Abstract
To recycle elemental molybdenum from waste molybdenum disilicide (MoSi2) heating elements, the MoSi2 was first disintegrated to MoO3 and SiO2 powders in air at a pest oxidation temperature of 500 °C. X-ray diffraction (XRD) patterns confirmed the completion of the pest oxidation reaction. The mixture of MoO3 and SiO2 powders were heated to 950 °C in a tube furnace to evaporate MoO3, and the XRD patterns of the residue showed that only SiO2 was left in the crucible, confirming that the MoO3 was removed through thermal evaporation. The collected MoO3 crystals had a striped morphology. Photocatalytic performance of MoO3 showed superior activity in comparison with commercial MoO3 and P25 for the degradation of methylene blue under visible light irradiation. The photocatalytic degradation activity of MoO3 synthesized by thermal evaporation at 950 °C was 99.25% in 60 min.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molybdenum disilicide (MoSi2) is an intermetallic compound widely used in oxidizing environments at high temperature [1]. The most important commercial application of MoSi2 is its use as heating elements in industrial furnaces. MoSi2 heating elements can be used in air and protective gas atmospheres to heat furnaces to high temperatures [2, 3]. Typically, the lifetime of the heating elements is 3–6 years, depending on the furnace heating practices. With the rapid development of high-temperature process, MoSi2 heating elements have been introduced into the glass industry, heat treatment, forging, ceramics and research and development industries [4, 5]. Although MoSi2 heating elements exhibit excellent life, the replacement is required due to (1) the increase in resistance over the time of use because of thickening of the surface silicon oxide, (2) the damage of oxide layer as a result of long-time erosion, volatilization and formation of micro-cracks in thermal cycling, or exposure to reducing atmospheres [6]. A large amount of waste MoSi2 has become available; however, to the best of our knowledge, the disposal of the waste MoSi2 and/or its recycling has been reported rarely in the literature.
MoSi2 is regarded as high-temperature structural material and generally used as heating elements. Nevertheless, MoSi2 undergoes accelerated oxidation at 400–600 °C and is disintegrated into powder. The phenomenon is termed as pest oxidation, which was first reported by Fitzer in 1955 [7,8,9]. In the past few decades, many researchers have studied the low-temperature oxidation behavior of MoSi2 and MoSi2-based composites [10,11,12,13,14,15,16,17]. Yanagihara et al. [18] found that MoSi2 underwent severe oxidation corrosion and was decomposed into MoO3 and SiO2 rapidly at 500 °C. Moreover, Chou et al. [19] reported that the pest oxidation products consisted of SiO2 clusters and MoO3 whiskers. Westbrook et al. [20] elucidated the mechanism of pest oxidation, and they reported that the preferential intergranular diffusion of oxygen contributed to the embrittlement of grain boundary. However, in this work, the pest oxidation was applied for the chemical separation of Mo and Si elements from the waste MoSi2 in forms of MoO3 and SiO2, respectively.
Molybdenum trioxide (MoO3) is a well-known n-type wide band gap (2.39–2.90 eV) semiconductor, which has found attractive prospects in photocatalysis [21,22,23]. Chithambararaj et al. [24] synthesized hexagonal molybdenum oxide (h-MoO3) nanocrystals with a flower-like hierarchical structure and studied the photocatalytic degradation of methylene blue (MB) under irradiation of visible or UV light. Kumar et al. [25] synthesized thermodynamically stable α-MoO3 nanoplates which exhibited strong photocatalytic degradation of MB and Rh-B up to 99% in the presence of sun light without using any oxidizing agents. A number of techniques have been reported for the deposition of MoO3 including pulse laser deposition [26], thermal evaporation [27], sputtering [28], sol–gel [29], spray pyrolysis [21], chemical vapor deposition [30, 31] and electrodeposition [32]. Zhou et al. [27] prepared orthorhombic MoO3 nanowires by thermal evaporation and oxidation without using any catalyst, and they found the stability of the emission current over time was within 10%, which indicated that MoO3 nanowires could be used as a cold cathode. Rahmani et al. [22] investigated the structural and gas sensing properties of MoO3 thin films which were prepared by thermal evaporation of MoO3 on the gold interdigital fingers on quartz substrates. Thermal evaporation is an advantageous method for producing highly crystalline and stratified structures [22] so that it was used to separate MoO3 from SiO2.
