Abstract
Eukaryotes, by the same combination of cytoskeleton and molecular motor, for example actin filament and myosin, can generate a variety of movements. For this diversity, the organization of biological machineries caused by the confinement and/or crowding effects of internal living cells, may play very important roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cytoskeletal system, consisting of actin filaments, microtubules and molecular motors together with their collaborating proteins, is a native smart micro machinery. It is involved in a number of dynamic activities regarding morphogenesis, transporting, movement, and force generation, in a wide range of the biological hierarchy, for example, from swimming of unicellular organism to movements of multicellular organism that are caused by muscle contraction. In order to unveil the underlying mechanism on these self-controlled motions with various motilities in living cells, cell-sized giant liposomes incorporating purified actin filament (F-actin) accompanied with molecular motor myosin or its derivatives (HMM or S-1) inside is examined, which was prepared through spontaneous transfer of a droplet across oil/water interface (Takiguchi et al. 2011; Yoshikawa et al. 2011). Novel scenario on transformation of the self-assembly system with these motile proteins is reported.
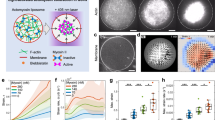
From in vitro studies, it has been established that actoHMM (assembly consists of F-actin and HMM), as well as F-actin, forms bundle structures in the presence of relatively large amounts of inert polymers, e.g., methylcellulose and PEG. This bundling force under the crowding environment has been interpreted in terms of the depletion effect. In the bundles, a large number of F-actins closely aligned in parallel with overlaps. The bundles of actoHMM or F-actin are usually non-polar. Accompanied by the generation of the bundles, these proteins cause spindle-like structures as exemplified in Fig. 1 (upper). For generating the actin sliding motility with molecular motors, ATP, a chemical fuel, is required. In our experiment, the addition of Mg-ATP into the complexes of HMM and F-actin under the crowding condition caused transformation of their organized structure owing to the sliding motion between F-actins and HMM. Successively, each bundle showed contraction, elongation, bending or twisting (Tanaka-Takiguchi et al. 2004). Following these shape change or active motion, each bundle tended to split longitudinally into several bundles in a stepwise manner, while the newly formed bundles remained associated with one end where the barbed end (B-end) of F-actins gathered. The product, an aster-like assembly of actoHMM (Fig. 1, bottom), was stable, that is, the individual bundles never contracted upon the further addition of ATP. It is to be noted that similar aster-like assemblies of F-actin are found in living cells (Arai and Mabuchi 2002).
Transformation of assembling system with actin and molecular motor in the bulk solution (Takiguchi et al. 2011)
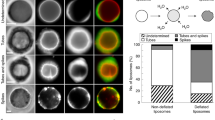
ActoHMM bundles together with methylcellulose at the concentration sufficient for maintaining bundle formation were successfully encapsulated into giant liposomes, and they changed the morphology and distribution inside the liposomes after the supply of ATP through the pores of α-hemolysin (Takiguchi et al. 2011). In some of the liposomes, we observed the formation of aster-like structure (Fig. 2, bottom left), whose shape was essentially the same as that formed through the contraction of actin bundle in the bulk solution (Fig. 1). Interestingly, actoHMM bundles in most liposomes were redistributed onto the periphery of the inner surface, so-called cortex (Fig. 2, bottom right). Almost all of encapsulated bundles were transformed into either asters or cortexes. The changes observed in the encapsulated actoHMM can be attributed to the active sliding between F-actins and HMM, because both the ATP and α-hemolysin, which creates the membrane channel to introduce ATP into liposomes, were required. One should note that during these experiments, the morphology of the giant liposomes did not show any notable changes. Liposomes entrapping aster were larger than those entrapping cortex (Fig. 3). While keeping the concentration of F-actin constant, increase of HMM concentration leaded the tendency for the cortex formation (Fig. 3). In the cases of when almost all of encapsulated bundles were transformed to either aster or cortex, the critical liposome diameter, to ensure the formation of only aster inside liposomes, was increased with increasing of HMM concentration. When the HMM concentration was further increased, all bundles were transformed to cortex after the addition of ATP in all liposomes, regardless of the liposomal size. It has been known that the ratio between the characteristic length of an elastic rod-like structure and the diameter of a spherical space is one of the dominant factors that decide the distribution of rod-like elastic structures in a spherical space. HMM, which has two actin-sliding motor domains, functions as a crosslinker between F-actins. This feature of HMM may make the encapsulated actoHMM bundles larger and longer. The nature of the myosin (as well as HMM) adsorbing on any surface is another factor for the actoHMM bundles to localize on the inner surface, when the HMM concentration exceeds the maximum binding amount of actins. It may be of scientific importance to extend the study in cell-sized phospholipid vesicles entrapping high concentrations of biopolymers and to explore the effect of compartment. The dynamic assembly of actin bundles by the molecular motor-induced active sliding is affected strongly by the geometrical confinement. The buckling of actin filaments during the process forms a cortex structure, but not an aster. Therefore, it is reasonable to conclude that the cortex is produced in smaller liposomes or in liposomes where the actoHMM bundles are localized on the surface. Transformations of the encapsulated actoHMM assembly observed after the ATP supply were quite different between the presence and absence of methylcellulose. In the absence of the depletion agent, such as methylcellulose in this report, dispersion of actin bundles took place similarly to that in the bulk solution, even under the confined environment. On the other hand, in the presence of methylcellulose, formation of the cortex newly was noted, in addition to the aster structure that existed in the control bulk solution (Fig. 2). The difference is, thus, attributed to the effect of crowding condition under relatively high concentration of methylcellulose in the liposome. This result suggests that crowding condition will contribute to the reactions between the polymers, such as cytoskeletons and molecular motors, which are taking place in living cells.
