Abstract
ZTS has been grown using a slow evaporation technique at ambient temperature, and the l-alanine-doped ZTS was likewise prepared. Powder X-ray diffraction (XRD), Fourier Transform Infrared spectroscopy (FTIR) and Vickers’s micro hardness test have all been used to analyse growing crystals. Both pure ZTS and l-Alanine-doped ZTS crystals have an orthorhombic structure. l-alanine doped ZTS had greater second harmonic generation (SHG) conversion efficiency. l-alanine doped ZTS crystals had a higher hardness than a pure ZTS sample. UV spectral analysis indicates that both crystals have excellent UV and visible transmission. Doping with l-alanine resulted in an increase in the melting point of the pure ZTS crystal.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With their notable potential in telecommunication, optical computing, optical data storage, and optical information processing, the semi-organic nonlinear optical materials with outstanding second-order optical nonlinearities have recently seen attention in their synthesis (Wang et al. 1999). ZTS is a semi-organic, nonlinear optical substance belonging to the family of semi-organics (Marcy et al. 1992). ZTS may be prepared in water at room temperature (Andreetti et al. 1968; Newman et al. 1990). ZTS forms an orthorhombic non-centerosymmetric space group Pca21 with four formula units in the unit cell. a = 11.178 Å, b = 7.765 Å, and c = 15.594 Å (Moitraand and Kar 2007). The three thiourea (tu) groups (tu1, tu2, tu3) and (SO4)2− ions are all part of the structure of ZTS. The structure of each tu group is based on a carbon atom connecting to two sulphur atoms and two nitrogen atoms. The two nitrogen atoms each have two hydrogen atoms attached to them. Like every Zn2+ ion, three sulphur atoms (in the three tu groups) and an oxygen atom in the sulphate ion are tetrahedrally coupled to a Zn2+ ion. The Zn-tetrahedral group is more deformed than the SO4− ion group. This unusual hydrogen bonding network is also part of the framework of ZTS. A high laser damage threshold, a broad transparency range, and a low dielectric constant at higher frequencies are some of the unique characteristics of ZTS (Chithambaram and Krishnan 2006). Foreign impurities used in doping pure crystals are increasingly intriguing since they may greatly alter the characteristics of the material. A general conclusion from several studies on organic impurities is that they may affect crystal formation, surface phenomena, structural flaws, and optical quality (Moitraand et al. 2007). Because most of the amino acids have nonlinear optical characteristics because of the donor amino group NH3+, the acceptor carboxyl group COOH− and intermolecular charge transfer is also conceivable, it is usually not feasible to detect the optical nonlinearity using these amino acids alone (Stryer 1995). To support this, researchers have found that by using amino acids as dopants, there was an improvement in the material characteristics, particularly with regards to nonlinear optical and ferroelectric capabilities (Chithambaram 2011). Now that you have seen this, motivated by the aforementioned ideas, the growth and characterisation of zinc (tris) thiourea sulphate (ZTS) doped with l-alanine (tris) has been covered in depth in the current research study.
2 Synthesis and crystal growth
Tris (Zn) thiourea (TU) was made from the acid form of the element, thiourea, and the inorganic salt, Zinc heptahydrate. When Thiourea interacts with zinc sulphate, it produces the following results:
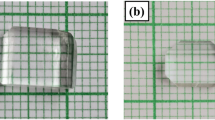
A magnetic stirrer was used to completely dissolve the necessary quantities of thiourea and zinc sulphate heptahydrate. A white crystalline precipitate was produced when the mixture was heated to 50 °C for an hour. To prevent decomposition, the temperature of the mixture was kept at 50 °C, and it was continuously stirred. Before filtering out the white powder of ZTS, it was vacuum-dried. White powder ZTS was dissolved in a beaker, after which it was quadruple distilled to form a saturated solution. Once the solvent had evaporated, the solution was dispersed into four dishes. The three percent l-alanine dishes were treated with l-alanine in amounts of 1, 3, and 5 mol percent, while the fourth was left untreated (for pure ZTS).Clear seed crystals were discovered in all the dishes after 2 weeks. To remove the impurities, they were recrystallized twice. Seed collection was done by gradual evaporation, and the SHG test was performed on the seeds using Kurtz and Perry. The 1 mol% l-alanine doped ZTS samples had a greater SHG conversion efficiency than the 3 and 5 mol% l-alanine doped ZTS samples. Thus, for large-scale development, a high-quality seed was taken that had 1 mol% l-alanine doped ZTS single-crystal doped with l-alanine. Figure 1 has shown the pure and l-alanine-doped ZTS crystals.
