Abstract
This study delves into the impact of lithium (Li3+) and silver (Ag7+) ion irradiation on the structure and luminescence of Dy3+ doped and Dy3+/Eu3+ co-doped Gd2MoO6 phosphors, synthesized via the hydrothermal method. To explore the influence of ions with varied mass and energy, 30 MeV Li3+ and 100 MeV Ag7+ ions were employed at different fluences. We elucidate the induced effects based on defect formation and the role of these ions’ linear energy transfer (LET) within the irradiated material. SRIM software estimates the depth profile of the ions. Irradiation of the Gd2MoO6 phosphors with Li3+ and Ag7+ ions resulted in the formation of disordered lattices or tracks, modifying their structural, optical, and luminescence properties, which were analyzed by various techniques, including X-ray diffraction, scanning electron microscopy, diffuse reflectance, and photoluminescence. Thermoluminescence (TL) tests and calculations of trapping parameters were conducted to evaluate the dosimetric potential. The findings reveal a more pronounced effect of silver ions compared to lithium ions on the structural and luminescence behavior of doped and codoped Gd2MoO6 phosphors due to their higher atomic weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, rare earth molybdate materials with the formula R2MoO6 (R = rare earth) that have three different types of monoclinic, cubic, and tetragonal polymorphs have drawn the attention of research because of their fascinating luminescent properties due to their stable structure, excellent chemical and thermal stability (Blasse 1968; Chebyshev et al. 2021; Hou et al. 2009). Molybdates are preferred as host materials because they feature a prominent charge transfer band (CTB) in the near-UV or blue spectrum, along with a high quenching concentration of activator ions (Chen and Liu 2013). Due to the low phonon energy of the molybdate host, the introduction of rare earth ions makes these materials promising for display and lighting applications by enabling high radiative transitions, leading to outstanding luminescence (Chen et al. 2011). Moreover, molybdates offer the advantage of a scalable and cost-effective preparation technique, enhancing their accessibility for various applications. The monoclinic scheelite-type Gd2MoO6 structure has previously been investigated as a useful host lattice for rare earth ions (R) due to efficient energy transfer (ET) from the MoO66- group to R3+, which aids in efficient photoluminescence emission (Chen et al. 2011). As a result, extensive research with rare earth doped Gd2MoO6 phosphors for various color light emissions has been conducted (Huang et al. 2013; Li et al. 2006, 2009). Recent research has investigated the potential of Gd2MoO6 doped with Eu3+ for fingerprinting applications (Park et al. 2019) and white light-emitting diodes (WLEDs) (Chen et al. 2021). This exploration raises questions about the versatility of these materials beyond LEDs, suggesting that further investigation into their potential applications could be a promising research avenue. To date, there is a lack of adequate experimental or theoretical evidence for the effects of ionizing radiation on Gd2MoO6 phosphors. Ionizing radiation is a way to induce extrinsic defects in any material, which can influence its various properties including color emission which is an important point for any phosphor material. One method is to irradiate the materials with Swift heavy ions (SHIs).
The authors have previously demonstrated the effect of SHIs on the structural, optical, and luminescence behavior of undoped Gd2MoO6 material (Dutta et al. 2016a, b). It is well known that the basic performance of SHI is mainly focused on material modifications and the development of nanostructures (Kumar et al. 2009) (Mishra et al. 2007), along with the creation of a significant number of defects (Mishra et al. 2007; Avasthi 1997; Mehta 1997). It accelerates various modifications, such as structural, optical and luminescence behaviors of different types of materials (Popov and Balanzat 2000) (Baubekova et al. 2020). The underlying basics related to the irradiation of SHIs and their dependence on the nature of the materials and the energy (Ansari et al. 2021); Ridgway et al. 2011) remain to be established in the case of these phosphor materials.
Keeping this in mind, the present research work aims at investigating Dy3+ and Eu3+ co-doped Gd2MoO6 phosphors for their ion-induced structural, optical, and luminescence behavior compared to their virgin counterparts for the first time using 30 MeV Li3+ and 100 MeV Ag7+ with different ion fluences. The primary goal in choosing the aforementioned ions for irradiation was to examine the relationship between ions in the periodic table with lower mass and energy and those with higher mass and energy. In order to understand the impact of two different ions in two different parts of the periodic table, the Li ion with a 30 MeV energy has been chosen as the element with the lowest energy, and the 100 MeV Ag ion has been chosen as the element with the highest energy. The ion-induced characteristics are discussed on the basis of the formation of defects concentration and the role of linear energy transfer (LET) of these ion species on this material with ion irradiation. Thermoluminescence (TL) studies and trapping parameters were calculated to explore their dosimetry properties.
