Abstract
Isothermal oxidation experiments with cladding tube segments of Zircaloy-4 (Zr-1.3%Sn) in oxygen–nitrogen model mixtures were performed at 800, 1000, and 1200 °C for 6, 1 h, and 15 min, respectively. The gas compositions varied between 0 and 100 vol% nitrogen including 1 and 99 vol%. A strong accelerating effect of nitrogen on the oxidation kinetics was seen for a wide range of boundary conditions. At 800 °C, oxidation in all mixtures with 1–99 % nitrogen resulted in higher reaction rates compared to the pure gases, especially after transition from protective to non-protective oxide scales. At 1000 and 1200 °C, only starvation of oxygen in mixtures with low oxygen contents resulted in lower rates compared to pure oxygen. The oxide scales formed in the mixtures were very porous due to the formation of zirconium nitride at the metal-oxide interface and its oxidation during continuing reaction. The extension of the oxide-nitride zone increased with temperature and with nitrogen content in the gas mixture. Nitrogen seems also to affect the pre-transition reaction kinetics. The mechanism of the faster oxidation kinetics of zirconium alloys in atmospheres containing nitrogen will be discussed in this paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zirconium alloys are widely used in water-cooled nuclear power plants as structure materials and fuel claddings due to their low cross-section for absorption of thermal neutrons, good mechanical properties and high corrosion resistance at operational conditions (e.g. 360 °C, 15 MPa for PWRs). However, oxidation becomes significant at elevated temperatures beyond 800–1200 °C as during accident scenarios and causes degradation of the mechanical properties of the materials as well the release of hydrogen and heat. Indeed, the main source of hydrogen causing the explosions during the nuclear accidents in the Fukushima Daiichi plants was the zirconium-steam reaction. The chemical energy released by this reaction may be higher than the residual decay heat after emergency shutdown and may cause temperature runaway e.g. during reflood of an overheated reactor core [1].
The high-temperature oxidation of zirconium alloys in various atmospheres has been extensively studied during the last decades, e.g. [2–7]. The oxidation in steam, the most relevant atmosphere in nuclear power plants, is described by cubic (for temperatures below 1000 °C) or parabolic (at temperatures beyond 1000 °C) kinetics determined by the diffusion of oxygen (vacancies) through the oxide scale and in the metal. Breakaway oxidation causes a transition from the initially (sub-)parabolic to a rather linear kinetics for temperatures below 1050 °C after the oxide scale thickness has reached a critical value.
Air oxidation may become relevant in case of open or failed reactor pressure vessels as well as during accidents of spent fuel pools and transport casks [8, 9]. Nitrogen is used for inerting BWR containments and for pressurizing of emergency core cooling systems. Hence, air oxidation of zirconium alloys was investigated in numerous studies as well, e.g. [10–15]. It was found that the formation of zirconium nitride at the metal-oxide interface and its re-oxidation with continuing reaction causes the formation of very porous oxide scales and hence significantly accelerates oxidation kinetics. Globally, oxygen is preferably consumed and the oxygen/nitrogen ratio may change during air oxidation. So far, there is no systematic study available of the dependence of the oxidation kinetics of zirconium alloys on the oxygen/nitrogen ratio. The present paper will provide oxidation data for Zircaloy-4 in oxygen–nitrogen mixtures covering the whole range from pure oxygen to pure nitrogen including 1 and 99 % nitrogen in the mixture at 800, 1000, and 1200 °C.
Experimental Procedures
All experiments presented here were conducted in a commercial thermal balance (NETZSCH STA-409) with a vertical tube furnace and balance below the furnace, coupled via a capillary with a quadrupole mass spectrometer (NETZSCH Aeolos); details are described elsewhere [16]. The gases (Ar, O2, N2) are supplied via Bronkhorst® flow controllers at the lower part of the vertical tube furnace. Argon flows through the balance containment into the furnace; the reaction gases are directly injected into the reaction tube to prevent contamination of the balance and to ensure a well-defined gas mixture in the furnace. All gases used are highly pure with less than 1 ppm (Ar, N2) and 10 ppm (O2) impurities. The gas flow rates applied were 10 l/h (O2+N2) and 3 l/h Ar at standard conditions.
