Abstract
The oxidation behavior of two ferrite/martensite (F/M) steels, including a novel 9–12 % Cr modified F/M steel named SIMP steel and a commercial T91 steel, were studied in air at 700 °C for up to 1,000 h. The oxides formed on the two steels were characterized and analyzed by XRD, XPS, SEM and EPMA. The results showed that the oxide formed on SIMP steel was single-layer including flake-like Cr2O3 with Mn1.5Cr1.5O4 spinel particles, while the oxide on T91 steel exhibited a double layers structure consisting of an outer hematite Fe2O3 layer and an inner Fe–Cr spinel layer. The reason why the SIMP steel showed better high temperature oxidation resistance than T91 steel was analyzed to be due to the higher Cr and Si contents that could form compact and continuous oxide layer on the steel. Based on all the results, a kinetic model describing nucleation, growth and degradation of the oxide scale formed on surfaces of the two steels was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
9–12 % Cr ferritic/martensitic (F/M) steels have advantages of lower cost, lower thermal expansion coefficient and better resistance to high temperature oxidation than austenitic stainless steels, which make them an attractive choice applied in many energy applications, in particular power plants and the accelerator driven sub-critical system (ADS) [1, 2].
A novel 9–12 % Cr modified F/M steel named SIMP steel has been developed recently by our team according to the harsh environment caused by the liquid Lead–Bismuth Eutectic (LBE) interaction with the containment at high temperature in ADS application, and the general idea of designing SIMP steel can be divided into three aspects: (1) contents of Cr and Si are increased to further improve the corrosion resistance to liquid LBE as refer to the Russian EP823 steel [3, 4]; (2) lower activation Ta and W elements are substituted for higher activation Nb and Mo elements [5–7], and the concentration of activating elements such as Ni and Mo are reduced according to the China Low Activation Martensitic (CLAM) steel [8]; (3) the Cr and Ni equivalent is controlled to obtain a single martensitic microstructure in order to improve the creep behavior.
The aim of the present work was to study the high temperature oxidation behavior of SIMP steel at 700 °C in air for up to 1,000 h. The oxide formed on the experimental steels was characterized, the microstructure of the oxide scale formed on the steels was analyzed, and the reason that SIMP steel showed better high temperature oxidation resistance compared to T91 steel was discussed. Considering all these results, a kinetic model describing nucleation, growth and degradation of oxide scale on the surface of experimental steels in air was proposed.
Experimental Procedures
Two F/M steels were studied in this work, including newly developed SIMP steel and a commercial T91 steel. Table 1 shows the chemical compositions of the two experimental steels. The SIMP steel was melted in a 500 kg vacuum induction melting furnace. The ingot was firstly forged at 1,200 °C, and then hot-rolled at 1,100 °C to sheets of 14 mm thick. The SIMP steel was normalized at 1,050 °C for 0.5 h, and then tempered at 760 °C for 1.5 h followed by air cooling. The as received commercial T91 steel, made in Japan, was normalized at 1,050 °C for 20 min, and then tempered at 780 °C for 60 min followed by air cooling. The above heat treatments created the steels containing a lath martensite microstructure, and Cr23C6 was precipitated along the lath and the prior austenite grain boundary [9].
Samples with size of 10 mm × 10 mm × 10 mm were cut directly from the SIMP and T91 steels using a spark erosion technique, polished by SiC papers up to 2000 grade, then washed by ethanol and finally dried. Samples were ultrasonically cleaned, weighed, and physically measured prior to starting the oxidation test. All the samples were placed in a roasted crucible in order to avoid weight loss by spallation. Continuous isothermal oxidation tests were conducted in air at 700 °C for up to 1,000 h. After testing, samples were removed from the furnace, air cooled and weighed at room temperature on a balance with accuracy better than 10−3 g. The weight change was determined from the difference the sample before and after oxidation testing divided by the sample surface area. The weight gain of samples was indicative of oxygen ingress and formation of oxide on the steels.