2 Materials and methods
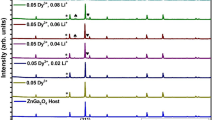
The synthesis of Dy3+ doped and Dy3+/ Eu3+ co-doped Gd2MoO6 and their optimized values have been reported in detail previously (Dutta and Sharma 2016). The SHI irradiation facility was used at the Inter University Accelerator Center, New Delhi. Stopping and Ranging of Ions in Matter (SRIM) software (Stoller et al. 2013) was used to estimate the range of 30 MeV Li3+ and 100 MeV Ag7+ ions where the ion range was estimated at 66.7 μm and 8.70 μm for the synthesized phosphors, respectively. The width of the pellets was between 66 μm and 8 μm, corresponding to the respective ion irradiations such that ions can pass throughout the pellets, excluding the possibility of the implantation of ions. The irradiation fluences used were 1 × 1011- 1 × 1013 ions/cm2 of 30 MeV Li3+ and 1 × 1011- 5 × 1012 ions/cm2 of 100 MeV Ag7+. The complete information about SHI irradiation is also reported in our previous study (Li et al. 2006). The structural and morphological characterizations of the pristine and irradiated phosphors were confirmed using X-ray diffraction (XRD) and field effect scanning electron microscopy (FESEM) on a Bruker D8 Focus and a Hitachi S-3400 N, respectively. The elemental analysis was carried out using an energy-dispersive X-ray (EDX) spectrometer from ThermoScientific. The luminescence spectra were recorded by a Hitachi F-2500 fluorescence spectrophotometer. The TL glow curves were obtained at a heating rate of 5 ºC/s using a Harshaw TLD reader (model 3500).
3 Results and discussion
3.1 Theoretical analysis
The linear energy transfer (LET) calculated (Table 1) using the SRIM code (Avasthi 1997) was further used to simulate the depth profiles in the SHI (Li3+ and Ag7+) induced Dy3+ and Dy3+/ Eu3+ Gd2MoO6 phosphors. Since the LET value is higher for Ag ions and the range is smaller as compared to Li ions, the effect of the Ag ion is more superficial than that of the Li ion, which breaches deeper in the phosphor pellets. The width of the phosphor pellets is made smaller than the SHI range so that the ions pass through the material transferring their kinetic energy to the host electrons through electron phonon coupling.
This causes an increase in the material’s temperature over its melting point and then sudden quenching transpires, creating different defects inside the material (Hodgson et al. 1980; Peña-Rodríguez et al. 2015; Som et al. 2013). The LET value calculated for the Ag7+ ion is higher, suggesting a more pronounced effect of disorder in both the doped and co-doped phosphors. The two energy losses as a function of beam energy - the nuclear (Sn) energy and electronic (Se) energy losses associated with ion irradiation are shown in Fig. 1 for the irradiated phosphors. Figure 1 shows the variation of Sn and Se as a function of energy for the ion irradiated material. According to the figure, Se outperforms Sn for both doped and co-doped phosphors at energies of 30 MeV and 100 MeV, which correspond to Li ion and Ag ion irradiation, respectively. Thus, in the MeV energy range, Se is responsible for the defects produced and the reordering of the trapping parameters leading to the structural, optical and luminescence modifications in the present materials.
