Abstract
We evaluate the structural, optoelectronic, and mechanical characteristics of the novel Rb2CaSnCl6 and Rb2CaSnI6 through density functional theory within the WIEN2K framework. The formation energies and volume optimization provide evidence for the structural stability of these compounds. The optimized lattice constants for Rb2CaSnCl6 and Rb2CaSnI6 are found to be 11.21 Å and 12.45 Å, respectively while their respective formation energies are calculated as − 2.5 Ry and − 1.6 Ry. Similarly, the grounds state energies are calculated − 31,184.32 Ry and − 111,074.60 Ry. We find that Rb2CaSnCl6 is an insulator with a bandgap energy of 3.50 eV when the modified Becke-Johnson (mBJ) approximation is applied and that Rb2CaSnI6 is directly semiconducting with a wide bandgap energy of 2.70 eV. Evaluations of mechanical stability show that both materials are mechanically stable and their constituent atoms are bound together by ionic interactions, indicated by the positive value of Cauchy’s pressure: 46. 32 and 21.93 for Rb2CaSnCl6 and Rb2CaSnI6, respectivley. Nevertheless, our investigation showed that Rb2CaSnI6 behaves as a ductile material while Rb2CaSnCl6 is brittle. We also investigate a variety of optical characteristics such as dielectric function, refraction, reflectivity, energy loss function, and extinction coefficient. The static dielectric function was found to be 2.9 and 4.3 for Rb2CaSnCl6 and Rb2CaSnI6, respectively. Rb2CaSnI6 exhibited greater optical conductivity, indicative of it being a better metallic than the Rb2CaSnCl6. The optical investigation suggests that these materials have a great deal of promise for cutting-edge optoelectronic applications, especially in the ultraviolet (UV) spectrum due to their large electronic band gap. These results offer a solid basis for future research into these intriguing materials by experimentalists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It has been longed for decades to obtain novel compounds with diverse characteristics which have driven intensified quest for such materials and manifested in the present cutting-edge progress in the technology. Among these compounds (Khattak et al. 2022a; Saddique et al. 2022), the halide perovskites (Khattak et al. 2022b) have been considered distinctive due to their potential use in multiple fields such as optoelectronics (Paul et al. 2023), photovoltaics (Jena et al. 2019) and solid-state lighting (Adjokatse et al. 2017). This has been followed by the shifting attention to their double form, i.e., double halide perovoksites (Muscarella and Hutter 2022; Ali et al. 2023a, b; Husain et al. 2023; Rahman et al. 2023), as double halide perovskites offer a better alternative: their properties can be tailored by altering their composition. Double halide perovskites drew a massive interest to be studied as they are adaptable extraordinarily and demonstrate promising electrical, structural, mechanical and structural characteristics (Muscarella and Hutter 2022; Ju et al. 2018). The double halide perovskites based on lead have been found useful for applications in solar cell devices (Rosales et al. 2017). However, since the lead is toxic, such perovskites can’t be fully exploited (Ren et al. 2022). Therefore, this has triggered the investigation of lead-free double perovskites which have the same advantages offered by the double perovskites and are free from the downsides posed by the lead-based double halide perovskites (Gao et al. 2021).
Lead-free double perovskites with the formula A2BIBIIIX6 have garnered attention for their potential in diverse sustainable and renewable energy applications (Saeed et al. 2022; Alnujaim et al. 2022; Islam et al. 2021). Here A represents a cation belonging to the s/p block; BI and BIII also denote cations but form the p/d/f section while an anion is represented by X, typically halides or oxides ions (Mir and Gupta 2021; Thawarkar et al. 2021). Such compounds have been proved useful because they have wonderful physical characteristics: they are modifiable and have appropriate band gap energies and low effective charge carrier masses, high optical absorption coefficients, extensive diffusion lengths, strong defect tolerance, and adaptable compositional flexibility (Grätzel 2014; Barrows et al. 2014; Xing et al. 2013). These compounds offer a vast array of potential combinations, each with a wide spectrum of optoelectronic and thermoelectric characteristics. Over the recent years, double perovskites have been extensively explored for applications in photoluminescence, photovoltaics, and thermoelectric (Soni et al. 2022; Bhorde et al. 2021; Tang and Tang 2023). Notably, double perovskites containing tin (Sn) have demonstrated excellent properties. Landini et al. (2022) investigated Cs2GeSnCl6 and Cs2GeSnBr6 in a double perovskite structure with a space group of Fm3m, determining band gaps of 1.35 eV and 0.85 eV, respectively. Spectroscopic limited maximum efficiency calculations indicated values of 27% and 21% for Cs2GeSnCl6 and Cs2GeSnBr6 respectively, positioning them as effective absorbers for solar cells. Mukaddar and Saurabh (Sk and Ghosh 2022) employed the DFT approach to report on the structural, optoelectronic, and photovoltaic properties of Cs2GeSnCl6, revealing a direct band gap of 0.91 eV and a power conversion efficiency of 16.35%. The structural, mechanical, optical, and thermoelectric properties of Cs2GeSnX6 (X = Cl, Br) have been investigated, suggesting their suitability for optoelectronic and thermoelectric applications (Behera and Kumar Mukherjee 2023). These findings underscore the potential of Sn-based double perovskites as alternatives to lead-based perovskites. However, the quest for a better alternative to that is still underway. In this regard, Rb2CaSnX6 (X = Cl, I) remains unexplored within this category where the alkali atom such as Rb offers stability through charge transfer like in fullerene intercalated compounds (Poloni et al. 2008a, b). This study, employing density functional theory (DFT), addresses this gap by systematically investigating the structural, electronic, and optical properties of these compounds. We explored the electronic characteristics of Rb2CaSnX6 (X = Cl, I) in detail, analyzing their electronic band structures, using state-of-the-art Density Functional Theory (DFT) computations. We carefully investigate the bandgap, which is a crucial characteristic defining its optoelectronic value. We also widen our focus to include these materials’ mechanical characteristics: how they react to outside pressures and situations using simulation approaches, with a focus on characteristics like ductility, hardness, and flexibility. This research not only fills a theoretical void in the scientific literature but also serves as a reference for future studies on Rb2CaSnX6 (X = Cl, I) double perovskite compounds. The results highlight the feasibility of utilizing Rb2CaSnX6 (X = Cl, I) compounds in electronic devices.