The experiments were conducted with Zircaloy-4, the classical cladding alloy for pressurized water reactors (PWR) with approx. 1.3 wt% tin, 0.2 wt% iron and 0.1 wt% chromium as main alloying components. Tube segments 2 cm long (10.75 mm outer diameter, 0.725 mm wall thickness) were cut from longer tubes, deburred and ground at both ends and cleaned in an ultrasonic bath of acetone. The samples were positioned on an alumina plate vertically in the furnace of the thermal balance. They were open at the ends; consequently, reactions of the gases with the inner and outer surfaces of the tube segments were possible, but with differing thermo-hydraulic boundary conditions due to the bottom-up gas flow.
For isothermal oxidation tests the samples were heated in argon atmosphere up to the defined temperature with a rate of 20 K/min. The oxidizing gas mixture was switched on after 10 min in the isothermal phase when the temperatures were controlled at a constant level. A small mass gain of around 0.1 wt% was observed during the pre-phases in argon which was neglected during the further analyses of the experiments. The change to oxidizing conditions led to a temporary increase of sample temperatures due to the highly exothermic reaction. This non-isothermal period was not considered for the determination of kinetic rate constants. The experiments were finished with a cool-down phase in flowing argon with an initial cooling rate of approx. 30 K/min down to 600 °C becoming slower at lower temperatures determined by the heat capacity of the furnace. Experiments were conducted at 800, 1000, and 1200 °C with 6, 1 h, and 15 min oxidation times, respectively, resulting in significant, but not complete oxidation of most of the samples. A wide range of oxygen–nitrogen compositions were considered between the pure gases by at most 10 % steps and including 1 and 99 % of nitrogen in the mixture.
Macro-photos were taken of all specimens after the tests. For post-test examinations the specimens were embedded in epoxy resin, cut, ground and polished for metallographic examinations by optical microscopy and SEM-EDX.
Results
Thermo Gravimetry (TG)
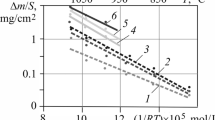
Specific mass gains versus time of all tests are summarized in Fig. 1. The strong effect of nitrogen on the oxidation kinetics becomes clear at a first glance. So, at 800 °C all TG curves obtained in mixtures are significantly above the two curves obtained in the pure gases. This is also true for the higher temperatures, but less pronounced and excluding experiments with low oxygen contents resulting in oxygen starvation.
For all temperatures the mass gains in nitrogen are by far the lowest compared to all other experiments. They are by 1–2 orders of magnitudes lower than in oxygen. At 800 °C, the mass gains obtained in the mixtures are by a factor of 3–6 higher compared to oxygen, including the mixtures with 1 and 99 % nitrogen. The mass gain curves of the mixtures initially behave similar to the one in oxygen up to the transition from sub-parabolic to linear or even accelerating kinetics, as can be seen in Fig. 1b. The transition time is around 2000 s for all tests in atmospheres between 10 and 98 % nitrogen, which corresponds to a mass gain of 15–20 g/m2 and a calculated critical oxide scale thickness of around 10 µm. The transition was later for very low nitrogen concentrations (5200 s for 1 % N2; 4700 s for 5 % N2) and for the highest nitrogen concentration applied (3100 s for 99 % N2). The transition time was not reached in the test in pure oxygen; according to earlier research it should have been expected at around 25,000 s [3].
The series of TG curves obtained at 1000 °C looks a bit more complex. Again the mass gain in pure nitrogen is by far the lowest, being almost 50 times lower than the one in pure oxygen. And again, most of the curves (all for nitrogen concentrations less than 70 %) show a transition from sub-parabolic to linear or faster kinetics. The transition time is around 1000 s for all these tests including the one under pure oxygen corresponding to a critical oxide scale thickness of about 60 µm. Very low oxygen concentrations (≤5 %) in the gas atmosphere result in linear kinetics almost from the initiation of the oxidation phase which is an indication for oxygen starvation, i.e. an oxygen deficit to meet the oxidation kinetics. Interestingly, the mass spectrometer signal does not show significant consumption of the supplied oxygen, meaning that the starvation occurred only locally at the sample surface but not in the whole reaction chamber. With increasing oxygen content the initial mass gain (partly affected by the temporary temperature rise) becomes more significant, whereby tests under 10 and 20 % oxygen reveal a different, more linear behavior compared to the tests in mixtures containing more than 30 % oxygen.
