Abstract
The fast and accurate detection of the blood components is very necessary before initiating the treatment of patients. In this article, a decagonal Solid Core Photonic Crystal Fiber (SC-PCF) sensor with very high sensitivity and low confinement loss for efficiently detecting the blood cells is proposed. The solid core with air hole ring gives an extra edge for sensing the biomedical analytes due to the easy infiltration of liquid in the holes of the core. The material used in the background of the PCF is TOPAS. A Perfectly Matched Layer (PML) is used as boundary condition in outer layer of cladding. The Full-Vectorial Finite Element Method (FV-FEM) is used for solving the Maxwell equation and numerical analysis of the model in the THz regime. In results, the highest sensitivity in the terahertz spectrum for glucose, plasma, WBC and RBC are 84.55%, 85.09%, 85.62% and 87.68% respectively. Specifically, more than 85.09% sensitivity for all the blood components makes this sensor very useful for rapid blood sensing. This paper also analyzes the low confinement loss (CL), high effective mode area, low birefringence and beat length at operating frequency in the terahertz spectrum (1THz–4THz) for all the blood components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the past few decades, optical biosensors have attracted researcher’s interest because of its higher sensitivity, design flexibility, scalability and long-lasting capability. The PCF based biosensor is a methodical device used to detect various biological stuff like cancer tissues, antibodies, enzymes and acids. Because of the different refractive indices of the different components. The PCF based sensors can also be used for sensing the blood components like glucose, plasma, water, hemoglobin, WBC and RBC, etc.
The health of the human body is largely dependent on the composition of the blood cells. The blood is a combination of plasma and blood corpuscles in the ratio of 11:9 respectively. The plasma contains water and protein in the ratio of 9:1 and blood corpuscles consist of leukocytes and thrombocytes and erythrocytes in the ratio of 1:99 (Chopra et al. 2016). The Erythrocyte also contains RBC, which is rich in hemoglobin. These blood components can be analyzed by using optical sensing technology, in which photons are allowed to interact with the different blood components. The intensity of light changed according to the refractive indices of different blood cells like RBC, WBC, Glucose and Plasma (Jin et al. 2006; Obayya et al. 2016), etc. The correct blood cell composition makes the body healthy and ambiguities in these cell compositions may create some ailment diseases. So, the early detection of these diseases can save many lives. The PCF based sensors are used to detect the disorders of blood cell with high sensitivity, low cost, small size and more reliability.
Photonic Crystal Fiber (PCF) is an optical fiber with periodically arranged microstructures air holes through the entire length of the fiber (Knight 2003). The special property of the PCF such as Endlessly Single Mode (ESM), Single Silica Material (SSM) and flexible air hole array design makes it a smart device in optical sensing technology. The liquid or gas infiltration within the air holes adds the extra sensing capability of the PCF. In solid core PCF, the propagation of light through the core of the fiber is accomplished by the Index Guiding (IG) mechanism (Zhang et al. 2017). These properties of PCF make it useful for sensing the chemical or biochemical substances like Glucose, Cholesterol, Red Blood Cells and White Blood Cells, Hemoglobin, Plasma and water content in the blood. The PCF based sensors can also be used for sensing physical parameters such as temperature, strain, force, etc.
In literature, Knight et al. in 1996 reported the first hexagonal PCF based gas sensor (Knight et al. 1996). Later on, the octagonal (Ademgil 2014), decagonal (Abdur Razzak et al. 2007), hybrid cladding (Ademgil and Haxha 2015) and honey comb structure (Hou et al. 2013) have been reported but with low sensitivity. Thereafter, chemical and bio-chemical sensors have also been reported for detecting the components like alcohol (Etu Podder et al. 2018), salinity (Vigneswaran et al. 2018), pH (Li et al. 2013), DNA (Sun et al. 2014), cholesterol (Soylemez et al. 2015) and glucose (Luo et al. 2014) using PCF. In biomedical applications, PCF based sensors can be very useful for detecting and diagnosing diseases in the initial stage. P. Sharan et al. reported a circular SC-PCF blood sensor to detect the various blood components and obtained the maximum sensitivity of 66.47% for hemoglobin at optical wavelength (Sharma and Sharan 2015). V. Kaur et al. (2019) have reported a SC-PCF with sensing ring in 2019 and obtained the high sensitivity for RBC (66.47%) and WBCs (62.72%) in near infrared region. The PCF based sensor in near infrared region could not achieve the sensitivity more than 70% as per the results reported. To overcome this problem, Terahertz sensors have shown a great potential. At high frequency, the interaction between photons and liquid molecules is enhanced which leads to improving sensing performance.