3.2 X-ray powder diffraction (XRD) analysis
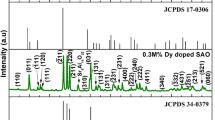
Figures 2(a) and 2(b) display the XRD spectra of the irradiated doped and co-doped Gd2MoO6 phosphors for the 5 × 1012 ions/cm2 ion fluence, respectively with the XRD of the virgin phosphors, indexed according to JCPDS pdf no. 24–0423 (Dutta and Sharma 2016)
The absence of any impurity peak even after high ion fluence irradiation indicates that SHI irradiation has no effect on phase. However, after ion irradiation, the full width at half maximum (FWHM) of Gd2MoO6: Dy3+ phosphors decreased. The reduction in FWHM seems to contradict the crystalline nature of the sample. This analogous contradictory behavior has been previously documented in studies involving ZnO subjected to irradiation by 100 keV Ne and 1.2 MeV Ar ions (Matsunami N,Sataka M 2003 a b). Generally, polycrystalline host matrices exhibit a high density of defects, with grain boundaries being significantly more defective than the grain interiors (Matsunami N,Sataka M 2003 c). Hence, the reorganization of defects in the grain boundary region during the passage of energetic projectiles is more probable. Therefore, after ion irradiation, the distribution of grain orientation becomes more precise, resulting in a decrease in the FWHM of the XRD peak (Matsunami N,Sataka M 2003 b d). However, in the case of Gd2MoO6: Dy3+/ Eu3+ phosphors, the FWHM values were observed to increase after ion irradiation. The widening of the XRD peaks shows a reduction in the crystallite size and is also related to the presence of lattice stresses. These non-uniform stresses are caused by the formation of point defects. The energy lost by the high intensity ion beam during the irradiation process causes certain defects, and the crystalline quality worsens with increasing ion dosage (Dutta and Sharma 2016). When compared to the doped phosphor, the co-doped phosphor appeared to be more affected by the ions. This may be attributed to the intensification of already existing defects inside the phosphor because of the co-doping effect of the Eu3+ ion.
3.3 FESEM and elemental analysis
Figure 3 depicts the FESEM images of virgin and ion irradiated Dy3+ and Dy3+/ Eu3+ phosphors. The fluence rate for Li ion irradiation was used as 1 × 1013 ions/cm2, while the ion fluence for Ag ion irradiation was 5 × 1012 ions/cm2. FESEM images of the virgin, Li-ion, and Ag-ion irradiated Dy3+doped Gd2MoO6 phosphors are shown in Fig. 3 (a-c), respectively
The virgin phosphor shows agglomerated particles. SHI irradiation disrupts the agglomerated particles into irregular particles with reduced sizes. The decrease in particle size might be attributable to the fragmentation of larger grains caused by SHIs irradiation. Similar behavior was observed for Dy3+/ Eu3+ co-doped Gd2MoO6 phosphors. Figure 3(d-f) shows the FESEM images of the virgin, Li ion, and Ag ion irradiated Dy3+/ Eu3+ co-doped Gd2MoO6 phosphors. The change in size and morphology after ion irradiation is more evident for co-doped Gd2MoO6 where the clustered rod like structures (Fig. 3(d) breaks down to smaller irregular size particles due to the ions (Fig. 3(e-f) (Dutta et al. 2016a, b). The EDS spectra of the phosphors before and after ion irradiation are shown in Fig. 4 where a, b, c display the doped phosphor and d, e, f exhibit the co-doped Dy3+/Eu3+ Gd2MoO6. There are no signs of Li or Ag ions in the spectra of b, c, and e, f that ensure irradiation in the virgin doped (a)/ co-doped (d) phosphors, indicating that the ions just transit through the host, causing various forms of defects inside that modify the properties without structurally and chemically changing the materials (Hodgson et al. 1980).
3.4 Optical characterizations
The comparative diffuse reflectance (DR) spectra of Dy3+ and Dy3+/ Eu3+ Gd2MoO6 phosphors are shown in Fig. 5. The spectra after irradiation are shown in the inset. The strong band around 260 nm for the doped phosphor can be attributed to the MoO66- groups and the host charge transfer absorptions (Dong et al. 2014). After Eu3+ co-doping, a small shift in the peak with a slight hump around 250 nm is due to the stronger Eu- O charge transfer effect.