2 Materials and methods
The studied compounds were subjected to computational analysis using the WIEN2K code. We applied the FP-LAPW method, as described by Blaha and his coworkers (Blaha et al. 1990). Notably, our calculations did not consider the effects of spin–orbit coupling (SOC), following the approach outlined by Vijayakumar and Gopinathan (1996).
For structural optimization, we employed the GGA method, initially introduced by Kohn and Sham (1965). This method has been coded in the WIEN2K, one of the DFT simulation codes, as documented by Blaha et al. (2019). To attain the structural optimization of the materials in terms of geometry and conduct calculations related to mechanical and optoelectronic characteristics, we utilized a Monkhorst–Pack sampling scheme. Specifically, a k-point sampling grid with dimensions of 10 × 10 × 10 was employed to adequately sample the Brillouin zone (BZ).
Throughout the structural optimization process, we adjusted both ionic coordinates and lattice volumes to ensure that residual forces remained below 0.01 eV.
A convergence criterion of 10–4 eV was established for self-consistent calculations related to energy.
In the analysis of optical and electronic properties, we employed the Tran Blaha modified Becke Johnson (TB-mBJ) potentials as introduced by Koller et al. (2012) To distinguish between core and valence states, a cutoff energy of − 6.0 Ry was determined. Additionally, we chose the parameters RMT × Kmax = 7.0. We established the 0.001-Ry convergence criterion, i.e., the convergence was obtained by becoming the total energy of the system constant, following the self-consistent calculations. The optical properties were determined by utilizing the OPTIC package integrated into Wien2k where the “Joint density of states” method has been employed, which was adapted with the inclusion of the pertinent dipole matrix elements as specified in the provided references (Ambrosch-Draxl et al. 1995; Abt et al. 1994; Ambrosch-Draxl and Sofo 2006). The elastic properties were determined by exploiting the IRelast package, interfaced with the Wien2K (Jamal et al. 2018). We characterized the optical characteristics of the compounds by calculating the complex dielectric function \([\varepsilon \left(\omega \right)]\) as given by Eq. (1).
where \({\varepsilon }_{1}\left(\omega \right)\) and \({\varepsilon }_{2}\left(\omega \right)\) represent photon dispersion and energy absorption capacity, respectively. The \({\varepsilon }_{2}\left(\omega \right)\) is given by Eq. 2:
where, \(k\) is the direction of polarization of the \(E\) field with a full isotropic type of average value in the polycrystalline materials, \(\Omega\) represents the unit cell volume, \(\omega\) denotes frequency of incident light while \(c, v\), denote the conduction and valence bands, respectively.
The \({\varepsilon }_{1}\left(\omega \right)\) and \({\varepsilon }_{2}\left(\omega \right)\) were used to calculate the energy loss function [\(L(\omega )\)], given by Eq. (3).
The absorption coefficient [\(I(\omega )\)] was computed using Eq. (4):
Equation (5) was used to calculate the reflectivity,
The refractive indices \(n(\omega )\) was determined by Eq. (6),
Equation (7) was employed to compute the extinction coefficient \(k(\omega )\),
The real optical conductivity \({\sigma }^{real}(\omega\)) was found while using Eq. (8),
where \({\varepsilon }_{0}\left(\omega \right)\) is the static dielectric function.
3 Results and discussions
3.1 Structural and mechanical properties
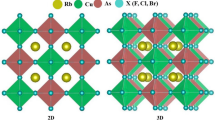
The selected halide-perovskites, Rb2CaSnX6 (X = Cl, I), have a cubic structure with the Fm-3 m (No. 225) space group. The structure of the studied materials is given in Fig. 1.
The fractional coordinates of the Rb, Ca, Sn and X atoms (X = Cl, I) are (0.25, 0.25, 0.25), (0, 0, 0), (0.5, 0.5, 0.5), and (0.25, 0, 0), respectively, and they correspond to the 8c, 4a, 4b, and 24e Wyckoff locations. Figure 1 illustrates the 40 atoms in the unit cell of the double perovskite halides Rb2CaSnX6 (X = Cl, I) with four formula units. The ground state energy of all three composites is negative; Eo characterizes the structural stability of Rb2CaSnX6 (where X = Cl, I).
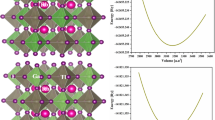
Structural optimization or geometry optimization was performed to compute the ground-state energy. For this purpose, the energy-volume curve is fitted with the Birch-Murnaghan equation of state (EOS), which provides the structural properties (Tyuterev and Vast 2006) of the compounds such as bulk modulus (B0) and the derivative of the bulk modulus, ground state volume and lattice parameter (a0). Figure 2 displays the fitted Birch-Murnaghan equation of states to the data of the energy-volume curve.
