Abstract
Today, cancer disease is a significant reason for the death of many patients. In many cases, cancer is diagnosed after metastasized during the body. Thus, the earlier detection gives a better opportunity for treatment and cure. A simple 1-D binary photonic crystal with a defect layer is proposed with the structure (Si/SiO2)N/Defect/(Si/SiO2)N as a detector for cancerous cells. The defect layer is taken here as the patient's blood sample. Compared with the normal blood sample, the cancerous samples lead to a considerable change in the refractive index. This index variation leads to a shift in the resonant mode position which can be used to diagnose cancer cells. The transfer matrix method is employed to analyze the structure. The number of periods, defect layer thickness and incident angle are investigated to maximize the sensitivity. The sensitivity is calculated at optimized conditions and found as 2400.08 nm/RIU. This sensitivity is extremely high when compared to the most recent biosensors. All the sensor performance parameters are calculated and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to medical science's failure to provide a complete treatment for cancer disorders, cancer diagnosis research has gotten a lot of attention during the previous several decades all over the world. These malignant cells grow abnormally and form tumours, which can be detected by a variety of medical tests. To reduce the deadly effects of cancer, it is critical to discover it at an early stage and to do so, a variety of clinical investigations are available in healthcare organisations to anticipate the current cell report (Katz and Edelson 2009). However, understanding the optical, chemical, and mechanical properties of a living cancer cell can lead to numerous interesting new insights into the cell's biology (Suresh 2007). The interaction of light with cells has recently yielded useful knowledge about changes in the optical characteristics of various cells. Most crucially, the cell's refractive index has gotten a lot of attention, which has expanded the possibilities for detecting cell abnormalities. According to cancer science, normal cells' refractive indices are lower than cancerous cells, which can be used as a useful indicator in the identification and diagnosis of malignant cells (Backman et al. 2000).
Photonic sensors are specifically intended to detect a variety of bio analytes by successfully converting the bio-entity into an electrical form that can be examined using a spectrometer (Danaie and Kiani 2018). Electrical characteristics and electric field analysis are used to examine the differences between normal and malignant cells (Panda and Devi 2020). Furthermore, the refractive indices of various analytes emerge as a critical parameter in the development of precise biosensors (Derbali et al. 2011; Vijayalakshmi et al. 2021; Islam et al. 2021). Recently, a few studies have been published that are based on the tabular optical properties of a variety of tissues, but this table may not provide enough information about living cells. The effective refractive index of discrete live cells has been determined using several optical (Bashkatov et al. 2011; Kim and Wilson 2011) approaches such as solution matching (Barer 1957) and refractometry analysis (Lue et al. 2006). Even though the aforementioned references reveal fascinating results, the paucity of research on the cell's local refractive index is a matter of concern. Furthermore, the sensing devices and procedures are somewhat sophisticated in terms of design, making them challenging to implement in practice. Apart from that, none of the studies in the literature has looked at sensor sensitivity, resolution, quality factor, and nonlinear coefficient all at the same time. Furthermore, the aforementioned sensor measurement parameters are insufficient to satisfy the current sensing scenario's difficulty. Although there are various strategies for detecting cancer cells in the literature, the current study is based on an examination of a binary photonic crystal for detecting normal and cancerous cells in a short amount of time. In a recent work, the authors have analysed the sensing of cancerous cells by investigating electric field distribution in a simple 2D photonic crystal fiber structure. Two clusters of cell lines, human immortalized normal oral keratinocyte which in normal cells group and YD-10B cells which are cancerous, were considered (Woo June Choi 2010). In a recent study, early cancer cell detection has been investigated by using an SPR-based photonic crystal fiber (PCF) biosensor structure (Yasli 2021). For numerical analysis, the finite element method with properly matched layers is used. The refractive index fluctuations of cancer cells are detected using spectral interrogation and amplitude approaches. Mollah et al. have presented a twin-core PCF for early detection of blood cancer based on the variation of the refractive index between normal and cancerous blood cells (Aslam Mollah et al. 2020). The analytes of the cancerous and normal cells have been considered at 80% and 30–70% in liquid form, respectively. The samples have been infiltrated into the central air hole. Using the finite element method, the change in the coupling length and transmitted spectrum of the proposed biosensor for normal and malignant cells have been observed. A metamaterial biosensor based on two loops of hexagonal gold layers on a polyamide substrate has been proposed for cancer cell early detection (Azab et al. 2021). Dual-core PCFs have been proposed (Ayyanar et al. 2018; Gyan Prakash Mishra 2020) for the detection of cancer cells in cervical, breast and basal parts.