In the present work, a simple and low-cost method, pest oxidation followed by thermal evaporation, for recycling of MoO3 from waste MoSi2 was investigated. The MoO3 was recovered effectively to realize the recycling of Mo from wasted MoSi2 heating elements. The process is effective for resource, environment and economy, and the photocatalytic performance of MoO3 for degradation of organic dyes in the water would be discussed.
Experimental Procedures
Preparation of MoO3
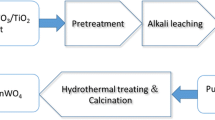
Figure 1 shows the illustration of recycling of MoO3. The waste MoSi2 heating element was broken in the jaw crusher and ground into powder in the roller ball mill for 24 h with a mean particle size of about 1.4 µm. The waste MoSi2 powder was weighed (m0) before calcination, then calcined in tube furnace with a heating rate of 10 °C/min and held for 180 min in air at 450–550 °C. After calcination, the oxidized powders were cooled down to room temperature naturally and weighed as mT (T = 450 °C, 475 °C, 500 °C, 525 °C and 550 °C), and the mass gain (M) of powder was calculated using Eq. (1):
Besides, the effect of holding time was explored under the similar conditions with oxidation of 500 °C for 30–210 min, the weight of powder after calcination was weighed as mt (t = 30 min, 60 min, 90 min, 120 min, 150 min, 180 min and 210 min) and the mass gain (M) of powder was calculated by Eq. (2):
The oxidation reaction of MoSi2 powder calcined in the air is indicated by Eq. (3) [19, 20]:
Equation (3) shows that the oxidation results in formation of MoO3 and SiO2.
After calcination process, the oxidized powders were put in the crucible which was placed in the tube furnace, and the powder was heated to different temperatures (700–950 °C) at a heating rate of 8 °C/min and held for 2 h before furnace cooling. The MoO3 was evaporated from the mixture of MoO3 and SiO2, transported with flowing argon in tube furnace and collected at the glass substrate placed where the temperature gradient was high, which was marked with red circle dash lines in Fig. 1. To figure out the effect of temperature on the amount of MoO3 evaporated, the powder was weighed and the mass loss of powder was calculated after thermal evaporation cycle.
The quantitative elemental analysis of waste MoSi2, recycled products and residue after thermal evaporation was performed using X-ray fluorite spectroscopy (XRF, S8 TIGER). The phase composition of recycled MoO3 was determined by X-ray diffraction (XRD) on a Bruker D8 Advance machine with Cu target. The microstructures were characterized by scanning electron microscopy (SEM, SU8220).
Photocatalytic Performance of MoO3
Photocatalytic experiments were carried out to degrade methylene blue (MB) in a photocatalyst aqueous suspension system, which was exposed to visible light. The initial concentration of MB was 20 mg/L. The solution containing photocatalysts was stirred in the dark for 30 min to establish a relative adsorption–desorption equilibrium between photocatalyst powders and MB solution. The photocatalytic degradation was conducted in a 50-ml glass vessel, and a 150 W halogen tungsten lamp was located 20 cm above the surface of the liquid. During irradiation, 6 ml mixture solution was withdrawn at every 10-min intervals and then centrifuged to separate photocatalysts from the mixture solution. The photocatalytic performance experiments of commercial MoO3 (10 µm, 99.9%, purity), nano-TiO2 (P25) were conducted under the same conditions as a comparison.