3 Characterizations
The structural, optical, thermal and mechanical characteristics of pure and l-alanine doped ZTS crystals were investigated. To validate the existence of functional groups in the crystal lattice, an FTIR spectrum was acquired using a Perkin-Elmer RX1 FTIR spectrometer in the wave number range 400–4000 cm−1 using the KBr pellet method. To determine the structure and estimate the lattice parameters, single crystal X-ray diffraction examination of the produced crystals was performed using ENRAF NONIUS CAD4 X-ray diffractometer MoKα (λ = 0.7170) radiation. Powder XRD patterns of pure and doped ZTS crystals were obtained using a Rich Seifert diffractometer with CuKα (λ = 1.5417) radiation. The UV–visible absorption of pure and l-alanine doped ZTS crystals were studied between 190 and 1100 nm using a Perkin Elmer Lambda 35 spectrophotometer. The NET SZCH STA 1500C version V4.30 thermal analyser was used to perform both differential thermal analysis (DTA) and thermo gravimetric analysis (TGA) on pure and l-alanine doped ZTS crystals. The SHG efficiency of pure and l-alanine doped ZTS crystals were evaluated using the Kurtz and Perry powder method. A Q-switched Nd: YAG laser beam with a wavelength of 1064 nm, an input pulse energy of 11.2 mJ/pulse, a pulse width of 8 ns, and a repetition rate of 10 Hz was utilised. Micro hardness testing was carried outby Static indentation tests were performed at room temperature on the fully developed faces of pure and alanine doped ZTS utilising a LeitzWetzlar hardness tester equipped with a Vickers’s diamond pyramidal indenter.
4 Results and discussion
4.1 FTIR spectral analysis
The Fig. 2 shows the recorded FTIR spectrum of pure ZTS. The presence of N–H absorption bands at higher frequencies during the production of metal Thiourea complexes shows that nitrogen to zinc bonds is absent and that the bonding between sulphur and zinc atoms is present. The 1625 cm−1 band is linked to NH2 deformation vibration. The presence of sulphate ions is indicated by a prominent absorption peak at 618 cm−1 (Nakamoto 1978). C=S is bound with NH2 in pure Thiourea, and the IR band for asymmetric stretching vibration of the C=S group typically occurs at 1417 cm−1. Because the metal in ZTS is bound to sulphur, the C=S bonding mode is altered to a lower value of 1399 cm−1. So, based on existing Thiourea data (Rajasekaran et al. 2001), the distinctive IR bands for the various chemical groups present in ZTS have been determined. Figure 3 shows the FTIR spectrum of l-alanine doped ZTS. When the IR spectra of pure and l-alanine doped ZTS are compared, there is a small shift in absorption bands, which may be related to the presence of l-alanine. In addition to the peaks seen in the pure ZTS, two additional peaks at 1303 and 539 cm−1 are observed in the l-alanine doped ZTS. These peaks are attributed to l-alanine C–H bending and COO− rocking. Thus, the FTIR spectrum of l-alanine doped ZTS indirectly confirmed the presence of l-alanine in the lattice of the ZTS crystal is shown in Table 1.
4.2 Single crystal X-ray diffraction analysis
The Table 2 shows the lattice parameter values obtained from single crystal XRD investigation for the produced crystals. This study showed that the crystals, both pure and alanine doped ZTS, had an orthorhombic structure.
4.3 Powder XRD analysis
The powder X-ray diffraction analysis was performed to validate the identification of the synthesised crystal as well as to detect changes in lattice parameters and diffraction pattern of the doped crystals. The powder samples were scanned in 0.02° increments for 2 s at a time interval of over a 2θ range of 10°–60°. In the XRD patterns, all of the detected reflection lines were catalogued. The XRD pattern of pure ZTS corresponds closely to the JCPDS dataset (File No. 76-0778). Figures 4 and 5 have shown the indexed XRD patterns of pure and l-alanine doped ZTS crystals. This study revealed that the majority of the main reflecting planes are present in both pure and doped crystals, with a small change in Bragg angle indicating the integration of l-alanine into the crystal lattice of pure ZTS.