There are several THz optical sensors have been reported for biomedical applications such as protein analysis (Cheng et al. 2020; Mobasser et al. 2020), DNA analysis (Fischer et al. 2002), and skin cancer detection (Rahman et al. 2013). The review of the sensing performance of the PCF based THz sensor is given as: A quasi-PCF based chemical sensor have reported in 2018 (Paul et al. 2018). In this article, TOPAS has been used as a base material of the quasi-PCF structure. The porous core of PCF was infiltrated with chemical liquid and observed the maximum sensitivity of 78% at 1.3 THz frequency. VS Chaudhary et al. in 2020 (Chaudhary and Kumar 2020) have reported a porous core chemical sensor with TOPAS as base material. The different pattern and different hole size in the cladding and porous core are increasing the complexity of the proposed PCF structure. The low sensitivity (70%) and complex structure were the major drawbacks of this proposal. Recently, the authors have proposed a novel liquid infiltrated PCF based sensor for cocaine sensing (Awadah et al. 2020). The large size of the ellipse in core increases the overall size of the PCF, which leads to the complex structure and increased cost of the sensor. Later, Shuvo Sen et al. reported a novel structure PCF based liquid sensor having hexagonal cladding with rotated hexacore (Shuvo Sen et al. 2020). In this, the authors have been obtained the maximum sensitivity of 82% at 1 THz frequency for the refractive index range of 1.33–1.36, the low refractive index range limits the application of the proposed sensor. A rectangular porous core PCF based sensor was proposed for a low refractive index range in (Al-Mamun Bulbul et al. 2020). The proposed structure has been analyzed in terms of a maximum power fraction of 65%, which means the sensitivity will never cross the 80% mark. K. Ahmed et al. in (2019) have reported a refractive index based blood components sensor and investigated the sensitivity, confinement loss and other parameters. The relative sensitivity response of 80.93%, 80.56%, 80.13%, 79.91% and 79.39% is achieved for the targeted analytes RBCs, hemoglobin, WBCs, plasma and water respectively at a frequency 1.5 THz but the complex design structure is a major drawback of this sensor.
To the best of our knowledge, the maximum sensitivity with porous core or solid core PCF is around 82% for chemical and 81% for biochemical analytes till date. Although, there are many of articles available for sensing the blood components, still there is a need to upgrade the performance of PCF based biosensor with fabrication friendly structure that can detect the biomolecules like a cancer cell, diabetes (glucose), blood cell compositions and other diseases in the early stages.
In this research paper, a highly sensitive solid core decagonal PCF with air hole ring is proposed for sensing the blood components. The blood components such as Plasma, Glucose, RBC and WBC are taken as analytes for filling in to the porous core. TOPAS is used as a base material for the proposed sensor. The proposed paper promises for enhanced results like high sensitivity, low CL, etc. The proposed sensor has the simple geometrical structure as compare to discussed sensor which is easier to fabricate with the existing fabrication techniques. This paper is further organized as follows: Sect. 2 elaborates the structural design in detail, Sect. 3provides the numerical analysis in terahertz spectrum, Sect. 4 provides the simulation results and Sect. s5 concludes of the paper.
2 Structural design
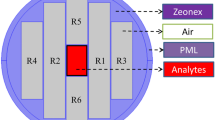
The cross-sectional view of the proposed decagonal PCF sensor is shown in Fig. 1a. The four decagonal circular air hole rings are in the cladding region helps to polarize the light in to the core region. The pitch of air hole is selected optimally to confine the optical energy into the core and for reducing the leakage of optical energy. The solid core consists of sensing the air hole rings, which can be infiltrated with the sensing analytes for providing better interaction of the evanescent field with the analytes. This contributes to the increase in the effective area resulting in better sensitivity.