Results and Discussion
Characterization of Waste MoSi2
The chemical composition of the waste MoSi2 powder determined by XRF in Table 1 shows that Mo and Si are the main components; O, W, and Al are present as major impurities. Feng et al. [1] reported that Mo, W and Si powders were mixed at a molar ratio of 1:2 [(Mo + W):Si] and 2.5 at.% and 5.5 at.% of Al were introduced into Mo–W–Si powders to improve the hardness, flexural strength and fracture toughness of MoSi2. The commercial Kanthal Super 1900 silicide contains tungsten, and Kanthal Super ER silicide comprises aluminum [33]. The addition of aluminum formed a thicker alumina scale at higher temperatures on Kanthal Super ER to protect the heating elements from corrosive reactions. Furthermore, during the shaping, silicide powders were mixed with clay binder to prepare MoSi2-base heating elements [33]. Thus, the presence of impurities, O, W, Al, largely depends on the processing of MoSi2 heating elements.
Effects of Oxidation Temperature and Holding Time
The effect of calcination temperature on the mass gain of waste MoSi2 powder in Fig. 2 shows that MoSi2 oxidizes rapidly at 450–550 °C [10, 11]. The mass gain of powder reaches the maximum of 63.8% at 500 °C, which means that the MoSi2 powder has undergone the most serious pest oxidation [11]. Above 500 °C, the mass gain decreases, which can be related to the formation of protective glass scale on the surface of material hindering the pest oxidation [6, 8,9,10,11].
Figure 3 presents the XRD patterns of waste MoSi2 powder calcined at 450 °C, 475 °C, 500 °C, 525 °C and 550 °C for 3 h. MoO3 was identified as the major crystalline phase; besides, unreacted MoSi2 peaks were detected after calcination. As such, the waste MoSi2 heating element powders can be used as a source of MoO3 for recycling. Just as the effect of calcination temperature on mass gain is exhibited in Fig. 2, MoSi2 powder is suffered from the most accelerated oxidation at 500 °C which corresponded to the maximum mass gain. It is evident from Figs. 2 and 3 that the calcination temperature of 500 °C is sufficiently high to transform MoSi2 to MoO3 as proposed by Eq. (3) by pest oxidation process.
Figure 4 shows the effect of holding time on the mass gain of waste MoSi2 powder calcined at 500 °C. When the holding time is between 30 and 90 min, the mass gain of calcined powder increases quickly, reaching 62.1% from 50.6%; with the prolonged holding time, it increases to 63.7% when holding for 210 min, which indicates that the extended holding time promotes the transformation of MoSi2 into MoO3 and SiO2. After calcination for 180 min, the oxidation reaction of MoSi2 was completed and mass gain does not change with the increase in holding time.
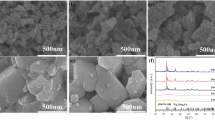
Figure 5 shows SEM micrograph of waste MoSi2 powder and the oxidized products calcined at 500 °C for 30 min, 120 min and 180 min. The waste MoSi2 powder consists of particles of 1–4 µm (Fig. 5a). It can be seen that when calcined at 500 °C for 30 min, the oxidized mixture is composed of needle-shaped MoO3 with 20 µm length and disintegrative SiO2 particle clusters. With the holding time prolonged, the number of MoO3 rods significantly increases and some rods stack together to form MoO3 aggregates when calcined for 180 min, as can be seen in Fig. 5d.
Effect of Sublimation Temperature
Table 2 exhibits the relationship between vapor pressure of MoO3 and temperature [34]. The vapor pressure increases as temperature rises, which climbs rapidly from 0.008 to 0.2333 kPa and 1.3467 kPa, respectively at 720 °C, 750 °C and 800 °C. This suggests that the temperature higher than 750 °C can volatilize MoO3 by evaporation from the MoO3–SiO2 powder mixture.
Figure 6 presents the effect of holding temperature on the evaporated mass of MoO3, which increases from 31.7 to 50.2% with the temperature range from 700 to 900 °C, respectively. As temperature increases to 900 °C, the evaporated mass increases. After 900 °C, the evaporated mass is stable and hits its maximum at 50.4% at 950 °C. Inset image in Fig. 6 presents the macrograph of recycled MoO3 recovered at 950 °C, and the recycled MoO3 shows transparent elongated strips of size of 2–10 mm.