4.4 UV–Vis-NIR spectral study
The majority of single crystals are utilised in optical applications. As a result, it is critical to investigate the optical transmission range of formed crystals. This research used pure and l-alanine doped crystals of uniform thickness. The optical transmission in order to wavelengths in the range of 200–1200 nm in the UV–Vis-NIR spectrum. Figure 6 of pure and l-alanine doped ZTS crystals show that l-alanine doping has no effect on the transmission region. When we look at the percentage of transmission, we see that l-alanine doped ZTS has a lower proportion than pure ZTS. Figure 6 depicts the absorption spectra of pure and l-alanine doped ZTS single crystals. According to the figure, both crystals exhibit a low UV cut-off at 265 nm. Absorption occurs in the UV area due to an electronic transition associated with the thiourea units of ZTS. Further it is possible for a molecule of organic l-Alanine to quickly absorb ultraviolet or visible light that excites electrons (making them high energy). Because the π and π* orbitals are delocalized (the energetic distance between them falls within the same energy), optical absorption rises with the smallest energy gap. At the same, the optical absorption in the NIR range begins to decline suddenly as the wavelength grows and the frequency decreases as energy decreases. This indicates that a large energy gap allows bonding electrons to be localised. The reduced transmittance of l-alanine doped ZTS compared to pure ZTS is due to its very higher optical absorbance (Ushasree et al. 1999). According to the UV–Vis-NIR spectral analysis, both pure and doped ZTS crystals exhibit excellent transmission in the UV and visible regions, which is an advantage for the crystals to be utilised in optoelectronic applications.
4.5 Thermal analysis
The Fig. 7 depicts the TGA/DTA curves of l-alanine doped ZTS. The TGA curve indicates the greatest weight loss between temperatures of 200 and 350 °C. This may be owing to the compound's volatile components, most likely sulphur oxide, and other contaminants being liberated. Because there is no phase transition until the material melts, the temperature range for using crystals for NLO applications is expanded. The lack of weight loss at 100 °C confirms the absence of water in the crystal lattice. The DTA curve revealed a strong endothermic peak at 281 °C, which corresponds to the melting temperature of the l-alanine doped ZTS. Here Pure ZTS shows no specific loss up to 234 ºC (Ramajothi and DhanuskodiandK 2004), indicating that the crystal lattice does not include water that vanishes owing to evaporation. As a result, weight loss may begin as soon as the temperatures drop. Doping to raise temperatures results in a displacement of Thiourea to l-alanine, this is verified by an endothermic peak seen above 281 °C as melting point and hence ascribed to l-alanine doped ZTS.These studies revealed that when the ZTS crystal is doped with l-alanine, the thermal stability enhanced for suitable device fabrications (Table 2).
4.6 SHG efficiency
The grown pure and l-alanine doped ZTS crystals were crushed to powder and placed in a micro capillary tube with a consistent diameter before being subjected to laser radiation. The emission of green radiation verified the generation of the second harmonic signal in the powder samples. A photomultiplier tube was used to measure the intensity of green light. The output of pure and l-alanine doped ZTS was measured to be 60 and 89 mV, respectively. As a result, l-alanine doped ZTS has a greater SHG conversion efficiency than pure ZTS.
4.7 Micro hardness studies
When it comes to the manufacturing of devices, Microhardness characterization is significant. A material's hardness is a measure of its resistance to local deformation. The Vickers’s micro hardness number was calculated using the equation Hv = 1.8544P/d2 (Pa) where Hv is the Vickers’s micro hardness number, P is the applied stress, and d is the indentation impression's diagonal length. Indentations were created on the crystals' well-developed faces, and micro hardness measurements were taken for applied stresses ranging from 10 to 50 g for a dwell period of 10 s. The micro hardness value was calculated by taking the average of numerous impressions produced with both diagonals measured. Figure 8 depicts the change in the hardness number with the indenter load. The hardness of l-alanine doped ZTS is greater than that of pure ZTS, as seen in the figure. The following is one potential reason for this behaviour, l-alanine occurs as a zwitterion in a solid form. As a result, the insertion of l-alanine into the crystal lattice of ZTS causes ionic vacancy. This imperfection acts as an impediment to dislocation motion, enhancing the crystal's hardness.