Weight changes of samples before and after high temperature exposures were measured by an electric balance with 0.1 mg in accuracy. The oxide formed on the surface of samples was identified by means of X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) using an installation coupled with Cu-Kα radiation source, and high definition scans were performed at a rate of 0.02°/s in the 2θ interval ranging from 15° to 85°. The surfaces of the SIMP and T91 steels were etched to reveal the microstructure by using a Vilella solution of 100 ml C2H5OH, 5 ml HCl and 1 g picric acid. The microstructure and morphology of the oxide on the two steels were observed on a scanning electron microscope (SEM) and an electron probe microanalysis (EPMA). Samples were coated with a thin layer of gold to allow charge dissipation during the analysis. Energy dispersive X-ray (EDX) analysis was conducted on the samples when required.
Results
Microstructure
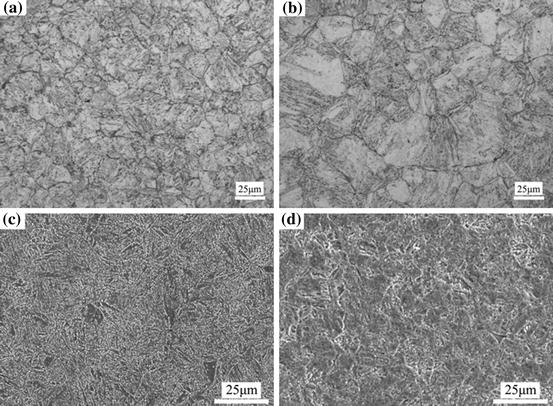
Figure 1 shows the SEM images of the tempered martensite structures of SIMP and T91 steels. In both SIMP and T91 steels, the carbides formed at the prior austenite and martensitic lath boundaries. The grain size of SIMP steel was slightly smaller than that of T91 steel and the carbides formed in SIMP steel were obviously more than those in T91 steel, as the carbon and chromium concentrations in SIMP steel were much higher than T91 steel, especially the carbon in SIMP steel was more than twice as much as that in T91 steel.
Oxidation Kinetics
Curves of weight gain versus oxidation time at 700 °C in air for up to 500 h are given in Fig. 2. It is obvious that the weight gain of unit area of oxidized samples was increased with increase of oxidation time, and the weight gain of SIMP steel was much less than that of T91 steel after oxidizing for 50 h. The weight gain curve of SIMP steel exhibits a parabolic feature for the duration of the test and the parabolic constant is 4.15 × 10−4 mg2 cm−4 h−1.
It is interesting that the weight gain curve of T91 steel generally had three distinct stages. The first 50 h of oxidation, which was almost the same as SIMP steel, was the first stage possibly caused by a selective oxidation of chromium. The second stage was a rapid oxidation stage lasting from 100 to 200 h. After 200 h of oxidation, it came to the third stable oxidation stage.
XRD Analyses
The X-ray diffraction analyses of the samples also provide important information on the oxidation phenomena. Phase analyses on oxide by XRD on SIMP and T91 steels after oxidation at 700 °C for 200, 500 and 1,000 h are shown in Fig. 3. The oxidized products on the surface of SIMP steel are composed of Cr2O3 and Mn1.5Cr1.5O4, and the intensity of Mn1.5Cr1.5O4 increased as the oxidation time increased, while only the peak of Fe2O3 was found on the surface of T91 steel, which indicates a different oxidation behavior compared with SIMP steel. The Fe–Cr peak can be found on the XRD patterns for SIMP steel and had little change as the oxidation time increased, indicating that the oxide formed on SIMP steel was much thinner than that on T91 steel.
X-ray Photoelectron Spectroscopy
It is important to note that the thickness of the sample analyzed by XPS is very small, depending on the mean distance covered by the emitted electrons that are related to their energy. The analyzed thickness, when the X-ray beam is perpendicular to the sample surface, is between 0.5 and 10 nm [10]. Thus, the information obtained from the XPS analysis comes from the very outer surface of the oxide layer. When the beam angle is reduced by tilting the sample, the information becomes more and more characteristic from the surface of oxide [11].
The XPS analysis was taken on surface oxide formed on SIMP steel oxidized for 208 h, and the XPS peaks and their reconstruction are shown in Fig. 4. For nearly all the cases, two components should be considered to rebuild the experimental chromium peak (Fig. 4a). The energy of the more intense peak corresponded to the Cr3+ in Cr2O3 while the energy of the small peak indicated the presence of chromium in the mixed Cr1.5Mn1.5O4 oxide at very outer surface, which was confirmed by the XRD analysis. Three components were necessary for manganese (Fig. 4b). Due to their binding energy, it might be confirmed that the first peak corresponded to the Mn2+ (i.e., a MnO compound), the second to Mn bounded in the mixed oxide (Cr1.5Mn1.5O4), and the last one corresponded to a satellite. The silicon and iron were not detected by XPS on the oxidized surface of SIMP steel.