Figure 7 shows the XRD patterns of recycled products and the residue, and only single-phase MoO3 is identified regardless of the heating temperature (Fig. 7a). No other diffraction peaks are present. Figure 7b demonstrates the XRD patterns of residual products in the crucible after evaporation procedure. When heating at 700 °C and 800 °C, the crystalline phases are SiO2 and MoO3 in the crucible, suggesting that MoO3 has not volatilized completely. With the increase in temperature to 900 °C, the SiO2 peaks become the strongest, and single-phase SiO2 is found after heating at 950 °C. The XRD in Fig. 7 confirms the removal of MoO3 from the mixture of MoO3 and SiO2 by thermal evaporation process.
Photocatalytic Performance
As shown in Fig. 8, the optical properties of recycled MoO3 and commercial MoO3 were characterized by UV–Vis diffuse reflectance spectroscopy (DRS). The optical absorption performance of semiconductors is evaluated on the basis of the band gap energy (Eg) as calculated by Eq. (4) [35]:
In Eq. (4), α, h, v, A and Eg represent the absorption coefficient near absorption edge, Planck constant (unit: eV), the light frequency, the absorption constant and the absorption band gap energy, respectively [35]. The value of n is determined by the type of optical transition in the semiconductor (n = 1 for direct transition and n = 4 for indirect transition). According to previous reports, MoO3 pertains to direct transition and the value of n is set as 1 [35]. The Tauc plot of the corresponding samples is shown in the inset of Fig. 8, and the band gap energies of recycled MoO3 and commercial MoO3 are 2.95 eV and 3.10 eV, respectively. As is shown, the band gap energy of the recycled MoO3 is narrower than that of commercial MoO3, and the narrower band gap energy helps improve the photocatalytic property of MoO3. Furthermore, some other characterization such as photoluminescence spectra (PL), surface area and defect will be used to deeply explore the photocatalysis mechanism in the following researches.
Figure 9 exhibits the photocatalytic performance of nano-TiO2 (P25), commercial MoO3 (10 µm, 99.9%, purity) and recycled MoO3 at 950 °C, which was evaluated by the degradation of methylene blue (MB) under visible light irradiation. The MB degradation efficiency (%) was calculated by Eq. (5) [36]:
where D is the degradation efficiency and C0 and Ct are initial and residual concentration of dyes at different times. As shown in Fig. 9, the degradation of MB by the commercial MoO3 and P25 is 5.52% and 11.18%, after irradiating for 60 min. The degradation activity of the recycled MoO3 at 950 °C is 99.25% which is far superior to commercial MoO3 and P25. Inset in Fig. 9 shows the corresponding colors of MB with recycling MoO3, among which − 30 refers to the solution before dark reaction and 0 refers to the initiation of photocatalytic reaction. Finally, the blue color solution changed to colorless within the period of 60 min. The recycling MoO3 in this work demonstrated a good photocatalytic activity for photodegradation of MB under visible light.
Concluding Remarks
MoO3 was recycled from waste MoSi2 heating elements, and the recycling MoO3 presented good functional properties. Specifically, when the waste MoSi2 powder calcined at 500 °C and held for 180 min, that is to say, using the pest oxidation temperature, the waste MoSi2 transformed into the mixture of MoO3 and SiO2 with the maximum transformation rate. Then, the pest oxidation products were heated to specific temperature to evaporate the MoO3. The evaporated mass was stable above 900 °C, and MoO3 was evaporated completely from MoO3 and SiO2 mixture. The recycling MoO3 demonstrated stronger photocatalytic degradation of MB up to 99.25% in visible light, which was higher than commercial MoO3 and P25. This study proved that recycled MoO3 with good photocatalytic degradation of MB could be effectively recovered from waste MoSi2.