As could be expected, oxygen starvation also affected the experiments at 1200 °C up to at least 10–20 % oxygen in the mixture. Significant initial consumption of the injected oxygen was observed by mass spectrometry for the experiments under less than 50 % oxygen in the mixture. Mass gain in pure nitrogen is the lowest again and all experiments in mixtures with less than 80 % nitrogen resulted in higher mass gains compared to pure oxygen. In contrast to the experiments at 800 and 1000 °C, no transition to accelerated kinetics was observed in all experiments at 1200 °C.
Figure 2 summarizes the results of the thermo-gravimetric tests showing the mass gain in dependence on the gas composition for all three temperatures. The steep gradient in the edge areas as well as the broad maximum between 10 and 90 % nitrogen becomes visible for the 800 °C test series. The effect of small nitrogen contents in the gas is less pronounced at the higher temperatures, and as already discussed above, the right range of this diagram with low oxygen concentrations is affected by oxygen starvation at least from 80 % nitrogen concentration.
Post-test Appearance and Metallography
Selected macrographs of the samples after the oxidation experiments are shown in Fig. 3. At a first glance, the stronger degradation of the samples oxidized in mixtures is visible. At 800 °C, and to a certain degree, also at 1000 °C, the surface of the samples oxidized in mixtures looks clod-shaped with a dense network of cracks. All three samples annealed in nitrogen look almost unchanged with golden or bluish tempering colors. Most samples reveal a non-homogeneous surface. This is caused by the local initiation of nitrogen attack as it was observed in earlier experiments on air oxidation of zirconium alloys [12, 15]. Furthermore, and especially for gas mixtures with low concentration of one gas and increasing with temperature, the composition and therefore the oxidation conditions may change with the height of the sample. All samples of the 1200 °C series annealed in oxygen-rich atmosphere (<90 % nitrogen) are axially cracked and slightly distorted due to these cracks.
The micrographs of the 800 °C series are quite similar for all samples oxidized in mixtures with nitrogen concentrations between 1 to 99 %, and differing pictures for the samples annealed in the pure gases, as to be seen in Fig. 4. The oxide scale of the 100 % oxygen sample is only about 5 µm thick and it is dense and protective. The partial spalling of the oxide seen in Fig. 4 occurred post-test during preparation of the embedded sample as can be clearly seen by the constant thickness of the oxygen-stabilized α-Zr(O) phase below the oxide and its smooth interface to the remaining former β-Zr phase. As expected from the TG results, the sample annealed in nitrogen is only slightly affected and reveals an about 2 µm nitrogen-stabilized α-Zr(N) layer at the surface. No zirconium nitride scale was detected at that sample. The oxide layers formed in the gas mixtures are between 50 and 100 µm thick. They consist of a thin, relatively dense external oxide layer with less than 10 µm thickness, which was formed initially. The remaining part of the oxide is lamellarly structured with a dense network of circumferential cracks. In addition, almost through-going radial cracks cross the oxide layers corresponding to the clod-shaped surface structure seen in the macrographs. The magnified micrographs of the metal-oxide interface make visible very tiny (around 1 µm), golden-colored zirconium nitride (ZrN) particles in the oxide near to the metal-oxide interface. One example is given in Fig. 7 (top) of the sample annealed at 800 °C in a mixture with 50 % nitrogen; but ZrN was found in all samples annealed in oxygen–nitrogen mixtures. As already seen in the macrographs, also the micrographs reveal partly inhomogeneous oxide structures, especially for the samples with small oxygen content in the gas mixture layers, including areas with dense oxide, see Fig. 4.