In this work, simple methodologies and compact structures are employed which can be easily implemented with newly emerging technologies. Normal cells, as well as five caner cells, are considered. Jurkat, Hela, PC12, MDA-MB231 and MCF-7 cancer cells are treated as analytes. The sensor performance parameters are all considered such as quality factor, figure of merit, detection limit, detection accuracy, signal-to-noise ratio, standard deviation, sensor resolution and dynamic range to enhance the novelty of the current research.
2 Design and theoretical model
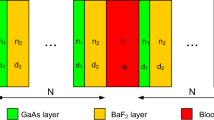
One-dimensional (1D) binary defective photonic crystal is proposed for cancer cell detection as shown in Fig. 1. The photonic crystal has the structure (AB)N/D/(AB)N. Layers A and B are chosen as Si and SiO2, respectively. D is the defect layer which is either a normal or cancerous cell. Layer D lies midway between two identical period numbers (N). The thicknesses and refractive indices of the layers are d1, d2 and dD and n1, n2, and nD, for Si, SiO2 and the defect layers, respectively.
A wide choice of different techniques to simulate photonic crystals is described in the literature. In the specific case of 1D photonic crystals, the transmission spectrum can be simulated using many different numerical methods such as finite difference time domain methods and finite element methods. The transfer matrix method is a simple and flexible technique to analyze the optical properties of 1D photonic crystals. We consider a system consisting of air/multilayer/substrate. The parameters related to air and substrate media are just their refractive indexes, no and ns, respectively. With Es and Hs are the electric and magnetic fields in the substrate, we can write the incident fields (E0 and H0) in terms of Es and Hs as
where \({F}_{j}\) is the characteristic matrix of one layer and fij are the matrix elements. Fj can be written as
\({\delta }_{j}\) is the phase variation of the light wave due to propagating through the jth layer
where \({n}_{j}\), dj \({\theta }_{j}\) are the refractive index, thickness and angle of incidence of the layer. \({\theta }_{j}\) can be written in terms of the initial incidence angle \({\theta }_{0}\) as
\({\gamma }_{j}={n}_{j}\mathrm{cos}({\theta }_{j})\) in transverse electric (TE) wave whereas \({\gamma }_{j}=\mathrm{cos}({\theta }_{j})/{n}_{j}\) in transverse magnetic (TM) wave and \({n}_{0}\) is the refractive index of the incidence medium. The transfer matrix F0 for one period consisting of two layers A and B can be written as F0 = FA FB. The full transfer matrix F of a defective binary photonic crystal can be expressed as
where FD is the transfer matrix of the defect layer and Fij are the elements of the total transfer matrix F.
The transmission coefficient can be written as
and the transmittance can have the form
The reflection coefficient can be written as
and the reflectance can have the form
For transverse electric (TE) waves, \({\gamma }_{in}={\gamma }_{out}= \mathrm{cos}({\theta }_{0})\) since the binary photonic crystal is assumed to be surrounded by air.
Based on the above theoretical equations, properties of both photonic bandgap and defect mode of a defective binary photonic crystal can be investigated.
3 Results and discussion
3.1 Cancer cell sensor
A binary photonic crystal is assumed with the structure (Si/SiO2)N/D/(Si/SiO2)N whose refractive indices are 3.3 for the Si and 1.46 for the SiO2. The thicknesses of the layers are taken as \({d}_{1}=117 \mathrm{nm}\) and \({d}_{2}=265\) nm, respectively. The wavelength of the incident radiation is taken from 800 to 2300 nm and the number of periods is taken as N = 5. The transfer matrix method is employed to treat the current problem. A code is generated using WOLFRAM MATHEMATICA 11.2 software to find the reflectance profile. All the above equations are fed to the Mathematica code. OriginPro 8.5 is used to plot the figures. Normal incidence is first considered in which θ0 = 0 and D is either the normal or cancerous cell. The transmission spectrum through the proposed binary photonic crystal without any defect layer is shown in Fig. 2a. A photonic bandgap of a width of 890 nm can be seen with left and right edges at wavelengths of 1221 nm and 2111, respectively. Figure 2b shows the transmission spectrum through the structure when the normal cell layer of thickness 1D is treated as a defect layer, where D = d1 + d2 (d1 = 117 nm, d2 = 265 nm). The width of the photonic bandgap becomes 1016.01 nm where the left and right edges are at wavelengths of 1202 nm and 2218.01 nm, respectively. A defect mode can be seen in the figure at a resonant wavelength of 1685.54 nm. An enlarged view of the defect mode is plotted in Fig. 2c when the normal cell layer is treated as a defect layer. The full width at half maximum of the resonant peak is \(FWHM=0.45 nm\) where the wavelengths of left and right edges are 1685.31 nm and 1685.76 nm. The quality factor (QF) is found as 3745.64 which was calculated by the following equation:\(QF=\frac{{\lambda }_{peak}}{FWHM}\), where \({\lambda }_{peak}\) is the resonant wavelength.