Morphology of Oxides
Figure 5 shows the SEM microstructure of the surface oxide formed on SIMP and T91 steels at 700 °C for 200, 500 and 1,000 h, respectively. The morphologies of the oxide formed on SIMP and T91 steels were different. The morphology of the oxide formed on T91 steel was rather loose and porous while the oxide on SIMP steel presented continuous potato flake-like oxide and granular particles. The potato flake-like oxide was 3–5 μm in size and did not change as the oxidation time increased, however, the granular particles grew up as the oxidation time increased. The oxide formed on T91 steel was Fe2O3 (Hematite), and the potato flake-like oxide and granular particles formed on SIMP steel were Cr2O3 (Eskolaite) with some Mn and Cr1.5Mn1.5O4 characterized by EDX and X-ray diffraction analysis.
Figure 6 shows SEM-BSE images of the oxide layer on SIMP and T91 steels after exposure in air at 700 °C for 200, 500 and 1,000 h, respectively. The oxide scale formed on SIMP steel was a single-layer of Cr2O3 (Eskolaite) with Cr–Mn spinel (Mn1.5Cr1.5O4) particles, while the oxide scale on T91 steel exhibited a double layers structure consisted of Fe2O3 (Hematite) outer layer and Fe–Cr spinel inner layer. The ratio of the thickness of the outer hematite layer over the inner Fe–Cr spinel layer was roughly equal to 1.3 for T91 steel, which is slightly higher than 1.2 reported elsewhere [12], and the ratio remained constant during the whole oxidation process.
The oxide layer thickness on SIMP was rather smaller than that on T91 steel. The thickness of oxide scale on T91 steel increased from 78 μm of 200 h oxidation to 157 μm of 1,000 h oxidation, while the oxide scale thickness on SIMP steel increased from 1.4 to 1.9 μm and did not change much for the duration of the test. However, the Cr–Mn spinel particles formed on SIMP steel increased during the oxidation test, and the silicon which was enriched at the interface between oxide scale and SIMP matrix was measured to be 2.85 wt% by EDX analysis, which was almost twice as much as that in SIMP steel. Pores which almost located at the interface between outer layer and inner layer on T91 steel were segregated and grew up as the oxidation time increased, and spallation of the outer hematite layer was observed at the rib joint of samples of T91 steel.
To obtain a better characterization of the oxide scale formed on SIMP and T91 steels, EMPA analyses were conducted. The element distributions in the oxide scale on SIMP and T91 steels oxidized in air at 700 °C for 1,000 h are shown in Figs. 7 and 8. The element distributions in the oxide scale formed on SIMP and T91 steels were totally different. A continuous and compact chromia layer was formed on SIMP steel and silicon was enriched beneath the Cr2O3 layer, whereas the outmost was rich in both manganese and chromium, which was in agreement with XRD and XPS analyses. The element distribution in the oxide scale on T91 steel presented a double layers structure with outer layer enriched of Fe and inner layer enriched of Fe and Cr, while manganese was found in the whole oxide scale and silicon was only observed in the inner Fe–Cr spinel layer. Chromium and silicon in the inner Fe–Cr spinel were in layered distribution as the steel was rolled, and they were enriched at the grain boundaries.
Discussion
Differences in Oxidation Resistance
The high temperature oxidation resistance of SIMP steel should be rather better than that of T91 steel as it was found that the oxide thickness on SIMP steel was only about 2 μm while that on T91 steel was about 157 μm after oxidation in air at 700 °C for 1,000 h. The oxides on the two steels were different as the oxide on SIMP steel was single protective chromia layer with Mn1.5Cr1.5O4 spinel particles while that on T91 steel was a double layers structure with an outer hematite layer and an inner Fe–Cr spinel layer. The difference on high temperature oxidation resistance for the two steels should be attributed to the different chemical compositions of the steels, which have been studied quite extensively [13–19], especially contents of chromium and silicon in SIMP steel is much higher than those in T91 steel.