The micrographs taken from the samples of the 1000 °C series (Fig. 5) look different at a first glance. Breakaway oxidation is already seen at the sample oxidized in oxygen which is in correspondence with the commonly known early breakaway oxidation at 1000 °C. An approx. 20–25 µm thick dense and columnarly grown oxide layer is covering the very porous residual oxide with lamellar structure. The sample annealed in nitrogen is again only slightly affected; a 5–10 µm thick α-Zr(N) layer is covering the bulk material. All samples oxidized in oxygen–nitrogen mixtures show three sub-layers within the oxide consisting of a, compared to the 800 °C series, much more pronounced oxide-nitride two-phase layer at the metal interface, a dense external ZrO2 scale and in between a very porous oxide area. The columnarly grown external oxide is about 20–40 µm thick and radially cracked. The density of these cracks increases with rising nitrogen content of the gas mixture during annealing. The subjacent oxide is interspersed with mainly circumferential cracks, whereby the morphology is much rougher as for the samples of the 800 °C series. The same is true for the oxide-nitride two-phase region nearby the metal with an extension of 40–80 µm. The golden-colored zirconium nitride is clearly visible in the micrographs in Fig. 5. A more detailed micrograph of one sample of the 1000 °C series (80 % nitrogen) is shown in Fig. 7 (mid).
Semi-quantitative (standardless) element analyses of the two-phase zone of samples of the 1000 °C series by SEM-EDX indicated the formation of “ZrO2” and “ZrN” with 64 % oxygen and 45 % nitrogen, respectively.
Compared to the 800 and 1000 °C series the micrographs of the samples annealed at 1200 °C look much more heterogeneous, except for the samples in the pure gases, as can be seen Fig. 6. As expected for this temperature, the 120 µm thick oxide formed in oxygen is dense and columnar. Two metal phases can be distinguished: the brittle, oxygen stabilized α-Zr(O) phase adjacent to the oxide and the central former (at 1200 °C) β-Zr phase with less oxygen content. Comparable to the samples at lower temperatures, the sample annealed in nitrogen shows no superficial zirconium nitride, but only an about 20 µm thin nitrogen-enriched α-Zr(N) zone. The samples annealed in oxygen–nitrogen mixtures behaved quite complex. At low nitrogen contents, the external oxide scales look similar to the one formed in pure oxygen; except that they are a bit thicker (130–160 µm). Starting from 40 % nitrogen, and further increasing with decreasing oxygen content, surface areas are partly completely oxidized besides areas with protective oxide scale. At 90 % nitrogen, local nitride formation underneath the oxide scale was observed, and at higher nitrogen concentrations in the reaction gas, mixed oxide-nitride scales are seen, which become thinner, but richer in ZrN with increasing nitrogen concentration. These samples reveal thin α-Zr(O,N) layers below the oxide/nitride.
In contrast to the experiments at 800 and 1000 °C, the inner surfaces of the tube samples differ from the external ones. Porous oxide-nitride layers were formed at the inner surface of all samples annealed under gas mixtures with 10–80 % nitrogen. The samples are completely oxidized in these areas for samples annealed under gas mixtures with 40–80 % nitrogen. In Fig. 6 only one micrograph per sample was selected showing either one or the other region of such samples with heterogeneous oxidation. A magnified view of one example of a completely oxidized sample is given in Fig. 7 (bottom). No inner oxidation was observed for samples annealed in gas mixtures with 90 % and higher nitrogen concentrations.
Analysis and Discussion of Experimental Results
The experimental results presented in chapter 3 highlight the strong effect of nitrogen on the oxidation of zirconium alloys (of Zircaloy-4 in these special experiments) over a wide range of gas composition and the complex high-temperature reaction behavior of the simple ternary system Zr–O–N.
The kinetics of the oxidation of zirconium and its alloys can be described by the following equation [17]:
where Δm is the mass of oxygen absorbed by the specimen, S the sample surface, k the rate constant and t the oxidation time at constant temperature. The exponent n equals ½ for the parabolic oxidation kinetics applicable for protective oxide scales. In this case the oxidation rate is determined by the diffusion of oxygen (vacancies) through the oxide scale. At lower temperatures cubic or even sub-cubic oxidation kinetics (n = 1/3 and n < 1/3, respectively) are observed. When the oxide scale loses its protective effect, oxygen and other reactive gases may be transported much faster through pores and cracks to the metal, thus accelerating the oxidation to linear kinetics (n = 1) or even faster one, if, for instance, breakaway starts locally and the non-protective surface increases or the permeability of the porous oxide changes with time.