After ion bombardment, the DR band demonstrates a prominent distinction with a slight shift in band positions for the Dy doped phosphor as compared to the Eu co-doped phosphor. These alterations are attributed to the ions creating a number of defect states in the phosphors. Also, the reflectance drops for the Ag ion irradiated phosphors, signifying more scattering. The Kubelka-Munk theory (Meena et al. 2022) was used to calculate the band gaps of the phosphors and is shown in Fig. 6. The inset provides the band gap variation with changes in the ion fluences for the two ions. The bandgap of the ion irradiated phosphors decreased when compared to their virgin counterparts, but the variation with ion fluence is different. The band gap values with increasing ion fluence are given in Table 2. The band gap was observed to decrease slightly with increasing ion fluences for Ag ions. This change in the band gap value might be attributed to structural disorder induced by irradiation and fluence, owing to the high electronic Linear Energy Transfer (LET) of ions [Abu-Hassan, L.H. Townsend1988–Dutta, S., Som, S., Kunti 2017]. Conversely, the trend in band gap variation differs for Li ion irradiation, showing a slight increase in band gap with Li ion fluence. The lower LET of Li ions as they pass through the molybdate host could be the primary reason for the distinct manifestations of structural disorder and their effects. It is challenging to fully elucidate such phenomena as the impact of heavy ions on any material is multifaceted, and predicting outcomes with certainty is difficult.
3.5 Photoluminescence studies
Figure 7 displays the photoluminescence (PL) spectra of the phosphors before (Fig. 7(a) and (b) and after ion irradiation (Fig. 7(c) and (d). In Fig. 7(a) the excitation spectra of the Gd2MoO6 phosphors range from 200 to 500 nm recorded for the yellow emission of Dy3+ (4F9/2→ 6H13/2 transition) at 577 nm which is the prevailing emission of Dy3+ and in Fig. 7 (b) the emission spectra are documented from 400 to 700 nm for an excitation wavelength of 351 nm. The peaks in the excitation spectra centering at 325 nm, 351 nm, 365 nm, 390 nm, 428 nm, 450 nm, and 475 nm are caused by f-f transitions of Dy3+ ions corresponding to the (6H15/2→6P3/2), (6H15/2→6P7/2), (6H15/2→6P5/2), (6H15/2→4I13/2), (6H15/2→4G11/2), (6H15/2→4I15/2) and (6H15/2→4F9/2) respectively. The two dominant emissions of Dy3+ at 488 and 577 nm corresponding to (4F9/2→6H15/2) and (4F9/2→6H13/2) transitions were further joined by a new peak at 612 corresponding to the 5D0→7F2 of Eu3+ in the co-doped Gd2MoO6 phosphor. The detailed study of the co-doping effect on the molybdate phosphor is explained previously (Dutta and Sharma 2016). The fluence used for the comparison was 1 × 1011 ions/cm2. Though ion irradiation doesn’t affect the PL peak position, the intensity is higher than the virgin one due to the large number of defects created (Abu-Hassan and Townsend 1988); Dutta et al. 2017); Dong et al. 2012)
The response curve with the variation in ion fluence range is viewed in Fig. 8. The PL intensity is affected by the damage caused by SHIs. Primary radiative transitions are indicated by a high PL intensity. As the concentration of defects acting as color centres grows, the rate of radiative transition also increases. SHI-induced fragmentation increases with SHI fluence, resulting in an increase in defect density or color centers. Thus, the first rise in PL intensities over those of the virgin samples can be attributed to an uptick in defect concentration brought on by ion irradiations. Further, new defects or color centres may develop as a result of an increase in ion fluence (Dutta and Sharma 2016). At a certain ion fluence, these new centres may be annihilated together with the production of luminous quenchers, which then contribute to the reduction in PL intensity. Therefore, as ion fluence increases, the excitons’ energy in the SHI-irradiated phosphors can non-radiatively transfer to the ions’ luminous quencher, resulting in a decrease in PL emission intensity.
The color coordinates changes for ion irradiated and virgin phosphors are presented in Fig. 9 (a, d). The color coordinate for the virgin Gd2MoO6: Dy3+ phosphor is (0.20, 0.26), whereas the CIE coordinate for the virgin Gd2MoO6: Dy3+/ Eu3+ phosphor is (0.38, 0.30). The color coordinate of virgin Gd2MoO6: Dy3+ formed mainly due to the presence of two peaks around blue and yellow regions, as shown in Fig. 9 (a). The conjugation of blue to yellow emission can result in a variety of emission colours ranging from blue to near white. In the case of virgin Gd2MoO6: Dy3+ phosphor, the blue emission intensity was higher than the yellow emission, leading to the color coordinates around the blue region. When the Gd2MoO6: Dy3+ phosphors were irradiated with Ag and Li ions, the yellow emission intensity increased due to the enhancement of dipole- dipole interaction. Therefore, the emission color shifted towards the white region. The variation of emission coordinates with various ion fluences of Li and Ag is shown in Fig. 9 (b) and (c), respectively.