Figure 3 shows the transmission spectra through the binary photonic crystal when the cancerous cells are treated as defect layers. It is found that the resonant peak shifts to a higher wavelength region. The new wavelength positions of the defect mode are cell-dependent. The new wavelength positions are at 1702.92, 1703.8, 1705.10, 1706.85 and 1707.71 nm for Jurkat, Hela, PC12, MDA-MB231 and MCF-7 cells, respectively. The sensitivity can be calculated as \(\frac{\Delta \lambda }{\Delta n}\), where \(\Delta \lambda\) is the wavelength shift between the resonant peak position of the normal cell and cancer cell and \(\Delta n\) is the change of refractive index between the normal cell and cancer cell. The sensitivity was found as 434.5, 434.76, 434.66, 434.89 and 434.70 nm/RIU for the cells of Jurkat, Hela, PC12, MDA-MB231 and MCF-7, respectively. Table 1 presents the refractive indices of different cancerous cells, defect mode positions of each cell and sensitivity of the proposed binary photonic crystal to each cell.
3.2 Effect of the incident angle
3.2.1 Angle of incidence and sensitivity
The incident angle variation has an essential effect on the bandgap position according to the Bragg-Snell law (Dalmisa et al. 2019)
where m, \(\lambda\), d, \({\theta }_{0}\) and \({n}_{eff}\) are the order of diffraction (m is an integer), the free space wavelength, the interplanar spacing, the incident angle and the effective refractive index, respectively. The angle of incidence was changed from 0° to 89° in steps of 5°. The results for two angles of them are presented in Tables 2 and 3 which show the enhancement of sensitivity from 496.77 nm/RIU to 671.23 nm/RIU when the incident angle increases from 30° to 60°.
In Table 4, we calculated the average sensitivity of the proposed binary photonic crystal corresponding to different incident angles starting from θ0 = 0° to θ0 = 89° with an increment of 5°. The sensitivity attains a considerable enhancement as the incident angle increases from 0° to 85°. For further increase of the angle of incidence beyond 85°, insignificant enhancement of the sensitivity can be observed. So, the angle of 85° is taken as the optimum value of the incident angle. The relation between the sensitivity (S) to cancer cells and the incident angle (\({\theta }_{0}\)) of a proposed binary photonic crystal is shown in Fig. 4 (blue points). It can be fitted by the following equation
The fitting Eq. (11) is useful to predict the sensitivity of the cancer cell sensor at any value of the incident angle located between \({\theta }_{0}=0.0^\circ\) and \({\theta }_{0}=85^\circ\). The fitted sensitivity as a function of the incident angle (red curve) along with the data (blue points) is shown in Fig. 4. The matching between the data and the fitting equation is perfect.
3.2.2 Angle of incidence and photonic bandgap
The photonic bandgap can be explained by the transmission of light through certain frequencies is zero by multiple Bragg scattering. The results in Table 5 show that as the incident angle increases, the photonic bandgap width increases. At an incident angle of 0.0°, the photonic bandgap width is 1016.01 nm. When the incident angle increases to 89°, the photonic bandgap width increases to 1094.7 nm. The photonic bandgap width versus the incident angle is shown in Fig. 5. The photonic bandgap width is calculated as the difference between the wavelengths of the left and right edges at half maximum of transmission in nanometers.
The sensitivity enhancement with the increase of the incident angle can be attributed to the increase of the photonic bandgap with the increase of the incident angle. As the photonic bandgap increases, the emergence and shift of the resonant wavelength are more likely.
3.3 Effect of the number of periods
The number of periods of a photonic crystal can affect the sensitivity and bandgap width of a binary photonic crystal.