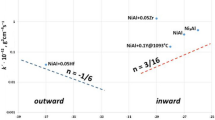
Most TG tests at 800 and 1000 °C in this study resulted in a transition from a, in general terms, decelerating to a rather linear or even accelerating kinetics. Exponents n of around 1/3 before transition and >1 after transition were determined from the TG curves. No transition was observed for the 1200 °C test series. Here the exponent n was determined to be around 0.6 for most of the tests. The post-test analyses revealed partially non-homogeneous oxidation of the sample surfaces which should have influenced the kinetic evaluation of the experiments. This non-homogeneity could be caused by different thermal–hydraulic boundary conditions at the external and internal surfaces of the tube segments, by changing gas composition along the sample due to consumption of oxygen, and by the inherent local initiation of nitrogen attack as described e.g. in [12, 15]. Although the experiments did not exhibit ideal parabolic to linear transitions, the rate constants of all tests were determined based on parabolic and linear reaction kinetics, i.e. assuming n = 0.5 and n = 1, respectively, allowing for comparison among each other and with literature values. Parabolic constants kp were calculated by linear fitting of the Δm = f(sqrt(t)) curves up to the begin of the transition and linear constants kl by linear fitting of the Δm = f(t) curves after transition till the end of the test or begin of saturation, i.e. until approaching complete oxidation of the metal. The results of this procedure are summarized in Table 1 and Fig. 8.
The parabolic rate constants for the mass gain by dissolution of oxygen in the metal and oxide formation rise with increasing nitrogen content in the reaction gas mixture for all three temperatures investigated. This trend is more pronounced at higher temperatures and it is an indication that nitrogen already affects the oxidation before the transition to breakaway. One mechanism explaining this behavior is the dissolution of nitrogen in the oxide. A partial substitution of oxygen ions by aliovalent nitrogen ions would lead to the formation of anion vacancies, as described e.g. by Lerch [18–20], Eq. 2, and thus influence the oxygen diffusion and oxidation kinetics.
An increasing solubility of nitrogen in the oxide with rising temperature could be responsible for the more pronounced dependence of the pre-transition kinetics on the nitrogen content in the gas mixture.
To understand the transition and the post-transition kinetics, phase diagrams of the system zirconium–oxygen–nitrogen are useful. The binary phase diagrams Zr–N [21] and Zr–O [22, 23] are comparable, at least at the Zr side. For the pure metal a phase transition takes place from hcp α-phase to bcc β-Zr at 863 °C; this transition temperature is reduced to around 800 °C for the pseudo-binary system Zircaloy-4-oxygen [24, 25]. The α-Zr phase is stabilized by the non-metals and can dissolve up to 30 at.% (7 wt%) oxygen and 23 at.% (4.4 wt%) nitrogen at 1200 °C. The solubility of the gases in the β-phases is much lower (<1 wt%). Three modifications of ZrO2 (monoclinic, tetragonal, cubic) are known on the oxygen-rich side of the Zr–O phase diagram with some hypo-stoichiometry in equilibrium with the oxygen-stabilized α-Zr(O). According to the Zr–N phase diagram only one phase, the cubic ZrNx with a broad range of composition (x = 0.7–1.3), is formed which is in equilibrium with the nitrogen-stabilized α-Zr(N) phase. Information in literature on the ternary phase diagram Zr–N–O is very scarce and mainly related to zirconium powder combustion and solid electrolytes [20, 26–28].
The discussion in this paper will be based on the known binary systems, except for the pO2/pN2 diagram (Fig. 9) showing the stability regions of Zr, ZrO2, and ZrN based on data for pure substances taken from Barin [29]. According to this diagram, ZrN is only stable at very low oxygen partial pressures, e.g. below 10−22 Pa at 930 °C and nitrogen partial pressure around 105 Pa. Such conditions can be never reached experimentally without applying reducing gases having in mind an oxygen partial pressure of about 10−1 Pa in the argon and nitrogen gases used. But such low oxygen partial pressures can be present locally at the metal-oxide interface where the Zr–ZrO2 equilibrium partial pressure, e.g. at 1000 °C, is about 10−30 Pa according to Fig. 9. Hence, if oxygen and nitrogen reach the metal-oxide interface by gas diffusion through any imperfection in the oxide scale or (less preferred) by solid state diffusion through the oxide and along the grain boundaries, the oxygen is first consumed. Only after complete consumption of the oxygen, and reaching e.g. oxygen partial pressures of less than 10−20 Pa at 1000 °C, nitrogen may react with the metal to form zirconium nitride, ZrN. Contrary to the reaction of nitrogen with as-received zirconium alloys, the nitriding kinetics of oxygen-stabilized α-Zr(O), i.e. ZrO0.4 in equilibrium with the oxide, is very fast, and can be described by Eq. 3 [30].