The excellent high temperature oxidation resistance of SIMP steel after prolonged oxidation at 700 °C, compared with T91 steel, should be attributed to three main reasons. The first reason was the more compact and continuous chromia layer grown by counter-current diffusion of chromium and oxygen depending strongly on the diffusion behavior of Cr along the grain boundary, which was much higher than that in the bulk [20]. As the grain size of SIMP steel was smaller than that of T91 steel (see Fig. 1), plus the higher Cr content, a compact and continuous chromia layer was formed on SIMP steel and did not peel off during the cycle oxidation. Furthermore, during the initial oxidation Cr was also oxidized, leading to formation of a Cr-depleted zone [21–23] under the oxide scale. Due to the slow mobility of Cr [24] the Cr-depleted zone was stable.
The second reason was related to the higher silicon content in SIMP steel (see Table 1), which was enriched at the substrate/scale interface after oxidation for 1,000 h (see Fig. 7). Small amount of silicon could reduce the high temperature oxidation rate in Fe–Cr steel [25–27]. Si in SIMP steel was possibly oxidized to form SiO2 and then reacted with FeO in the scale under lower oxygen partial pressure to produce Fe2SiO4. The existence of Fe2SiO4 provided a certain level of high oxidation resistance for steel, which impeded the diffusion of iron cations in scale [28]. Once a continuous layer of Fe2SiO4 was formed between the oxide scale and the substrate, the oxide scale would further impede the diffusion of iron cations from substrate to oxide scale. When the diffusion of iron was impeded, the amount of iron cations in scale would decrease. Consequently, the high oxidation rate of steel would be slowed down [26, 29, 30].
Furthermore, the formation of silicon oxide at the metal/scale interface could act as the initiation sites for chromia formation [15] and the Si increased the effective inter-diffusion coefficient of Cr in the metal matrix, so that the formation and the re-formation of the protective chromia layer was promoted [31], although the chromium content in SIMP steel was less than the minimum bulk concentration [32], which was necessary to form the protective chromia layer on the entire surface to prevent oxidation of the base material. Moreover the silica greatly enhanced the adhesive force of the chromia layer to the steel matrix [33].
The outmost oxide particles were Mn–Cr spinel Mn1.5Cr1.5O4 due to the high diffusion rate of manganese ions through the oxide, which was consistent with other alloys oxidized at high temperatures [34–36]. On the other hand, the formation of Mn–Cr spinel significantly reduced the evaporation of Cr [37]. However, a few of Mn2+ (see Fig. 4b), which was lower than Cr3+, dissolved in the chromia scale, which decreased the ion defeat concentration and improved the compact of the scale as Cr2O3 was a P-type semiconductor with surplus negative ions and insufficient positive ions [38].
Kinetic Model of Oxide Layer Growth
Figure 9 shows a kinetic model of the evolution of the oxide scale on SIMP and T91 steels exposed to air at 700 °C. At the first stage of nucleation, mechanical failure of the initial passive layer might take place [39], thus leading to the nucleation and growth of initial Cr oxide encountering with [O], which came from the decomposition of oxygen after absorbed at the surface of the steels. X = 0, which was slightly beneath the initial chromia, was the initial surface of the two steels as chromia layer grew by counter-current diffusions of chromium and oxygen. Such Cr2O3 layer resulted in a slight Cr depletion in the steel close to the internal oxide-metal interface. The consistency and integrity of the initial chromium oxide layer depends on the content of alloy composition. The slowest kinetic should be attributed to the formation of chromia layer plus original passive layer, which may hinder the diffusion of cations and anion during the first stage of oxidation on the two steels. This is the reason that the oxidation kinetic of two steels kept a lower rate level in the first oxidized 50 h.
At the second stage, structure of the oxide scale changed as the oxidizing time increased due to the different compositions of the two steels, especially the chromium and silicon contents. The oxide scale on SIMP steel remained single protective chromia layer structure and the silicon was enriched beneath the chromia layer, which had positive effect on the oxidation resistance due to the higher silicon content [31]. Mn–Cr spinel was formed by the high diffusion rate of manganese ions through the oxide [35]. However, the oxide scale on T91 steel changed into a double layers structure with the outer Fe2O3 layer and the inner Fe–Cr spinel layer as the initial chromia layer could not impede the iron and oxygen diffusions due to the less consistency and compact characteristic of the chromia layer.