5 Conclusion
The Slow evaporation technique was used to produce transparent single crystals of pure ZTS and l-alanine doped ZTS. The lattice parameters of the grown crystals were calculated using single crystal X-ray diffraction analysis. Powder X-ray diffraction studies confirm the presence of the substituted amino acid l-alanine in the case of l-alanine doped ZTS crystal by changes in relative intensity and peak locations. The functional groups contained in l-alanine doped ZTS crystals were evaluated using an FTIR spectrum. The micro hardness study on the well-developed plane of the doped ZTS crystal shows that the hardness values are substantially greater than the pure ZTS crystals. According to the UV–vis-NIR spectral analyses, there is no difference in the transmission window for l-alanine doped ZTS compared to pure ZTS. The SHG conversion efficiency measurement reveals that the dopant l-alanine improves ZTS's SHG efficiency.
Research Data Policy and Data Availability Statements
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Andreetti, G.D., CavalcaandA, L., Musatti.: The crystal and molecular structure of tris(thiourea)zinc(II) sulphate. Acta Cryst. Sect B 24, 683 (1968)
Chithambaram, V., Jerome Da, S., Krishnan, S.: Synthesis, optical and dielectric studies on Novel semi organic nonlinear optical crystal by solution growth technique. Journal of Alloys and Compounds 509, 4543–4546 (2011)
Chithambaram, V., Krishnan, S.: Synthesis, optical and thermal studies on novel semi organic nonlinear optical Urea Zinc Acetate crystals by solution growth technique for the applications of optoelectronic devicesOpt. Laser Technol. 55(14), 18–20 (2014)
Marcy, H.O., Warren, L.F., Webb, M.S., Ebbers, C.A., Velsko, S.P., Kennedy, G.C., Catella, G.C.: Second-harmonic generation in zinc tris(thiourea) sulfate. Appl. Opt. 31, 5051–5060 (1992)
Moitra. S., Kar, T.: Growth and characterization of nonlinear optical crystal zinc tris (thiourea) sulphate in presence of l-arginine. Opt. Mater. 30, 508–512 (2007)
Nakamoto, K.: IR Spectra of Inorganic and Coordination Compounds, 2nd edn. Wiley, New York (1978)
Newman, P. R., Warren, L.F., Cunningham, P., Chang, T.Y., Copper, D.E., Burdge, G.L., Polak, P., Dinglesand, C.K.: Adv. Organic Solid State Materials [C.Y. Chiang, P.M. Chaikan and D. Cowan (eds.)] Materials Research Society Symposium Proceedings, 173, 557 (1990)
Rajasekaran, R., Ushasree, P.M., Jayavel, R., Ramasamy, P.: Growth and characterization of Zinc Thiourea Chloride (ZTC): a semiorganic nonlinear optical crystal. J. Cryst. Growth 229, 563–567 (2001)
Ramajothi, J., DhanuskodiandK, S.: Nagarajan, Crystal growth, thermal, optical and microhardness studies of tris (thiourea) zinc sulphate - a semiorganic NLO material. Cryst. Res. Technol 39, 414–420 (2004)
Stryer, L.: Biochemistry, 4thedn. New York: W.H. Freeman and Company (1995) chap 2.
Ushasree, P.M., Jayavel, R., Ramasamy, P.: Growth and characterisation of phosphate mixed ZTS single crystals. Mater. Sci. Eng. B 65, 153–158 (1999)
Wang, X.Q., Xu, D., Yuan, D.R., Tian, Y.P., Yu, W.T., Sun, S.Y., Yang, Z.H., Fang, Q., Lu, M.K., Yan, Y.X., Meng, F.Q., Guo, S.Y., Zhang, G.H., Jiang, M.H.: Synthesis, structure and properties of a new nonlinear optical material: zinc cadmium tetrathiocyanate Mater. Res. Bull. 34, 2003–2011 (1999)
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
GM: Validation, TB: Formal analysis, AC: Methodology, VC: Writing, review & editing. This is the original work for my PhD research work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marudhu, G., Baraniraj, T., Chandravadhana, A. et al. Influence of l-alanine doping on zinc (TRIS) thiourea sulphate (ZTS) single crystals. Opt Quant Electron 54, 700 (2022). https://doi.org/10.1007/s11082-022-04130-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-04130-8