The structural parameters obtained by fitting the Birch Murnaghan state of the equation to the data are listed in Table 1, which have also been compared with those of other similar previously-reported compounds. Due to the rising ionic radii of the X-anion from Cl to I, the a0 increases from 11.21 Å to 12.45 Å for Rb2CaSnCl6 to Rb2CaSnI6. The formation energies of the compounds were computed through the Eq. (9) given in the work of Khattak et al. (2023):
where \({E}_{{Rb}_{2}CaSn{X}_{6}}\) is the total energy of the compound, a, b, c and d shows the number of individual atoms. \({E}_{Rb}, {E}_{Ca}, {E}_{Sn} and {E}_{X}\) represent the energy of Rb, Ca, Sn and X (Cl, I). The negative formation energy of the compounds indicates their stability.
The determination of thermodynamic stability often relies on the computation of Gibbs free energy (G). In this study, the calculated values for G using the Gibbs2 computational code (Blanco et al. 2004) were determined to be − 3.28 × 107 kJ/mol for Rb2CaSnCl6 and − 5.63 × 107 kJ/mol for Rb2CaSnI6. The negative values obtained affirm the thermodynamic stability of both double perovskite compounds. In thermodynamics, a negative Gibbs free energy indicates the spontaneous and energetically favorable nature of a reaction under specific conditions. Consequently, the negative G values for Rb2CaSnCl6 and Rb2CaSnI6 suggest their thermodynamic stability under the given computational parameters.
The determination of elastic constants is crucial to investigating a material’s elastic properties since it shows how the material responds to outside forces and sheds light on its mechanical properties. These constants establish whether the material is mechanically stable and tough or not. The energy is applied in accordance with the lattice strain for maintaining the volume constants(State and Physics: Ashcroft, Neil W., Mermin, N. 2022). The IRelast package was exploited for the computation of the elastic constants (Jamal et al. 2018). C11, C12 and C44 are the elastic constants (Cij) in the case of a cubic structure. Equations detailed in the work of Khattak et al. (2022a) were used to find these parameters. With the halogen atom switching from Cl to I, a decrease in the bulk, shear and Young’s moduli of Rb2CaSnX6 (X = Cl, I) was noted. The Born-Huang stability condition is used to examine the materials in the cubic phase whether they are mechanically stable or not (Karki et al. 1997; Mouhat and Coudert 2014):
Both materials meet the Born-Huang stability criterion and all the stability standard results, confirming that the Rb2CaSnX6 (X = Cl, I) compounds are mechanically stable. The values of C12–C44, or Cauchy’s pressures, can be used to calculate the nature of chemical bonding between atoms. The positive values of Cauchy’s pressure for both materials in Table 1 suggest the ionic bonds among the atoms. To determine if the materials under consideration are brittle or ductile, we computed the Poisson’s. A material is classified as brittle if its Poisson’s ratio value is less than 0.26 GPa; otherwise, it is ductile. Similarly, a material is brittle if the computed Pugh ratio is less than 1.75 GPa; otherwise, it is ductile. Table 2’s estimated Poisson’s and Pugh ratio values indicate that Rb2CaSnCl6 is brittle while ductility is exhibited by Rb2CaSnI6.
3.2 Electronic properties
A material’s band structure shows the permitted bands or energy levels that electrons can occupy, which is illustrated by a graph of energy vs. momentum. The energy dispersion and permitted electronic states in the material are revealed by the band structure. A material’s electrical, optical, and thermal properties are largely determined by its band structure. The electronic energy states distribution in a compound is described by the density of states (DOS). It shows how many different energy states are possible for each unit’s volume and energy range. The distribution and population of electrons at various energy levels are revealed by the DOS, which helps grasp the electronic transport, optical absorption, and thermal characteristics of compounds. Using the optimized lattice constants, computational research was done on the compounds’ electronic characteristics.
Figure 3 depicts the band structures that were properly analyzed to study the electronic characteristics of Rb2CaSnX6 (X = Cl, I). For Rb2CaSnCl6 and Rb2CaSnI6, the calculations revealed that they are direct-bandgap semiconductors with bandgap energies of 3.50 eV, and 2.70 eV, respectively. An increased lattice parameter causes a decrease in the ion–electron coulomb interaction of X-anions (X = Cl, I) from Rb2CaSnCl6 to Rb2CaSnI6, which in turn results in a decreased band gap while moving from Rb2CaSnCl6 to Rb2CaSnI6. The Coulomb interaction in the chlorine anion is greater than that in the iodine ion as the ionic radius of Cl is smaller than that of the I. The weaker ion–electron interaction in the I than in the Cl results in a decreased electronic bandgap for the Rb2CaSnI6.
Table 3 lists the calculated bandgap values of the present compounds with those of similar double perovskites, previously reported.
These results have several noticeable implications in various fields both scientifically and technologically: To design and develop new materials with tailorable properties, it is imperative to grasp their structural and elastic properties. The calculated structural and mechanical properties such as lattice constants, bulk modulus, and elastic constants can help in synthesizing and engineering the material for a particular purpose. The mechanical strength exhibited by their ductility and mechanical stability suggests that they can be used where toughness and flexibility are required. The revealed direct bandgap of these semiconductors indicates that these materials can be used as active materials in optoelectronic devices. Particularly, the Rb2CaSnI6 with the decreased band gap is promising for optoelectronic devices having more efficiency. Furthermore, the suitability of the materials for conversion technology and energy storage is decided by their bandgap. The calculated bandgap energies suggest their applications in energy devices such as memory devices because less energy is required for set and rest, making the memory storage devices more efficient.