PO2/PN2 stability diagram based on data for pure substances of Barin [29]. The stars in the right upper corner represent the experimental atmospheres
With continuing reaction, the ZrN moves away relative to the metal-oxide interface, leading to an increase of the surrounding local oxygen partial pressure and oxidation of the nitride according to Eq. 4 and Fig. 9.
The molar volumes of the involved phases are quite different (Vm,Zr = 14.0 cm3/mol, Vm,ZrN = 14.8 cm3/mol, Vm,ZrO2 = 22.0 cm3/mol). Thus, the reaction according to Eq. 4 is associated with a volume increase by 48 %, causing very high compressive stresses which are relieved by crack formation and finally leads to the formation of the very porous oxide layers. Furthermore, nitrogen is released by this reaction which is available for further nitride formation at the metal-oxide interface.
The mechanism described above is valid for all temperatures and gas compositions investigated, but it is of course affected by the experimental boundary conditions, as it is obvious in the micrographs in Figs. 4, 5, 6, and Fig. 7. The dimension of the ZrN containing regions strongly increases with rising temperature from about 1 µm at 800 °C to around 50 and 200 µm at 1000 and 1200 °C, respectively. Obviously, the ZrN stability region is extending with rising temperature. According to Fig. 9, zirconium nitride is stable for oxygen partial pressures below 10−25 Pa at 800 °C, 10−20 Pa at 1000 °C, and 10−16 Pa at 1200 °C. Even increasing, these oxygen partial pressures are so small that they may not solely explain the temperature dependence of the extension of the nitrides. The generally enhanced oxidation with higher temperatures causes stronger oxygen consumption and thus extended areas with locally very low oxygen partial pressures. The overall change of the oxide morphology from a very fine structure at 800 °C to much bigger oxide/nitride particles and pores/cracks may be due to the changing mechanical properties of the oxide affecting the critical oxide scale thickness before breaking.
As could be expected from the TG results, the oxygen/nitrogen ratio in the gas phase only slightly affected the morphology of oxide/(nitride) layer at 800 °C. No oxygen starvation was observed for the broad range of gas compositions and the consumption of nitrogen was very small. For the higher temperatures, more nitride is seen with increasing nitrogen content in the gas. Oxygen starvation was observed for lower oxygen concentrations influencing the morphology of the oxide/nitride layer. At 1000 °C breakaway oxidation which is known to occur in steam and oxygen due to the tetragonal to monoclinic phase transition in the oxide after 30–90 min opens the path for the nitrogen attack. No breakaway oxidation occurs at temperatures beyond 1050 °C. Hence, at 1200 °C, nitrogen could only meet the metal if (i) oxygen starvation takes place from the very beginning (before a protective oxide scale has been formed), (ii) radial cracks give local access to the metal, and nitride formation propagates from such failure positions or (iii) nitrogen diffuses through the dense oxide to the metal-oxide interface. The increasing parabolic rate constants may give indication for the latter mechanism whose discussion is beyond the scope of this paper. The micrographs in Fig. 6 point to global oxygen starvation for the tests in atmospheres with low oxygen contents (>95 % nitrogen) and show examples for local nitrogen attack, e.g. for the samples annealed in 50, 70, and 90 % nitrogen. The stochastic character of the nitrogen access due to ZrN nucleation and growth [15] is also illustrated in Fig. 6 by the comparably low and local ZrN formation seen for the specimen annealed at 1200 °C in 70 % nitrogen compared to the samples annealed in 60 and 80 % nitrogen.