At the third stage, the oxide scale on SIMP steel was still the single protective chromia layer like the second stage except the thickness of chromia layer slightly increases and the Cr–Mn spinel (Mn1.5Cr1.5O4 particle) grows. However, the outer hematite layer on T91 steel was spallation at the interface between hematite and Fe–Cr spinel, for which there were two reasons. First, the hematite layer was subjected to a high compressive stress after cooling from the oxidation temperature, and the primary source of the stress was the thermal mismatch between the oxide and the steel, which led to the development of mechanical stress in the oxide scale during the thermo-cycling process [40]. Second, a compressive stress in the oxide during its growth provided substantial contribution to the total residual compression at room temperature whenever the Pilling-Bedworth ratio [41] was more than 2 [42].
Conclusions
The oxidation behaviors of F/M SIMP steel and commercial T91 steel have been investigated at 700 °C for up to 1,000 h. The oxide scales formed on the two steels were characterized and the SIMP steel exhibited better high temperature oxidation resistance, compared with T91 steel, was analyzed. The main conclusions are drawn in the following:
-
1.
The oxide scale formed on SIMP steel was single-layer including flake-like Cr2O3 with Mn1.5Cr1.5O4 spinel particles, while the oxide scale on T91 steel exhibited a double layers structure consisting of an outer hematite Fe2O3 layer and an inner Fe–Cr spinel layer.
-
2.
The SIMP steel with better oxidation resistance than T91 steel was due largely to formation of a continuous and compact Cr2O3 layer combining with Cr1.5Mn1.5O4 particles on SIMP steel and the development of internal silica beneath the chromia layer, which should be attributed to the higher chromium and silicon contents in SIMP steel in comparison with T91 steel.
-
3.
A kinetic model describing the evolution of oxide scale on surfaces of SIMP and T91 steels during exposure to air is proposed, which includes the main stages of interaction: nucleation, growth and degradation of the oxide scale. With increase of the time at high temperature, the two steels exhibited different oxidation mechanisms, forming single and duplex oxide scale on SIMP and T91 steels, respectively.
References
R. Klueh and A. Nelson, Journal of Nuclear Materials 371, 37 (2007).
F. Masuyama, ISIJ International 41, 612 (2001).
O. Eliseeva, V. Tsisar, V. Fedirko and Y. S. Matychak, Materials Science 40, 260 (2004).
O. I. Eliseeva and V. P. Tsisar, Materials Science 43, 230 (2007).
A. Ioltukhovsky, M. Leontyeva-Smirnova, Y. Kazennov, E. Medvedeva, A. Tselishchev, V. Shamardin, A. Povstyanko, S. Ostrovsky, A. Dvoryashin and S. Porollo, Journal of Nuclear Materials 258, 1312 (1998).
A. Ioltukhovskiy, A. Blokhin, N. Budylkin, V. Chernov, M. Leont’eva-Smirnova, E. Mironova, E. Medvedeva, M. Solonin, S. Porollo and L. Zavyalsky, Journal of Nuclear Materials 283, 652 (2000).
A. Ioltukhovskiy, M. Leonteva-Smirnova, M. Solonin, V. Chernov, V. Golovanov, V. Shamardin, T. Bulanova, A. Povstyanko and A. Fedoseev, Journal of Nuclear Materials 307, 532 (2002).
L. Huang, X. Hu, C. Yang, W. Yan, F. Xiao, Y. Shan and K. Yang, Journal of Nuclear Materials 443, 479 (2013).
R. L. Klueh and D. R. Harries, High-Chromium Ferritic and Martensitic Steels for Nuclear Applications, (ASTM, West Conshohocken, 2001).
D. Briggs, J. C. Riviere, in: D. Briggs, M. P. Seah (Eds.), Practical Surface Analysis, Vol. 1, (Chapter 3), (Wiley, Chichester, 1996), p. 85–141.
A. M. Huntz, A. Reckmann, C. Haut, C. Sévérac, M. Herbst, F. C. T. Resende and A. C. S. Sabioni, Materials Science and Engineering 447, 266 (2007).
L. Martinelli, F. Balbaud-Célérier, A. Terlain, S. Bosonnet, G. Picard and G. Santarini, Corrosion Science 50, 2537 (2008).