3.3 Optical properties
When a material interacts with light, its optical properties include how it absorbs, reflects, transmits, and emits electromagnetic radiation in the visible, ultraviolet, and infrared regions. These characteristics are crucial for several applications in photonics, materials science, optoelectronics, and other related fields. A material’s electronic band structure largely determines its optical properties50.
The two compounds’ optical characteristics were examined, considering incident photon energies as high as 14 eV. The dielectric function ε(ω) spectrum for both materials are shown in Fig. 4, which offers information about how the material reacts to incident photons at different energies. As the losses are related to polarization effects, the material’s capacity to store electric energy is represented by ε1(ω) in Fig. 4(a). The dielectric function’s static frequency component, denoted by ε1(0), is 2.9 and 4.3 for Rb2CaSnCl6 and Rb2CaSnI6, respectively, in the zero-frequency range, which is consistent with the bandgap values shown in Fig. 3, i.e, for the compounds with increased bandgap value (Rb2CaSnCl6), the static dielectric function is lower than that for the compound with decreased bandgap (Rb2CaSnI6). For Rb2CaSnCl6 and Rb2CaSnI6, the real component’s maximum values, ε1, in the complex dielectric function are 5.1 at 3.74 (UV range) and 7.7 at 2.8 eV (visible range), respectively. The higher dielectric constant for Rb2CaSnI6 than for the Rb2CaSnCl6 can be attributed to multiple factors: (1) upon exposure to the external electric field in electromagnetic radiation, the electrons in Rb2CaSnI6 get more displaced than in the Rb2CaSnCl6, hence more electronic polarization effect takes place in the former than the latter; (2) more atomic polarizability (distortion of electron clouds around the atoms) occurs in Rb2CaSnI6 than in the Rb2CaSnCl6. It is evident from the ε1(ω) spectrum that the curve takes on negative values at higher energies, meaning that these materials reflect electromagnetic waves in this range of frequencies. The maximum value of 5. 1 ε1(ω) for Rb2CaSnCl6 indicates that the material has good dielectric characteristics in the UV range. This implies that the material is promising for applications where UV light responsiveness is required: like photodetectors and UV sensors. On the other hand, Rb2CaSnI6 with 7.7 as the maximum ε1(ω) value at 2.8 eV, i.e., in the visible range, suggests that it is suitable for photovoltaic cells and LEDs, the devices working within the visible spectrum. Additionally, Fig. 4(b) shows how the imaginary component ε2(ω) of the dielectric function behaves, representing how these compounds absorb incident radiation. The energy range of roughly 3–9 eV is where the dominant peaks in the spectrum are found. Notably, the imaginary component spectra in both materials show zero values up until the point at which absorption begins, which is the band gap energy corresponding to the photon energy, i.e., optical bandgap, which is nearly equal to their electronic bandgap shown in Fig. 3. For the optical transitions between the valence band maxima (VBM) and the conduction band minima (CBM), this is the transition point.
A material’s ability to link the induced current density and the induced electric field strength at different frequencies is known as optical conductivity. The optical conductivity σ(ω), both its real and imaginary parts, of the two compounds are shown in Fig. 5. Both materials’ optical conductivity spectra show a comparable pattern. The spectra in Fig. 5(a) clearly show that the optical conductivity is zero until the incident photon energy crosses the band gap energy. For Rb2CaSnCl6 and Rb2CaSnI6, the maximum peak of the real part of optical conductivity σ(ω) is observed to be 3.2 × 1015/sec and 4.0 × 1015/sec, respectively, i.e., with greater optical conductivity, the Rb2CaSnI6 demonstrates lesser insulation (or greater metallicity) than the Rb2CaSnCl6, which is consistent with the band structure profiles of both compounds given in Fig. 3. Similarly, the imaginary part of the optical conductivity is given in Fig. 5(b), where it is in the negative values up to around 11.63 eV and 6.57 eV for Rb2CaSnCl6 and Rb2CaSnI6, respectively. This suggests that rather than absorbing the incident light, both materials behave as a gain medium at the corresponding frequencies of these energy ranges, i.e., they can amplify the light through stimulated emission and are promising for laser and optical amplification.
The refractive index is a dimensionless measurement that expresses how well an optical medium bends light. It has a linear dependence on wavelength because of interactions between various wavelengths and the medium’s atoms. The refractive index n(ω) and spectra of the extinction coefficient, κ(ω), for Rb2CaSnCl6 and Rb2CaSnI6, are shown in Fig. 6(a) and (b), respectively. The refractive index at zero-incident photon energy is represented by the static refractive index, or n (0). The values of n (0) for Rb2CaSnCl6 and Rb2CaSnI6 are 1.7 and 2.1, respectively. For both materials, the n(ω) spectra show similar behavior: a steep decline that becomes negative at 11.36 eV and 9.26 eV for Rb2CaSnCl6 and Rb2CaSnI6, respectively. The negative refractive index beyond these energies shows these compounds exhibit negative refraction, i.e., the light starts traveling in the opposite direction to that observed commonly in the materials.