The brown circle represents the CIE coordinates of the virgin samples. The deep yellow triangle indicates the CIE coordinate of the phosphors irradiated with Li ions having an ion fluence of 5 × 1012 ions/cm2. The green cross indicates the CIE coordinate of the phosphors irradiated with Li ions having an ion fluence of 5 × 1012 ions/cm2. Furthermore, yellow emission intensity was intensified along with the appearance of another red emission for virgin Gd2MoO6: Dy3+/ Eu3+ phosphor. The combined emission color was near-white (Fig. 9(d)). When the Gd2MoO6: Dy3+/ Eu3+ phosphors were irradiated with Ag and Li ions, the blue emission intensity increased, and hence the emission coordinate shifted towards the blue region. The variation of emission coordinates with various ion fluences of Li and Ag is shown in Fig. 9 (e-f) respectively.
3.6 Thermoluminescence
3.6.1 TL glow curves analysis
The TL glow curves of Dy3+ doped and Dy3+/ Eu3+ co-doped Gd2MoO6 phosphors irradiated with 30 MeV Li3+ and 100 MeV Ag ion beam at varying ion fluence range mentioned previously are displayed in Fig. 10. A broad single peak at around 340 °C was found in all instances, but at varying intensities.
For Li ion, with the increase in ion fluence, the intensity of the glow peak of the doped Gd2MoO6 phosphor goes on increasing up to the maximum fluence value whereas for the co-doped phosphor the maximum intensity was obtained at 1 × 1012 ions/cm2. When the phosphors are irradiated with Ag ion, 1 × 1012 ions/cm2 was the fluence that produced maximum intensity for the doped phosphor but there was a decrease in intensity from the lowest to the highest fluence for the co-doped one. The first increase and subsequent decrease in TL intensity can be related to a preliminary increase in the quantity of intrinsic traps, as well as lattice disruption at greater fluence caused by strong electronic excitation. For the Ag ion irradiated co-doped Gd2MoO6 phosphors the TL intensity decrease can be credited to the lattice disorder effect due to the bulky Ag ion (Atomic Weight = 107.868) coupled with the rupture of the O- Mo bonding on the sample surface as well as higher LET value. These inconsistent variations of the TL glow curves can be further clarified by the Track Interaction Model (Horowitz et al. 1996). The TL response curves of the phosphors are shown in Fig. 11.
3.6.2 Trapping parameters
The defects/traps within the material formed after ion irradiation give rise to the TL glow curves. Different trapping parameters divulge the dosimetric behavior of the material after the heavy ions irradiation (Achard et al. 2011). Thermoluminescence glow curves were deconvoluted using the TLanal (Chung et al. 2005) computer program to gain a clear sense of where the traps were located and shown in Fig. 12.
The figure of merit (FOM) determining the quality of fitting was less than 2% for the current study. The kinetic parameters for the deconvoluted peaks were calculated using Chen Formulism (Chen and McKeever 1997); Chen and Winer 1970) and are summarized in Table 3. It was observed that the traps which correspond to the activation energy created for Li ions are less than 1 eV for both the phosphors (0.892 eV and 0.963 eV) whereas for Ag ion irradiated phosphors two peaks were obtained by deconvolution indicating two types of trap centers- one less than 1 eV and another higher. By summing up it can be concluded that when compared to the band gap of the materials, all the trap centers formed are superficial in nature and the deepest trap value is about 1.3 eV for the Ag ion in both the materials.