3.3.1 Number of periods and sensitivity
The number of periods was changed from 3 to 7. Some of the results can be seen in Tables 6 and 7. The average sensitivity has dropped from 444.45 nm/RIU to 434.27 nm/RIU as the number of periods increases from 3 to 6. The resonant peak position is 1687.65 nm when the normal cell is treated as a defect layer at a number of periods of 3. But when the cancerous cells are treated as defect layers, it is found that the resonant peak shifts to a higher wavelength region (redshift) as shown in Fig. 6. The new wavelength positions are at 1705.44, 1706.31, 1707.66, 1709.41and 1710.32 nm for Jurkat, Hela, PC12, MDA-MB231 and MCF-7 cells, respectively. The sensitivity is calculated and found as 444.75, 444.28, 444.66, 444.08 and 444.50 nm/RIU for the cells of Jurkat, Hela, PC12, MDA-MB231 and MCF-7, respectively.
Table 8 shows that as the number of periods increases, the sensitivity of the biosensor decreases. This can be attributed to the fraction of light in the analyte layer being less when the number of periods increases. The relation between the sensitivity of the cancer cell sensor (S) and the number of periods (\(\mathrm{N}\)) of a proposed binary photonic crystal can be fitted by the following equation
The fitting Eq. (12) can predict the sensitivity of the cancer cell sensor at any value of the number of periods located between \(N=3\) and \(\mathrm{N}=7\). When the number of periods increases beyond N = 7, the defect mode starts vanishing. The fitting equation of the sensitivity as a function of the number of periods (red curve) along with the data (blue points) is shown in Fig. 7. The matching between the data and the fitting equations is perfect.
3.3.2 Number of periods and photonic bandgap
Table 9 presents the bandgap width of the proposed binary photonic crystal and the wavelength positions of the left and right edges at different N starting from N = 3 to N = 10. When the number of periods increases the photonic bandgap width decreases. For a binary photonic crystal of N = 3, the photonic bandgap width is 1246 nm. When N becomes 10, the photonic bandgap width decreases to 889.4 nm. When N is less than 3, the photonic bandgap cannot be observed. The bandgap width versus the number of periods is shown in Fig. 8.
3.4 Effect of cancer cell layer thickness
3.4.1 Cancer cell layer thickness and sensitivity
The cancer cell layer thickness was changed from 1 to 13D. Some of the results can be seen in Tables 10 and 11. The average sensitivity has risen from 434.7 to 802.55 nm/RIU as the cancer cell layer thickness increases from 1 to 4D. Increasing the cancer cell layer from 7 to 13D shows a kind of saturation. A further increase of the cancer cell layer beyond 13D shows an insignificant improvement in the sensitivity.
Table 12 shows that as the cancer cell layer thickness increases the sensitivity of the biosensor is dramatically enhanced. In optimizing the proposed sensor, we consider the cancer cell layer thickness to be 13D. The relation between the sensitivity and the cancer cell layer thickness of a proposed binary photonic crystal can be fitted by the following equation
The fitted sensitivity as a function of cancer cell layer thickness (red curve) along with the data presented in Table 12 (blue points) is shown in Fig. 9. The matching between the calculated data and the fitting equations is excellent.
3.4.2 Cancer cell layer thickness and photonic bandgap
Table 13 shows that the photonic bandgap width oscillates as the cancer cell layer thickness increases. For example, as the cancer cell layer thickness increases from 1 to 2D, the photonic bandgap width decreases from 1018 to 905.2 nm and as the layer thickness increases from 2 to 3D, the photonic bandgap width increases from 905.2 to 1024 nm. The photonic bandgap width versus the cancer cell layer thickness is plotted in Fig. 10.
3.5 Cancer cell sensor optimization
Figure 11 shows the defect modes corresponding to different cells at the sensitivity optimized conditions (θ0 = 85°, \({d}_{f}\) = 13D and N = 3). When the normal cell is treated as a defect layer, the resonant peak position is at 1855.05 nm. It is found that resonant peak shifts to a higher wavelength region as cancerous cells are treated as defect layers. The new wavelength positions of the defect mode are cell-dependent. The new wavelength positions are at 1951.62, 1956.25, 1963.11, 1972.8 and 1976.68 nm for Jurkat, Hela, PC12, MDA-MB231 and MCF-7 cells, respectively. The sensitivity is found as 2414.25, 2409.52, 2401.4, 2390.4 and 2384.9 nm/RIU for the cells of Jurkat, Hela, PC12, MDA-MB231 and MCF-7, respectively. Table 14 presents the refractive indices of different cancerous cells, the defect mode positions of each and the sensitivity of the proposed binary photonic crystal to each cell.