The extinction coefficient describes how much light is absorbed by a substance at a given color and frequency and how much of the light’s intensity is lost as it travels through the substance. It gives details on how deeply a material can absorb light with a particular wavelength. Low extinction coefficient materials tend to transmit light, giving the impression that they are transparent, especially at certain wavelengths. Both the material and the incident light’s wavelength have an impact on the extinction coefficient. Light with energies below the band gap cannot induce electron transitions from the VB to the CB in semiconductor materials, which have high extinction coefficients. As a result, it’s possible that light won’t be absorbed.
The probability of photon absorption is determined by the possibility of photon-electron interaction during the electron’s energy band transition. By interacting with photons, only electrons at the valence band’s immediate edge can effectively absorb them. As a result, absorption is minimal if the photon’s energy closely resembles the band gap value. On the other hand, electrons with energies farther from the band gap can interact with photons as photon energy increases. Increased photon absorption results from a greater number of electrons interacting with the photon due to the wider range of electron energies. The absorption coefficients displayed by various materials have an impact on the materials chosen for solar cell design51.
The extinction coefficient in Figure (b) shows that over the energy range of 0–14 eV, the Rb2CaSnI6 demonstrates a higher extinction coefficient than the Rb2CaSnCl6.
Reflectivity is represented by Fig. 7(a) where the reflectivity R(ω) spectral curves for Rb2CaSnX6 (X = Cl, I) perovskites are displayed. Rb2CaSnCl6 and Rb2CaSnI6 have static reflectivity values of R(ω) of 0.06 and 0.001, respectively. There is no significant increase in the reflectivity until 12 eV and 9 eV for Rb2CaSnCl6 and Rb2CaSnI6, respectively. However, the R(ω) spectra for both compounds show an upward trend with increasing photon energy, afterward. In general, the Rb2CaSnI6 is more reflective than the Rb2CaSnCl6. Figure 7(b) shows the energy loss functions versus photon energy in the energy range of 0–14 eV. The energy loss function for both materials slightly fluctuates between 0 and 6 eV and for the higher energies than the 6 eV, there is a noticeable increase in it: the Rb2CaSnI6 exhibits a higher energy loss function than the Rb2CaSnCl6. This suggests that Rb2CaSnI6 dissipates more energy than Rb2CaSnCl6 during its interaction with electromagnetic radiation.
4 Conclusion
First-principles calculations within the WIEN2K framework were used to determine the structural, optoelectronic, and mechanical properties of the compounds under investigation. The optimized lattice constants for Rb2CaSnCl6 and Rb2CaSnI6 were determined to be 11.21 Å and 12.45 Å, respectively while their respective formation energies were found as − 2.5 Ry and − 1.6 Ry. Volume optimization and the negative value of formation energy supported the structural stability of both compounds. By using the mBJ approximation, the Rb2CaSnCl6 was found to be an insulator with a bandgap energy of 3.50 eV while Rb2CaSnI6 exhibited a direct semiconducting nature with a wide bandgap energy of 2.70 eV. It was also shown that both materials are mechanically stable, and the constituent atoms are bonded with each other by ionic bonds, indicated by the positive value of Cauchy’s pressure: 46. 32 and 21.93 for Rb2CaSnCl6 and Rb2CaSnI6, respectivley. The Rb2CaSnCl6 exhibited brittleness while the ductile nature of the Rb2CaSnI6 was revealed. The static dielectric function was found to be 2.9 and 4.3 for Rb2CaSnCl6 and Rb2CaSnI6, respectively. Rb2CaSnI6 exhibited greater optical conductivity, indicative of it being a better metallic than the Rb2CaSnCl6. The examination of different optical properties showed that these materials have a significant potential for advanced optoelectronic applications, especially in the ultraviolet (UV) region, especially given their wide electronic band gap. Experimentalists now have the chance to investigate these materials more thoroughly thanks to these findings.
Data Availability
No datasets were generated or analysed during the current study.