4 Conclusions
The current investigation reveals that the irradiation of 30 MeV Li3+ and 100 MeV Ag7+ ions with varying ion fluences is plausible for the creation of disordered lattices or tracks inside the doped and co-doped Gd2MoO6 phosphors, which eventually modified their structural, optical, and luminescence properties. Structurally, there is crystallinity loss of the phosphors with the reduction in particle size due to the increment in defect concentration. A decrease in the band gap for both phosphors is observed owing to the structure disorder and defect formation with ion irradiation and fluence. Following ion irradiation, PL peak intensities increased without any corresponding change in peak positions. Thermoluminescence behavior showed a single glow peak for Li ions and two peaks for Ag ions, and a non-linear response in the studied ion fluence range along with superficial traps, which mainly confirm their incompatibility for dosimetry purposes. All the effects were more pronounced for the Ag ion in creating lattice disorder or tracks inside the phosphors due to its bulkier nature and the high electronic LET of the ion.
Data availability
No datasets were generated or analysed during the current study.
References
Abu-Hassan, L.H., Townsend, P.D.: Luminescence Efficiency of Silica during Ion Beam Excitation. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 32, 293–298 (1988). https://doi.org/10.1016/0168-583X(88)90226-1
Achard, P., Adriani, O., Aguilar-Benitez, M., Alcaraz, J., Alemanni, G., Allaby, J., Aloisio, A., Alviggi, M.G., Anderhub, H., Andreev, V.P., et al.: Test of the τ-Model of bose–Einstein correlations and Reconstruction of the source function in Hadronic Z-Boson Decay at LEP. Eur. Phys. J. C. 71, 1648 (2011). https://doi.org/10.1140/epjc/s10052-011-1648-8
Ansari, A.A., Parchur, A.K., Nazeeruddin, M.K., Tavakoli, M.M.: Luminescent Lanthanide nanocomposites in Thermometry: Chemistry of Dopant ions and host matrices. Coord. Chem. Rev. 444, 214040 (2021). https://doi.org/10.1016/j.ccr.2021.214040
Avasthi, D.K.: Role of Swift Heavy ions in materials characterization and modification. Vacuum. 48, 1011–1015 (1997). https://doi.org/10.1016/S0042-207X(97)00114-0
Baubekova, G., Akilbekov, A., Feldbach, E., Grants, R., Manika, I., Popov, A.I., Schwartz, K., Vasil’chenko, E., Zdorovets, M., Lushchik, A.: Accumulation of Radiation defects and Modification of Micromechanical Properties under MgO Crystal Irradiation with Swift 132Xe ions. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 463, 50–54 (2020). https://doi.org/10.1016/j.nimb.2019.11.021
Blasse, G.: Crystal structure and fluorescence of compounds Ln2Me4+Me6+O8. J. Inorg. Nucl. Chem. 30, 2091–2099 (1968). https://doi.org/10.1016/0022-1902(68)80203-9
Chebyshev, K.A., Ignatov, A.V., Pasechnik, L.V., Selikova, N.I., Get`man, E.I.: Investigation of the Heterovalent Substitution Cadmium for Lanthanum in Molybdate La2MoO6. J. Chem. 5537048 (2021) (2021). https://doi.org/10.1155/2021/5537048
Chen, F., Liu, X.: Structure and Photoluminescence properties of La2Mo(W)O6:Eu3+ as red phosphors for white LED applications. Opt. Mater. (Amst). 35, 2716–2720 (2013). https://doi.org/10.1016/j.optmat.2013.08.015
Chen, R., McKeever, S.W.S.: Theory of Thermoluminescence and Related Phenomena;. WORLD SCIENTIFIC, ISBN 978-981-02-2295-6. (1997)
Chen, R., Winer, S.A.A.: Effects of various Heating Rates on Glow curves. J. Appl. Phys. 41, 5227–5232 (1970). https://doi.org/10.1063/1.1658652