“Global” oxygen starvation, i.e. mechanism (i), is also the reason for the nitrogen attack and much stronger oxidation of the inner surfaces of the samples annealed at 1200 °C in atmospheres with less than 90 % nitrogen in the gas mixture. The bottom-to-top flow of the gas mixture along the quasi one-side closed sample standing on an alumina plate creates differing thermo-hydraulic boundary conditions for the internal and external samples surfaces. Whereas the external surface is steadily flown by the gas mixture, the atmosphere in the tube segment is rather stagnant, and transport of the reaction gases in the tube segments is mainly determined by gas diffusion and convection. This non-prototypic effect is more relevant at higher the temperatures; in this study, it was only observed at 1200 °C. The focus of attention should be on the external surfaces because the fuel claddings in reactors are closed and hence only external surfaces are in contact with the oxidizing atmosphere.
Conclusion
The oxidation of Zircaloy-4 was investigated at 800, 1000, and 1200 °C in oxygen–nitrogen mixtures over the whole range of gas compositions between the two gases. The strong influence of nitrogen on the oxidation kinetics already seen in earlier studies [10–16] was confirmed for all test conditions. Zirconium nitride is formed at the metal-oxide interface where locally two conditions are fulfilled: 1) the thermodynamic boundary conditions, i.e. very low oxygen partial pressures, for the formation of ZrN are given and 2) the kinetics of the reaction of nitrogen with the oxygen-stabilized zirconium, as it is in equilibrium with the oxide, is very fast. The transport of the nitrogen to the metal-oxide interface may occur through imperfections in the initially formed oxide, or by anion diffusion through the sub-stoichiometric oxide. Furthermore nitrogen may attack zirconium in case of global oxygen starvation, i.e., complete consumption of the oxygen in the ambient atmosphere and hence non-existing protective zirconia scale. According to earlier studies [12], a preformed dense oxide layer prevents nitrogen attack as long as it is intact.
When zirconium nitride is formed once at the metal-oxide interface, with continuing reaction it is moving away relative to the Zr–ZrO2 interface, and consequently it is leaving its thermodynamic stability region because the local oxygen partial pressures rises. The oxidation of zirconium nitride to zirconium oxide according to Eq. 4 is associated with a volume increase by more than 48 % which is the main reason for the formation of very porous oxide scales. Once this mechanism, i.e. nitride formation and oxidation, has started it is self-sustaining because nitrogen is released by the oxidation of ZrN and available for further nitriding at the metal-oxide interface. Eventually, zirconium is completely converted to oxide; hence nitrogen acted like a catalyst.
The consequence for the overall reaction kinetics is a transition from (sub-)parabolic to linear or even accelerating reaction rates, at least if initially a protective oxide scale is formed. This study revealed the effect of temperature and gas composition on the mechanisms described above. Nitride formation increased with temperature and ambient nitrogen content, but even marginal nitride formation strongly affected oxidation kinetics, as impressively seen in the test series at 800 °C.
A more general conclusion from this work is that experiments on the oxidation kinetics of zirconium alloys must be conducted very carefully especially with respect to well controlled gas atmospheres. Nitrogen (air) must be excluded from the reaction gas as long as they are not used by intention.
Further studies on the oxidation of zirconium alloys in nitrogen-containing atmospheres have already been initiated taking into account ternary compounds in the system Zr–O–N [31] as well as more prototypic steam-nitrogen atmospheres [32].
References
M. Steinbrück, M. Große, L. Sepold and J. Stuckert, Nuclear Engineering and Design 240, 1714–1727 (2010).
G. Schanz, B. Adroguer and A. Volchek, Nuclear Engineering and Design 232, 75–84 (2004).
M. Grosse, Nuclear Technology 170, ,272 (2010).
M. Steinbrück, Oxidation of Metals 70, 317–329 (2008).
M. Steinbrück, N. Vér and M. Grosse, Oxidation of Metals 76, 215–232 (2011).
J. H. Back, K. B. Park and Y. H. Jeong, Journal of Nuclear Materials 335, 443–456 (2004).
M. Steinbrück, et al., Progress in Nuclear Energy 52, 19–36 (2010).
D. A. Powers, L. N. Kmetyk and R. C. Schmidt, A Review of Technical Issues of Air Ingression during Severe Reactor Accidents. Report NUREG/CR-6218, SAND94-0731, Sandia National Lab., (1994).