References
Abt, R., Ambrosch-Draxl, C., Knoll, P.: Optical response of high temperature superconductors by full potential LAPW band structure calculations. Phys. B Condens. Matter. 194–196, 1451–1452 (1994). https://doi.org/10.1016/0921-4526(94)91225-4
Adjokatse, S., Fang, H.H., Loi, M.A.: Broadly tunable metal halide perovskites for solid-state light-emission applications. Mater. Today 20(8), 413–424 (2017). https://doi.org/10.1016/J.MATTOD.2017.03.021
Ali, A., Khan, S.M., Khan, G., Gul, B., Tawfeek, M.A., Azam, S., Abbas, F., Zulfiqar, S., Khattak, S.A., Khan, T., Shah, S.K.: The spin-polarized electronic and optical properties of novel Ba2TaXO6 (X = Co, Fe, In) for optoelectronic applications: an ab-initio study. J. Solid State Chem. 326, 124211 (2023). https://doi.org/10.1016/J.JSSC.2023.124211
Ali, M.A., Musa Saad, M., Tighezza, A.M., Khattak, S., Al-Qaisi, S., Faizan, M.: First-principles calculations of novel lead-free X2GeSnI6 (X = Rb, Cs) double perovskite compounds for optoelectronic and energy exploitations. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/S10904-023-02901-8/METRICS
Ali, M.A., Saad, H.-E.M., Tighezza, A.M., Khattak, S., Al-Qaisi, S., Faizan, M.: First-principles calculations of novel lead-free X2GeSnI6 (X = Rb, Cs) double perovskite compounds for optoelectronic and energy exploitations. J. Inorg. Organom. Polymers Mater. 1, 1–11 (2023). https://doi.org/10.1007/S10904-023-02901-8
Ali, M.A., Alshgari, R.A., Awadh Bahajjaj, A.A., Sillanpää, M.: The study of new double perovskites K2AgAsX6 (X = Cl, Br) for energy-based applications. J. Taibah Univ. Sci. 17(1), 2170680 (2023d). https://doi.org/10.1080/16583655.2023.2170680
Alnujaim, S., Bouhemadou, A., Chegaar, M., Guechi, A., Bin-Omran, S., Khenata, R., Al-Douri, Y., Yang, W., Lu, H.: Density functional theory screening of some fundamental physical properties of Cs2InSbCl6 and Cs2InBiCl6 double perovskites. Eur. Phys. J. B 95(7), 1–16 (2022). https://doi.org/10.1140/EPJB/S10051-022-00381-2
Ambrosch-Draxl, C., Sofo, J.O.: Linear optical properties of solids within the full-potential linearized augmented planewave method. Comput. Phys. Commun. 175(1), 1–14 (2006). https://doi.org/10.1016/J.CPC.2006.03.005
Ambrosch-Draxl, C., Majewski, J.A., Vogl, P., Leising, G.: First-principles studies of the structural and optical properties of crystalline poly(para-phenylene). Phys. Rev. B 51(15), 9668 (1995). https://doi.org/10.1103/PhysRevB.51.9668
Barrows, A.T., Pearson, A.J., Kwak, C.K., Dunbar, A.D.F., Buckley, A.R., Lidzey, D.G.: Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition. Energy Environ. Sci. 7(9), 2944–2950 (2014). https://doi.org/10.1039/C4EE01546K
Behera, D., Kumar Mukherjee, S.: First-principles calculations to investigate structural, optoelectronics and thermoelectric properties of lead free Cs2GeSnX6 (X = Cl, Br). Mater. Sci. Eng. B 292, 116421 (2023). https://doi.org/10.1016/J.MSEB.2023.116421
Bhorde, A., Waykar, R., Rondiya, S.R., Nair, S., Lonkar, G., Funde, A., Dzade, N.Y., Jadkar, S.: Structural, electronic, and optical properties of lead-free halide double perovskite Rb2AgBiI6: a combined experimental and density functional theory study. ES Mater. Manuf. 12, 43–52 (2021). https://doi.org/10.30919/ESMM5F1042
Blaha, P., Schwarz, K., Sorantin, P., Trickey, S.B.: Full-potential, linearized augmented plane wave programs for crystalline systems. Comput. Phys. Commun. 59(2), 399–415 (1990). https://doi.org/10.1016/0010-4655(90)90187-6
Blaha, P., Schwarz, K., Madsen, G.K.H., Kvasnicka, D., Luitz, J., Laskowsk, R., Tran, F., Marks, L., Marks, L.: WIEN2k: An augmented plane wave plus local orbitals program for calculating crystal properties. Uniw. śląski, (2019)
Blanco, M.A., Francisco, E., Luaña, V.: GIBBS: isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic debye model. Comput. Phys. Commun. 158(1), 57–72 (2004). https://doi.org/10.1016/J.COMPHY.2003.12.001
Dimitrov, S.D., Durrant, J.R.: Materials design considerations for charge generation in organic solar cells. Chem. Mater. 26(1), 616–630 (2014). https://doi.org/10.1021/CM402403Z/ASSET/IMAGES/MEDIUM/CM-2013-02403Z_0012.GIF
Fox, M., Bertsch, G.F.: Optical properties of solids. Am. J. Phys. 70(12), 1269–1270 (2002). https://doi.org/10.1119/1.1691372
Gao, Y., Pan, Y., Zhou, F., Niu, G., Yan, C.: Lead-free halide perovskites: a review of the structure-property relationship and applications in light emitting devices and radiation detectors. J. Mater. Chem. A 9(20), 11931–11943 (2021). https://doi.org/10.1039/D1TA01737C
Grätzel, M.: The light and shade of perovskite solar cells. Nat. Mater. 13(9), 838–842 (2014). https://doi.org/10.1038/nmat4065