References
P. Feng, X. Qu, A. Farid, et al., Rare Metals 25, 225 (2006).
Y. Jiang, D. Feng, H. Ru, et al., Surface and Coatings Technology 339, 91 (2018).
Y. Zhang, Y. Li, C. Bai, et al., Ceramics International 43, 6250 (2017).
A. Makris, Industrial Heating 61, 46 (1994).
V. Bizzarri, B. Linder and N. Lindskog, American Ceramic Society Bulletin 68, 1834 (1989).
M. Samadzadeh, C. Oprea, H. Sharif, et al., International Journal of Refractory Metals and Hard Materials 66, 11 (2017).
D. Berztiss, R. Cerchiara and E. Gulbransen, Materials Science and Engineering: A 155, 165 (1992).
T. Chou and T. Nieh, Scripta Metallurgica et Materialia 26, 1637 (1992).
Z. Zaki, N. Mostafa and Y. Ahmed, International Journal of Refractory Metals and Hard Materials 45, 23 (2014).
Y. Liu, G. Shao and P. Tsakiropoulos, Intermetallics 9, 125 (2001).
P. Feng, X. Wang, Y. He, et al., Journal of Alloys and Compounds 473, 185 (2009).
F. Zhang, L. Zhang, A. Shan, et al., Intermetallics 14, 406 (2006).
J. Chen, C. Li, Z. Fu, et al., Materials Science and Engineering: A 261, 239 (1999).
S. Chevalier, F. Bernard, E. Gaffet, et al., Materials Science Forum 461–464, 439 (2004).
S. Knittel, S. Mathieu and M. Vilasi, Intermetallics 18, 2267 (2010).
K. Kurokawa, H. Houzumi, I. Saeki, et al., Materials Science and Engineering: A 261, 292 (1999).
C. McKamey, P. Tortorelli, J. DeVan, et al., Journal of Materials Research 7, 2747 (1992).
K. Yanagihara, K. Przybylski and T. Maruyama, Oxidation of Metals 47, 2 (1997).
T. Chou and T. Nieh, Journal of Materials Science 29, 2963 (1994).
D. Pope and R. Darolia, Materials Research Society Bulletin 21, 30 (1996).
A. Bouzidi, N. Benramdane, H. Tabet-Derraz, et al., Materials Science and Engineering: B 97, 5 (2003).
M. Rahmania, S. Keshmiri, J. Yu, et al., Sensor and Actuators B 145, 13 (2010).
I. Navas, R. Vinodkumar, K. Lethy, et al., Journal of Physics D: Applied Physics 42, 175305 (2009).
A. Chithambararaj, N. Sanjini, A. Chandra Bose, et al., Catalysis Science & Technology 3, 1405 (2013).
V. Kumar, K. Gayathri and S. Anthony, Materials Research Bulletin 76, 147 (2016).
S. Sunu, E. Prabhu, V. Jayaraman, et al., Sensor and Actuators B 94, 189 (2003).
J. Zhou, S. Deng, N. Xu, et al., Applied Physics Letters 83, 2653 (2003).
A. Prasad, P. Gouma, D. Kubinski, et al., Thin Solid Films 436, 46 (2003).
A. Prasad, D. Kubinski and P. Gouma, Sensors and Actuators B: Chemical 93, 25 (2003).
K. Gesheva and T. Ivanova, Chemical Vapor Deposition 12, 231 (2006).
S. Ashraf, C. Blackman, G. Hyett, et al., Journal of Materials Chemistry 16, 3575 (2006).
R. Patil, M. Uplane and P. Patil, Applied Surface Science 252, 8050 (2006).
K. Hellström, P. Persson and E. Ström, Journal of the European Ceramic Society 35, 513 (2015).
X. Liu, S. Wang, Q. Zhang, et al., Chinese Journal of Materials Research 24, 17 (2010).
S. L. Prabavathi, P. S. Kumar, K. Saravanakumar, et al., Journal of Photochemistry and Photobiology A 356, 642 (2018).
D. Zhou, Z. Chen, Q. Yang, et al., Solar Energy Materials and Solar Cells 157, 399 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51574241 and 51874305), the Swedish Foundation for Strategic Research (SSF) for Infrastructure Fellowship (RIF14-0083).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kong, G., Du, X., Cai, X. et al. Recycling Molybdenum Oxides from Waste Molybdenum Disilicides: Oxidation Experimental Study and Photocatalytic Properties. Oxid Met 92, 1–12 (2019). https://doi.org/10.1007/s11085-019-09909-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09909-x