Y. Niu, S. Wang, F. Gao, Z. Zhang and F. Gesmundo, Corrosion Science 50, 345 (2008).
H. Asteman and M. Spiegel, Corrosion Science 50, 1734 (2008).
F. H. Stott and F. I. Wei, Oxidation of Metals 31, 369 (1989).
N. Babu, R. Balasubramaniam and A. Ghosh, Corrosion Science 43, 2239 (2001).
G. B. Abderrazik, G. Moulin, A. Huntz and E. Young, Solid State Ionics 22, 285 (1987).
J. Nychka and D. Clarke, Oxidation of Metals 63, 325 (2005).
J. Mougin, M. Dupeux, L. Antoni and A. Galerie, Materials Science and Engineering 359, 44 (2003).
I. Kaur and W. Gust, Fundamentals of Grain and Interphase Boundary Diffusion, (Ziegler Press, Stuttgart, 1988).
B. A. Pint and I. G. Wright, Materials Science Forum, (Trans Tech Publications, Durnten, 2004), p. 799.
R. M. Deacon, J. DuPont, C. Kiely, A. Marder and P. Tortorelli, Oxidation of metals 72, 87 (2009).
E. Airiskallio, E. Nurmi, M. H. Heinonen, I. J. Vayrynen, K. Kokko, M. Ropo, M. P. J. Punkkinen, H. Pitkanen, M. Alatalo, J. Kollar, B. Johansson and L. Vitos, Corrosion Science 52, 3394 (2010).
P. C. Tortorici and M. Dayananda, Materials Science and Engineering: A 244, 207 (1998).
L. Mikkelsen, S. Linderoth and J. Bilde-Sørensen, Materials Science Forum, (Trans Tech Publications, Durnten, 2004), p. 117.
R. Pettersson, L. Liu and J. Sund, Corrosion Engineering, Science and Technology 40, 211 (2005).
G. Bamba, Y. Wouters, A. Galerie, F. Charlot and A. Dellali, Acta Materialia 54, 3917 (2006).
S. Liu, D. Tang, H. Wu and L. Wang, Journal of Materials Processing Technology 213, 1068 (2013).
A. Paúl, S. Elmrabet, L. Alves, M. Da Silva, J. Soares and J. Odriozola, Nuclear Instruments and Methods in Physics Research Section B 181, 394 (2001).
F. Riffard, H. Buscail, E. Caudron, R. Cueff, C. Issartel and S. Perrier, Materials Characterization 49, 55 (2002).
B. Li and B. Gleeson, Oxidation of Metals 65, 101 (2006).
V. B. Trindade, U. Krupp, B. Z. Hanjari, S. Yang and H.-J. Christ, Materials Research 8, 371 (2005).
A. M. Huntz, V. Bague, G. Beauple, C. Haut, C. Severac, P. Lecour, X. Longaygue and F. Ropital, Applied Surface Science 207, 255 (2003).
R. K. Wild, Corrosion Science 17, 87 (1977).
R. E. Lobnig, H. P. Schmidt, K. Hennesen and H. J. Grabke, Oxidation of Metals 37, 81 (1992).
M. K. Hossain, Corrosion Science 19, 1031 (1979).
G. R. Holcomb and D. E. Alman, Scripta Materialia 54, 1821 (2006).
K. Hauffe, Oxidation of Metals, (Plenum Press, New York, 1965).
N. Quan and D. Young, Oxidation of Metals 25, 107 (1986).
V. Tolpygo and D. Clarke, Acta Materialia 47, 3589 (1999).
N. Pilling and R. E. Bedworth, Journal of the Institute of Metals 29, 529 (1923).
T. Mitchell, D. Voss and E. Butler, Journal of Materials Science 17, 1825 (1982).
Acknowledgments
This work was financially supported by a sub-project (XDA03010301, XDA03010302) of Advanced Fission Energy Program-ADS Transmutation System, Chinese Academy of Sciences Strategic Priority Research Program (XDA03010000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Q., Liu, J., Wang, W. et al. High Temperature Oxidation Behavior of SIMP Steel. Oxid Met 83, 521–532 (2015). https://doi.org/10.1007/s11085-015-9532-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-015-9532-9