To show the efficiency of the proposed sensor, we calculated some performance parameters such as quality factor, figure of merit, detection limit, detection accuracy, signal-to-noise ratio, standard deviation, sensor resolution and dynamic range (Ahmed and Shaban 2020; Ahmed et al. 2021). Table 15 shows the calculated values of the sensor performance parameters.
Table 16 presents a comparison of the current work sensitivity with that of the most recent published biosensors. It shows the technique used and the sensitivity obtained. As can be seen, the current sensor achieves the highest sensitivity.
4 Conclusion
We have assumed a nano-detector using a 1D binary PC to detect cancerous cells. The effects of incident angle, number of periods and the defect layer thickness on the sensitivity and bandgap width of the proposed structure were investigated. By changing the blood sample from normal to cancer cells, the defect mode is shifted to a higher wavelength region. The sensitivity of the proposed design is considerably enhanced when the incidence angle and the defect layer thickness increase. The photonic bandgap width of the proposed binary photonic crystal increases when the incidence angle increases while it oscillates as the defect layer thickness increases. When the number of periods increases, both sensitivity as well as bandgap width decrease. The sensitivity reached 2400.08 nm/RIU at optimized conditions which is extremely high when compared to most recent papers published in the biosensing field. In addition, our sensor has a very simple structure, tunable design and low cost.
Finally, it is worth mentioning that the typical diameter of a human blood cell is around 7–8 μm. As a result, the defect layer thickness should be taken in this range to test the human cells efficiently. Moreover, the Thermo Scientific Multiskan SkyHigh Microplate Spectrophotometer is a good candidate that is capable of detecting the transmission in the proposed range. It is a spectrophotometer that is designed to be convenient and straightforward to use for virtually any photometric research application, including DNA, RNA, and protein detection as well as turbidity measurements.
References
Abohassan, K.M., Ashour, H.S., Abadla, M.M.: A 1D photonic crystal-based sensor for detection of cancerous blood cells. Opt Quant Electron 53, 356 (2021)
Ahmed, A.M., Elsayed, H.A., Mehaney, A.: High-performance temperature sensor based on one-dimensional pyroelectric photonic crystals comprising Tamm/Fano resonances. Plasmonics 16(2), 547–557 (2021)
Ahmed, A.M., Shaban, M.: Highly sensitive Au–Fe2O3–Au and Fe2O3–Au–Fe2O3 biosensors utilizing strong surface plasmon resonance. Appl. Phys. B Lasers Opt. 126(4), 1–10 (2020)
Aly, A.H., Zaky, Z.A.: Ultra-sensitive photonic crystal cancer cells sensor with a high-quality factor. Cryogenics 104, 102991 (2019)
Ayyanar, N., Raja, G.T., Sharma, M., Kumar, D.S.: Photonic crystal fiber-based refractive index sensor for early detection of cancer. IEEE Sens. J. 18(17), 7093–7099 (2018)
Azab, M.Y., Hameed, M.F.O., Nasr, A.M., Obayya, S.S.A.: Highly sensitive metamaterial biosensor for cancer early detection. IEEE Sens. J. 21(6), 7748–7755 (2021)
Backman, V., Wallace, M.B., Perelman, L.T., Arendt, J.T., Gurjar, R., Müller, M.G., Zhang, Q., Zonios, G., Kline, E., McGilligan, J.A., Shapshay, S., Valdez, T., Badizadegan, K., Crawford, J.M., Fitzmaurice, M., Kabani, S., Levin, H.S., Seiler, M., Dasari, R.R., Itzkan, I., Van Dam, J., Feld, M.S., McGillican, T.: Detection of preinvasive cancercells. Nature 406(6791), 35–36 (2000)
Barer, R.: Refractometry and interferometry of living cells. J. Opt. Soc. Am. 47(6), 545–556 (1957)
Bashkatov, A.N., Genina, E.A., Tuchin, V.V.: Optical properties of skin, subcutaneous, and muscle tissues: a review. J. Innovative Opt. Health Sci. 4, 9–38 (2011)
Choi, W.J., Jeon, D.I., Ahn, S.-G., Yoon, J.-H., Kim, S., Lee, B.H.: Full-field optical coherence microscopy for identifying live cancer cells by quantitative measurement of refractive index distribution. Opt. Express 18(22), 23285–23295 (2010)
