433).
V. Vrtílková, S. Linhart, and L. Novotný. Corrosion and thermo-mechanical properties of E110 alloys. Proceedings of Nuclear Fuel Workshop, Liberec, Czech Republic, 27–30 April, 2009.
M. Kiraly, K. Kulacsy, Z. Hozer, E. Perez-Fero, and T. Novotny, Journal of Nuclear Material 475, 2016 (27).
Y. Yan, B. E. Garrison, M. Howell, and G. L. Bell, Journal of Nuclear Material 499, 2018 (595).
J. Krejčí, V. Vrtílková, P. Gajdoš, and D. Rada, Nuclear Science and Technology 3, 2017 (18).
Y. Yan, J. R. Keiser, K. A. Terrani, G. L. Bell, and L. L. Snead, Journal of Nuclear Materials 448, 2014 (436).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript was authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Appendix A: Weight Gain Correlations for Zircaloy-4, E110, and Zircaloy-2
Appendix A: Weight Gain Correlations for Zircaloy-4, E110, and Zircaloy-2
Weight gain correlation for PWR alloy Zircaloy-4 by Cathcart and Pawel [2]:
where Wg is weight gain in g/cm2, T is temperature in K, and t is time in s.
Weight gain correlations for PWR alloy E110 by Yan et al. [18]:
where Wg is weight gain in g/cm2, T is temperature in K, and t is time in s.
Weight gain correlations for BWR alloy Zircaloy-2 in this work:
where Wg is weight gain in g/cm2, T is temperature in K, and t is time in s.
Rights and permissions
About this article
Cite this article
Yan, Y., Garrison, B.E., T. Nelson, A. et al. Correlations of the Steam Oxidation Rate Constant of BWR Alloy Zircaloy-2 at 800–1400 °C. Oxid Met 97, 227–239 (2022). https://doi.org/10.1007/s11085-021-10070-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-021-10070-7