Husain, M., Rahman, N., Sfina, N., Al-Shaalan, N.H., Alharthi, S., Alharthy, S.A., Amin, M.A., Tirth, V., Khan, R., Sohail, M., Azzouz-Rached, A., Khattak, S.A., Khan, M.Y.: The comparative investigations of structural, optoelectronic, and mechanical properties of AgBeX3 (X = F and Cl) metal halide-perovskites for prospective energy applications utilizing DFT approach. Opt. Quantum Electron. 55(10), 1–16 (2023). https://doi.org/10.1007/S11082-023-05187-9/FIGURES/9
Islam, M.N., Podder, J., Saha, T., Rani, P.: Semiconductor to metallic transition under induced pressure in Cs2AgBiBr6 double halide perovskite: a theoretical DFT study for photovoltaic and optoelectronic applications. RSC Adv. 11(39), 24001–24012 (2021). https://doi.org/10.1039/D1RA03161A
Jamal, M., Bilal, M., Ahmad, I., Jalali-Asadabadi, S.: IRelast package. J. Alloys Compd. 735, 569–579 (2018). https://doi.org/10.1016/J.JALLCOM.2017.10.139
Jena, A.K., Kulkarni, A., Miyasaka, T.: Halide perovskite photovoltaics: background, status, and future prospects. Chem. Rev. 119(5), 3036–3103 (2019). https://doi.org/10.1021/ACS.CHEMREV.8B00539/ASSET/IMAGES/MEDIUM/CR-2018-00539A_0060.GIF
Ju, M.G., Chen, M., Zhou, Y., Dai, J., Ma, L., Padture, N.P., Zeng, X.C.: Toward eco-friendly and stable perovskite materials for photovoltaics. Joule 2(7), 1231–1241 (2018). https://doi.org/10.1016/J.JOULE.2018.04.026
Karki, B.B., Ackland, G.J., Crain, J.: Elastic instabilities in crystals from ab initio stress—strain relations. J. Phys. Condens. Matter 9(41), 8579 (1997). https://doi.org/10.1088/0953-8984/9/41/005
Khattak, S.A., Wabaidur, S.M., Islam, M.A., Husain, M., Ullah, I., Zulfiqar, S., Rooh, G., Rahman, N., Khan, M.S., Khan, G., Khan, T., Ghlamallah, B.: First-principles structural, elastic and optoelectronics study of sodium niobate and tantalate perovskites. Sci. Rep. 12(1), 1–12 (2022). https://doi.org/10.1038/s41598-022-26250-7
Khattak, S.A., Abohashrh, M., Ahmad, I., Husain, M., Ullah, I., Zulfiqar, S., Rooh, G., Rahman, N., Khan, G., Khan, T., Salman Khan, M., Shah, S.K., Tirth, V.: Investigation of structural, mechanical, optoelectronic, and thermoelectric properties of BaXF3 (X = Co, Ir) fluoro-perovskites: promising materials for optoelectronic and thermoelectric applications. ACS Omega (2022b). https://doi.org/10.1021/ACSOMEGA.2C05845/ASSET/IMAGES/LARGE/AO2C05845_0011.JPEG
Khattak, S.A., Abohashrh, M., Ahmad, I., Husain, M., Ullah, I., Zulfiqar, S., Rooh, G., Rahman, N., Khan, G., Khan, T., Salman Khan, M., Shah, S.K., Tirth, V.: Investigation of structural, mechanical, optoelectronic, and thermoelectric properties of BaXF3 (X = Co, Ir) fluoro-perovskites: promising materials for optoelectronic and thermoelectric applications. ACS Omega 8(6), 5274–5284 (2023)
Kiran, S., Mumtaz, U., Mustafa, A., Imran, M., Hussain, F., Rasheed, U., Khalil, R.M.A., Khera, E.A., Nazir, A.: An ab initio investigation of the structural, mechanical, electronic, optical, and thermoelectric characteristics of novel double perovskite halides Cs2CaSnX6 (X = Cl, Br, I) for optically influenced RRAM devices. RSC Adv. 13(16), 11192–11200 (2023). https://doi.org/10.1039/D3RA00078H
Kohn, W., Sham, L.J.: Self-consistent equations including exchange and correlation effects. Phys. Rev. 140(4A), A1133 (1965). https://doi.org/10.1103/PHYSREV.140.A1133/FIGURE/1/THUMB
Koller, D., Tran, F., Blaha, P.: Improving the modified Becke-Johnson exchange potential. Phys. Rev. B Condens. Matter. Mater. Phys. 85(15), 155109 (2012)
Landini, E., Reuter, K., Oberhofer, H.: Machine-learning based screening of lead-free halide double perovskites for photovoltaic applications. (2022)
Mir, S.A., Gupta, D.C.: Structural and mechanical stabilities, electronic, magnetic and thermophysical properties of double perovskite Ba2LaNbO6: probed by DFT computation. Int. J. Energy Res. 45(10), 14603–14611 (2021)
Mouhat, F., Coudert, F.X.: Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 90(22), 224104 (2014). https://doi.org/10.1103/PhysRevB.90.224104
Muscarella, L.A., Hutter, E.M.: Halide double-perovskite semiconductors beyond photovoltaics. ACS Energy Lett. 7(6), 2128–2135 (2022)
Paul, T., Hiralal Makani, N., Sahoo, A., Singh Tanwar, L., Singh, M., Banerjee, R.: Halide perovskites for optoelectronic application. Mater. Today Proc. (2023). https://doi.org/10.1016/J.MATPR.2023.06.327
Poloni, R., Machon, D., Fernandez-Serra, M.V., Le Floch, S., Pascarelli, S., Montagnac, G., Cardon, H., San-Miguel, A.: High-pressure stability of Cs6 C60. Phys. Rev. B Condens. Matter Mater. Phys. 77(12), 125413 (2008). https://doi.org/10.1103/PHYSREVB.77.125413/FIGURES/2/MEDIUM
Poloni, R., Fernandez-Serra, M.V., Le Floch, S., De Panfilis, S., Toulemonde, P., Machon, D., Crichton, W., Pascarelli, S., San-Miguel, A.: Pressure-Induced Deformation of the C60 Fullerene in Rb6 C60 and Cs6 C60. Phys. Rev. B Condens. Matter Mater. Phys. 77(3), 035429 (2008). https://doi.org/10.1103/PHYSREVB.77.035429/FIGURES/10/MEDIUM