Diagram of the morphology of actoHMM inside the liposome observed after the ATP supply, as functions of liposomal size and the molar ratio of HMM and F-actin, at a fixed F-actin concentration of 10 μM (Takiguchi et al. 2011)
Nevertheless this study demonstrate the marked difference on the structure of actoHMM system in a closed cell-sized space from that in the bulk solution, particularly in the coexistence of highly concentrated hydrophilic polymer, suggesting the important role of confined environment and crowding condition for biological phenomena. The inner space of living cells is a space in which many organelles and solutes, native polymer such as proteins and nucleic acids, are present in dense. The results reported in this study would be helpful for a better understanding of the biological phenomena in intracellular space.
Unfortunately, any remarkable movement of these giant liposomes has never been observed (Takiguchi et al. 2011; Yoshikawa et al. 2011). In our system, to realize spontaneous motion by mimicking living cells, the following parameters should be resolved. (i) In living cells, a number of actin-associating proteins are involved in determining their morphology and motility. Therefore, other factors, for example, protein crosslinkers between F-actin and the membrane as well as between F-actins, should be required for liposomal morphogenesis or movement. (ii) It has been well known that actin dynamics are essential for cell morphology and motility. In addition, it has been reported that actin dynamics are affected by the surface under crowding conditions (Popp et al. 2007). Some regulators of actin dynamics may also serve as protein crosslinkers. As a future research, it may be interesting to examine the actual role of these biochemical species, by utilizing the experimental system reported herein. (iii) In this study, in order to simplify the system, we utilized HMM as a motor. However, over 20 classes of myosins have been identified. Since their precise roles and how they share those roles in vivo have remained unclear, those native myosin motors should be tested. (iv) So far, we used only limited lipids, mainly native or synthetic PC, as components of the liposome membrane. Biological membranes, however, consist of various types of lipid molecules, and it has been reported that phosphatidylserine or phosphoinositides are important for regulation of many actin-binding proteins. Therefore, the lipid composition needs to be reconsidered as well. The regulators for actin dynamics and crosslinks, the molecular motors, and the biological membranes, all elements existing in living cells are, of course, expected to become to show new function or behavior under the confined and crowding conditions (Yoshikawa et al. 2011; Takiguchi and Hayashi 2013).
References
Arai R, Mabuchi I (2002) F-actin ring formation and roles of F-actin cables in the fission yeast Schizosaccharomyces pombe. J Cell Sci 115:887–898
Popp D, Yamamoto A, Maéda Y (2007) Crowded surfaces change annealing dynamics of actin filaments. J Mol Biol 368:365–374
Takiguchi K, Hayashi M (2013) Construction of artificial motile cell model. 2013 International Symposium on Micro-NanoMechatronics and Human Science, MHS 2013. doi:10.1109/MHS.2013.6710453
Takiguchi K, Negishi M, Tanaka-Takiguchi Y, Homma M, Yoshikawa K (2011) Transformation of actoHMM assembly confined in cell-sized liposome. Langmuir 27:11528–11535
Tanaka-Takiguchi Y, Kakei T, Tanimura A, Takagi A, Honda M, Hotani H, Takiguchi K (2004) The elongation and contraction of actin bundles are induced by double-headed myosins in a motor concentration-dependent manner. J Mol Biol 341:467–476
Yoshikawa K, Nomura SM, Tsumoto K, Takiguchi K (2011) Construction of an in vitro model of a living cellular system. In: Luisi PL, Stano P (Eds) The minimal cell: the biophysics of cell compartment and the origin of cell functionality, Springer, New York, pp. 173–194
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takiguchi, K., Negishi, M., Tanaka-Takiguchi, Y. et al. Specific Transformation of Assembly with Actin Filaments and Molecular Motors in a Cell-Sized Self-Emerged Liposome. Orig Life Evol Biosph 44, 325–329 (2014). https://doi.org/10.1007/s11084-014-9395-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-014-9395-0