Dalmisa, R., Keskina, O.Y., Azem, N.F.A., Birlik, I.: A new one dimensional photonic crystal combination of TiO2/CuO form structural color applications. Ceram. Int. 45(17), 21333–21340 (2019)
Danaie, M., Kiani, B.: Design of a label-free photonic crystal refractive index sensor for biomedical applications. Photonics Nanostruct.-Fundam. Appl. 31(13), 89–98 (2018)
Derbali, J., AbdelMalek, F., Obayya, S.S.A., et al.: Design of a compact photonic crystal sensor. Opt Quant Electron 42, 463–472 (2011). https://doi.org/10.1007/s11082-010-9429-4
Hasan, M., Akter, S., Rifat, A.A., Rana, S., Ali, S.: A highly sensitive gold-coated photonic crystal fiber biosensor based on surface plasmon resonance. Photonics 4(1), 18–28 (2017)
Islam, M.R., Iftekher, A.N.M., Hasan, K.R., et al.: Design and numerical analysis of a gold-coated photonic crystal fiber based refractive index sensor. Opt Quant Electron 53, 112 (2021). https://doi.org/10.1007/s11082-021-02748-8
Katz, R., Edelson, M.: The cancer-fighting kitchen: nourishing, big-flavor recipes for cancer. Ten Speed Press; Crown Publishing Group, New York (2009)
Kim, A., Wilson, B.C.: Measurement of ex vivo and in vivo tissue optical properties: methods and theories optical-thermal response of laser-irradiated tissue, chapter 8, 2nd edn. Springer, Berlin (2011)
Lue, N., Popescu, G., Ikeda, T., Dasari, R.R., Badizadegan, K., Feld, M.S.: Live cell refractometry using microfluidic devices. Opt. Lett. 31(18), 2759–2761 (2006)
Mishra, G.P., Kumar, D., Chaudhary, V.S., Murmu, G.: Cancer cell detection by a heart-shaped dual-core photonic crystal fiber sensor. Appl. Opt. 59, 10321–10329 (2020)
Mollah, M.A., Yousufali, M., Ankan, I.M., Rahman, M.M., Sarker, H., Chakrabarti, K.: Twin core photonic crystal fiber refractive index sensor for early detection of blood cancer. Sens. Bio-Sens. Res. 29, 100344 (2020)
Nejad, H.E., Mir, A., Farmani, A.: Supersensitive and tunable nano-biosensor for cancer detection. IEEE Sens. J. 19(13), 4874–4881 (2019)
Panda, A., Devi, P.P.: Photonic crystal biosensor for refractive index based cancerous cell detection. Opt. Fiber Technol. 54, 102123 (2020). https://doi.org/10.1016/j.yofte.2019.102123
Patel, S., Parmar, J., Kosta, Y., Ladumor, M., Zakaria, R., Nguyen, T., et al.: "Design of graphene metasurface based sensitive infrared biosensor. Sens Actuators A Phys. 301, 111767 (2020)
Ramanujam, N.R., Amiri, I., Taya, S.A., Olyaee, S., Udaiyakumar, R., Pandian, A.P., Wilson, K.S.J., Mahalakshmi, P., Yupapin, P.P.: Enhanced sensitivity of cancer cell using one dimensional nano composite material coated photonic crystal. Microsyst. Technol. 25(1), 189–196 (2019)
Suresh, S.: Biomechanics and biophysics of cancer cells. Acta Biomater 3(4), 413–438 (2007)
Vijayalakshmi, D., Manimegalai, C.T., Ayyanar, N., et al.: Highly sensitive tri-path photonic crystal fiber plasmonic sensor based on hybrid layer of gold/platinum diselenide. Opt Quant Electron 53, 454 (2021). https://doi.org/10.1007/s11082-021-03092-7
Yasli, A.: Cancer detection with surface plasmon resonance-based photonic crystal fiber biosensor. Plasmonics 16, 1605–1612 (2021)
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work under the Research Groups Funding program grant code (NU/RG/SERC/11/16).
Funding
Deanship of Scientific Research at Najran University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Mathematical derivation and part of the editing were performed by MGD. The code and part of the editing were performed by SAT and IC. The first draft of the manuscript was written by SKP and OMR. Discussion of the results was performed by SAT and OMR. Revising the language of the paper in the revision stage, conducting all changes to the manuscript and performing the final editing of the paper AHMA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
No consent to participate is required for this study.
Consent for publish
No consent for publication is required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almawgani, A.H.M., Daher, M.G., Taya, S.A. et al. Highly sensitive nano-biosensor based on a binary photonic crystal for cancer cell detection. Opt Quant Electron 54, 554 (2022). https://doi.org/10.1007/s11082-022-03978-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-03978-0