I. Shepherd et al., Oxidation Phenomena in Severe Accidents (OPSA). Final Report, INV-OPSA(99)-P008, (2000).
M. Steinbrück, A. Miassoedov, G. Schanz, L. Sepold, U. Stegmaier and J. Stuckert, Nuclear Engineering and Design 236, 1709–1719 (2006).
M. Steinbrück and M. Böttcher, Journal of Nuclear Materials 414, 276–285 (2011).
M. Steinbrück, Journal of Nuclear Materials 392, 531–544 (2009).
C. Duriez, T. Dupon, B. Schmet and F. Enoch, Journal of Nuclear Materials 380, 30–45 (2008).
C. Duriez, M. Steinbrück, D. Ohai, T. Meleg, J. Birchley and T. Haste, Nuclear Engineering and Design 239, 244–253 (2009).
M. Lasserre, V. Peres, M. Pijolat, O. Coindreau, C. Duriez and J.-P. Mardon, Materials and Corrosion 65, 250–259 (2014).
M. Steinbrück, U. Stegmaier and T. Ziegler, Prototypical Experiments on Air Oxidation of Zircaloy-4 at High Temperatures. Forschungszentrum Karlsruhe, Report FZKA, 7257 (2007).
N. Birks, G. H. Meier and F. S. Pettit, Introduction to the High-Temperature Oxidation of Metals, 2nd ed, (Cambridge University Press, Cambridge, 2006). ISBN 978-0-521-48042-0.
M. Lerch, Journal of the American Ceramic Society 79, 2641–2644 (1996).
J.-S. Lee, M. Lerch and J. Maier, Journal of Solid State Chemistry 179, 270–277 (2006).
M. Lerch, et al., Progress in Solid State Chemistry 37, 81–131 (2009).
L. Gribaudo, D. Arias and J. Abriata, Journal of Phase Equilibria 15, 441–449 (1994).
P. Hofmann, S. Hagen, G. Schanz and A. Skokan, Chemical Interactions of Reactor Core Materials up to very High Temperatures. Report KFK-4485, Karlsruhe, (1989).
P. Liang, N. Dupin, S. G. Fries, H. J. Seifert, I. Ansara, H. L. Lukas and F. Aldinger, Zeitschrift für Metallkunde 92, 747–756 (2001).
H. M. Chung and T. F. Kassner, Journal of Nuclear Materials 84, 327–339 (1979).
N. Dupin, I. Ansara, C. Servant, C. Toffolon, C. Lemaignan and J. C. Brachet, Journal of Nuclear Materials 275, 287–295 (1999).
E. L. Dreizin, V. K. Hoffmann and E. P. Viecenzi, Journal of Materials Research 14, 3840–3842 (1999).
A. Ermoline, M. Schoenitz and E. L. Dreizin, Journal of Materials Research 21, 320–328 (2006).
M. Lerch, Journal of Materials Science Letters 17, 441–443 (1998).
I. Barin, Thermochemical Data of Pure Substances, (VCH Verlagsgesellschaft, Weinheim, 1995).
M. Steinbrück, Journal of Nuclear Materials 447, 46–55 (2014).
S. Park, L. Fernandez-Moguel, M. Steinbrück, J. Birchley, H. -M. Prasser and H. J. Seifert, A mechanism of nitridation process in the Zr-O-N system during air oxidation. The Nuclear Materials Conference NUMAT2014, 27–30 Oct 2014, Clearwater Beach, Florida.
M. Steinbrück, F. Oliveira da Silva and H. J. Seifert, High-temperature oxidation of Zircaloy-4 in steam-nitrogen mixtures. The Nuclear Materials Conference NUMAT2014, 27–30 Oct 2014, Clearwater Beach, Florida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinbrück, M., Schaffer, S. High-Temperature Oxidation of Zircaloy-4 in Oxygen–Nitrogen Mixtures. Oxid Met 85, 245–262 (2016). https://doi.org/10.1007/s11085-015-9572-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-015-9572-1