Chen, Y., Wang, J., Liu, C., Kuang, X., Su, Q.: A host sensitized reddish-Orange Gd2MoO6:Sm3+ phosphor for light emitting diodes. Appl. Phys. Lett. 98, 81917 (2011). https://doi.org/10.1063/1.3557065
Chen, Y., Lan, Y., Wang, D., Zhang, G., Peng, W., Chen, Y., He, X., Zeng, Q., Wang, J.: Luminescence properties of Gd2MoO6:Eu3+ nanophosphors for WLEDs. Dalt Trans. 50, 6281–6289 (2021). https://doi.org/10.1039/D1DT00547B
Chung, K.S., Choe, H.S., Lee, J.I., Kim, J.L., Chang, S.Y.: A computer program for the deconvolution of Thermoluminescence Glow curves. Radiat. Prot. Dosimetry. 115, 343–349 (2005). https://doi.org/10.1093/rpd/nci073
Dong, N., Yao, Y., Chen, F.: Optical waveguides in nd:GdVO4 crystals fabricated by Swift N3 + Ion Irradiation. Opt. Mater. (Amst). 35, 310–313 (2012). https://doi.org/10.1016/j.optmat.2012.09.024
Dong, S., Ye, S., Wang, L., Chen, X., Yang, S., Zhao, Y., Wang, J., Jing, X., Zhang, Q.: Gd3B(W,Mo)O9: Eu3+ red Phosphor: From structure design to Photoluminescence Behavior and near-UV White-LEDs performance. J. Alloys Compd. 610, 402–408 (2014). https://doi.org/10.1016/j.jallcom.2014.05.011
Dutta, S., Sharma, S.K.: Energy transfer between Dy3+ and Eu3+ in Dy3+/Eu3+-Co-doped Gd2MoO6. J. Mater. Sci. 51, 6750–6760 (2016). https://doi.org/10.1007/s10853-016-9962-z
Dutta, S., Sharma, S.K., Kumar, V., Som, S., Swart, H.C., Visser, H.G.: Ion-Induced modification of Structural, Optical and luminescence behaviour of Gd2MoO6 nanomaterials: A comparative Approach. Vacuum. 128, 146–157 (2016a). https://doi.org/10.1016/j.vacuum.2016.03.017
Dutta, S., Som, S., Kunti, A.K., Sharma, S.K., Kumar, V., Swart, H.C., Visser, H.G.: Ag7+ Ion Induced modification of morphology, Optical and Luminescence Behaviour of Charge compensated CaMoO4 Nanophosphor. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 384, 76–85 (2016b). https://doi.org/10.1016/j.nimb.2016.08.007
Dutta, S., Som, S., Kunti, A.K., Kumar, V., Sharma, S.K., Swart, H.C., Visser, H.G.: Structural and luminescence responses of CaMoO4 Nano Phosphors synthesized by Hydrothermal Route to Swift Heavy Ion Irradiation: Elemental and Spectral Stability. Acta Mater. 124, 109–119 (2017). https://doi.org/10.1016/j.actamat.2016.11.002
Hodgson, R.T., Baglin, J.E.E., Pal, R., Neri, J.M., Hammer, D.A.: Ion Beam Annealing of Semiconductors. Appl. Phys. Lett. 37, 187–189 (1980). https://doi.org/10.1063/1.91819
Horowitz, Y.S., Rosenkrantz, M., Mahajna, S., Yossian, D.: The Track Interaction Model for Alpha Particle Induced Thermoluminescence Supralinearity: Dependence of the Supralinearity on the Vector properties of the alpha particle Radiation Field. J. Phys. D Appl. Phys. 29, 205–217 (1996). https://doi.org/10.1088/0022-3727/29/1/031
Hou, Z., Lian, H., Zhang, M., Wang, L., Lü, M., Zhang, C., Lin, J.: Preparation and Luminescence properties of Gd2MoO6:Eu3+ nanofibers and nanobelts by Electrospinning. J. Electrochem. Soc. 156, J209 (2009). https://doi.org/10.1149/1.3138702
Huang, M.N., Ma, Y.Y., Huang, X.Y., Ye, S., Zhang, Q.Y.: The Luminescence properties of Bi3+ sensitized Gd2MoO6:RE3+ (RE = eu or sm) phosphors for Solar Spectral Conversion. Spectrochim Acta Part. Mol. Biomol. Spectrosc. 115, 767–771 (2013). https://doi.org/10.1016/j.saa.2013.06.111
Kumar, V., Swart, H.C., Ntwaeaborwa, O.M., Kumar, R., Lochab, S.P., Mishra, V., Singh, N.: Thermoluminescence Response of CaS:Bi3+ Nanophosphor exposed to 200 MeV ag + 15 Ion Beam. Opt. Mater. (Amst). 32, 164–168 (2009). https://doi.org/10.1016/j.optmat.2009.06.018