Rahman, N., Rauf, A., Husain, M., Sfina, N., Tirth, V., Sohail, M., Khan, R., Azzouz-Rached, A., Murtaza, G., Khan, A.A., Khattak, S.A., Khan, A.: Probing the physical properties of M2LiCeF6 (M = Rb and Cs) double perovskite compounds for prospective high-energy applications employing the DFT framework. RSC Adv. 13(23), 15457–15466 (2023). https://doi.org/10.1039/D3RA01451G
Ren, M., Qian, X., Chen, Y., Wang, T., Zhao, Y.: Potential lead toxicity and leakage issues on lead halide perovskite photovoltaics. J. Hazard. Mater. 426, 127848 (2022). https://doi.org/10.1016/J.JHAZMAT.2021.127848
Rosales, B.A., Hanrahan, M.P., Boote, B.W., Rossini, A.J., Smith, E.A., Vela, J.: Lead halide perovskites: challenges and opportunities in advanced synthesis and spectroscopy. ACS Energy Lett. 2(4), 906–914 (2017)
Saddique, J., Husain, M., Rahman, N., Khan, R., Zulfiqar, Iqbal, A., Sohail, M., Khattak, S.A., Khan, S.N., Khan, A.A., Reshak, A.H., Khan, A.: Modeling structural, elastic, electronic and optical properties of ternary cubic barium based fluoroperovskites MBaF3 (M = Ga and In) compounds based on DFT. Mater. Sci. Semicond. Process. 139(May), 106345 (2022). https://doi.org/10.1016/j.mssp.2021.106345
Saeed, M., Haq, I.U., Saleemi, A.S., Rehman, S.U., Haq, B.U., Chaudhry, A.R., Khan, I.: First-principles prediction of the ground-state crystal structure of double-perovskite halides Cs2AgCrX6 (X = Cl, Br, and I). J. Phys. Chem. Solids 160, 110302 (2022). https://doi.org/10.1016/J.JPCS.2021.110302
Sk, M., Ghosh, S.: 16.35 % efficient Cs2GeSnCl6 based heterojunction solar cell with hole-blocking SnO2 layer: DFT and SCAPS-1D simulation. Optik 267, 169608 (2022). https://doi.org/10.1016/J.IJLEO.2022.169608
Soni, Y., Rani, U., Shukla, A., Joshi, T.K., Verma, A.S.: Transition metal-based halides double Cs2ZSbX6 (Z = Ag, Cu, and X = Cl, Br, I) perovskites: a mechanically stable and highly absorptive materials for photovoltaic devices. J. Solid State Chem. 314, 123420 (2022). https://doi.org/10.1016/J.JSSC.2022.123420
Solid State Physics: Ashcroft, Neil W., Mermin, N. David + Free Shipping. https://www.amazon.com/Solid-State-Physics-Neil-Ashcroft/dp/0030839939 (Accessed 2022–11–16)
Tang, T., Tang, Y.: First principle comparative study of transitional elements Co, Rh, Ir(III)-based double halide perovskites. Mater. Today Commun. 34, 105431 (2023). https://doi.org/10.1016/J.MTCOMM.2023.105431
Thawarkar, S., Rondiya, S.R., Dzade, N.Y., Khupse, N., Jadkar, S.: Experimental and theoretical investigation of the structural and opto-electronic properties of Fe-doped lead-free Cs2AgBiCl6 double perovskite. Chem. A Eur. J. 27(26), 7408–7417 (2021). https://doi.org/10.1002/CHEM.202004902
Tyuterev, V.G., Vast, N.: Murnaghan’s equation of state for the electronic ground state energy. Comput. Mater. Sci. 38(2), 350–353 (2006). https://doi.org/10.1016/J.COMMATSCI.2005.08.012
Vijayakumar, M., Gopinathan, M.S.: Spin-orbit coupling constants of transition metal atoms and ions in density functional theory. J. Mol. Struct. Theochem. 361(1–3), 15–19 (1996). https://doi.org/10.1016/0166-1280(95)04297-0
Xing, G., Mathews, N., Sun, S., Lim, S.S., Lam, Y.M., Graẗzel, M., Mhaisalkar, S., Sum, T.C.: Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 342(6156), 344–347 (2013). https://doi.org/10.1126/SCIENCE.1243167/SUPPL_FILE/XING.SM.PDF
Acknowledgements
The authors would like to thank the Researchers Supporting Project Number (RSP2024R448), King Saud University, Riyadh, Saudi Arabia. The authors would like to thank the National Research Program for Universities (NRPU), Higher Education Commission Pakistan, No. 20-15131/NRPU/R&D/HEC/2021. The authors would like to thank National Research Program for Universities (NRPU), Higher Education Commission Pakistan, No. 20-15128/NRPU/R&D/ HEC/2021.
Author information
Authors and Affiliations
Contributions
Shaukat Ali Khattak: software, data curation: conceptualization, project administration, writing—original draft. Saikh Mohammad Wabaidur: data curation, Asma A. Alothman: software, data curation. Mudasser Husain: conceptualization, project administration. Malak Azmat Ali: conceptualization, project administration. Nasir Rahman: conceptualization, project administration. Irfan Ullah: conceptualization, project administration. Syed Zulfiqar: data curation. Gul Rooh: software, data curation. Tahirzeb Khan: software, data curation. Gulzar Khan: project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khattak, S.A., Wabaidur, S.M., Alothman, A.A. et al. DFT study of structural, elastic and optoelectronic characteristics of novel Rb2CaSnX6 (X = Cl, I) double halide perovskites for optoelectronic applications. Opt Quant Electron 56, 1384 (2024). https://doi.org/10.1007/s11082-024-07318-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-07318-2