Li, G., Wang, Z., Quan, Z., Liu, X., Yu, M., Wang, R., Lin, J.: Sol–Gel Growth of Gd2MoO6:Eu3+ nanocrystalline layers on SiO2 spheres (SiO2@Gd2MoO6:Eu3+) and their luminescent properties. Surf. Sci. 600, 3321–3326 (2006). https://doi.org/10.1016/j.susc.2006.06.018
Li, H., Zhang, S., Zhou, S., Cao, X., Zheng, Y.: Crystalline size effect on the Energy transfer from Mo – O groups to Eu3+ ions in R2MoO6:Eu (R = La, Gd, and Y) crystals. J. Phys. Chem. C. 113, 13115–13120 (2009). https://doi.org/10.1021/jp903647v
Meena, M.L., Lu, C.-H., Som, S., Chaurasiya, R., Lin, S.D.: Highly efficient and thermally stable Eu3+ activated phosphate based phosphors for WLEDs: An experimental and DFT Study. J. Alloys Compd. 895, 162670 (2022). https://doi.org/10.1016/j.jallcom.2021.162670
Mehta, G.K.: Swift Heavy ions in materials Science—Emerging possibilities. Vacuum. 48, 957–959 (1997). https://doi.org/10.1016/S0042-207X(97)00102-4
Mishra, Y.K., Kabiraj, D., Avasthi, D.K., Pivin, J.C.: Swift Heavy Ion-Induced dissolution of gold nanoparticles in silica matrix. Radiat. Eff. Defects Solids. 162, 207–213 (2007). https://doi.org/10.1080/10420150601132883
Park, J.Y., Chung, J.W., Yang, H.K.: Versatile fluorescent Gd2MoO6:Eu3+ nanophosphor for latent fingerprints and anti-counterfeiting applications. Ceram. Int. 45, 11591–11599 (2019). https://doi.org/10.1016/j.ceramint.2019.03.030
Peña-Rodríguez, O., Olivares, J., Bányász, I.: Optical properties of Crystalline and Ion-Beam Amorphized Bi12GeO20: Relevance for Waveguide Applications. Opt. Mater. (Amst). 47, 328–332 (2015). https://doi.org/10.1016/j.optmat.2015.05.050
Popov, A.I., Balanzat, E.F.: Centre Production in CsI and CsI–Tl crystals under Kr Ion Irradiation at 15 K. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 166–167, 545–549 (2000). https://doi.org/10.1016/S0168-583X(99)00789-2
Ridgway, M.C., Giulian, R., Sprouster, D.J., Kluth, P., Araujo, L.L., Llewellyn, D.J., Byrne, A.P., Kremer, F., Fichtner, P.F.P., Rizza, G., et al.: Role of thermodynamics in the shape Transformation of embedded Metal nanoparticles Induced by Swift Heavy-Ion Irradiation. Phys. Rev. Lett. 106, 95505 (2011). https://doi.org/10.1103/PhysRevLett.106.095505
Som, S., Sharma, S.K., Lochab, S.P.: Morphology, Ion Impact, and kinetic parameters of Swift Heavy-Ion-Induced Y2O3:Dy3+ phosphor. Phys. Status Solidi. 210, 1624–1635 (2013). https://doi.org/10.1002/pssa.201329197
Stoller, R.E., Toloczko, M.B., Was, G.S., Certain, A.G., Dwaraknath, S., Garner, F.A.: On the Use of SRIM for Computing Radiation damage exposure. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 310, 75–80 (2013). https://doi.org/10.1016/j.nimb.2013.05.008
Acknowledgements
Authors are highly thankful to Pelletron group at IUAC, New Delhi, India, for providing ion beam and other facilities.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.D., S.S., S.K.S; methodology, S.D.; software, S.D., and M.L.M.; validation, M.L.M., and S.D.; formal analysis, S.D., S.S.; investigation, S.D.; data curation, S.D., and M.L.M.; writing—original draft preparation, S.D., M.L.M.; writing—review and editing, S.S., S.K.S.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, S., Som, S., Meena, M.L. et al. Probing the luminescence behavior of Dy3+/Eu3+ co-doped gadolinium molybdate phosphors under the impact of swift heavy ions. Opt Quant Electron 56, 942 (2024). https://doi.org/10.1007/s11082-024-06739-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-06739-3