In the described structure, the radius of air hole R = 40 µm, diameter d = 2R = 80 µm in both core and cladding. The same diameter of the air holes in the core as well as in cladding makes the fabrication uncomplicated. The distance between the center of one hole to the center of another hole is called pitch (Ʌ) and Ʌ = 86 µm. The air filling ratio d/Ʌ = 0.93 is maintained in hole layers in cladding as well as in the core of the PCF. The distance between each layer in the cladding region is 248 µm. The core radius is taken as Rc = 178 µm, the large radius of core accentuates the light to confine into the core running through the entire length of the fiber and the width of the sensing ring is considered as 98 µm. A perfectly matched layer (PML) is used as the boundary condition to absorb the radiation in the outer surface of the PCF sensor. The material used in the background of the PCF is TOPAS instead of silica as it has exceptional optical transparency, biocompatibility and high resistance to alkalis and acids. The Refractive index of the infiltrated sample like Glucose, plasma, WBC and RBC are 1.34, 1.35, 1.36 and 1.40 respectively (Jin et al. 2006; Kaur and Singh 2019; Natesan et al. 2019). The proposed THz sensor has a compact size structure as compare to previously reported THz sensors.
Figure 1b shows the practical implementation of the proposed refractive index (RI) sensor; the laser light is launched into the designed PCF with the help of single mode fiber (SMF) and fiber couplers. The blood cells can infiltrate into the sensing holes by the selective filling method (Zhao et al. 2018). When the optical power is transmitted through the fiber, the RI of the blood cells modulates the intensity of light, the intensity modulation can be analyzed by the optical spectrum analyzer (OSA). The output of the OSA can be investigated by the computer to obtain the performance parameter like sensitivity, CL, etc.
Fabrication Possibilities There are many techniques available for the fabrication of PCF, out of these the most suitable techniques are sol–gel, stack and draw, 3D printing, extrusion and capillary stacking to fabricate circular shaped air holes (Bise and Trevor 2005; Ebendorff-Heidepriem et al. 2014; Ghazanfari Li et al. 2017). The proposed decagonal SC-PCF based bio-sensor contains only circular shaped air holes both in the core as well as in the cladding region. So, the proposed decagonal SC-PCF is more feasible to fabricate by employing sol–gel and capillary stacking fabrication technique due to their equal radius circular holes.
This type of fabrication friendly decagonal shaped SC-PCF structure as RI sensor is proposed for the first time for detecting the glucose, plasma, WBC, RBC with high sensitivity. The structure of this sensor is compact in size and easy to fabricate as compare to previously reported sensors. The optical behavior through the mathematical analysis of the proposed sensor is discussed in the next section.
3 Numerical analysis at THZ frequency
For numerical analysis of the proposed PCF sensor, FEM is the most popular method (Prabhakar 2012). FEM is used to solve Maxwell’s equation in wave propagation, less complexity and computational time are the main advantages of this method and it is best suited to analyze the photonic devices. For a given material, the Effective mode index (\({n}_{eff}\)) is the ratio of the velocity of light in a vacuum (c) to the velocity of light in the guided medium (\({v}_{pm}\)) in the direction of propagation. Mathematically, the effective mode index can be evaluated by the following expression (Liu and Salemink 2014)
Here \(\beta_{pm}\) is the propagation constant and K0 is the wavenumber in free space, it can be derived as,
And the cladding holes are filled with air and the core holes are infiltrated with sensing analytes such as RBC, WBC, plasma and glucose with RI of 1.40, 1.36, 1.35 and 1.34 respectively. On the basis of the RI of the sensing analytes, the intensity of light is modulated. The intensity modulation can be offered by the following equation (Biswas et al. 2019),
where \(I_{0}\) denotes the Intensity of light for incident wave and \(I\) denotes the intensity of light for transmitted wave, \(r\) denotes the relative sensitivity, \(\varepsilon\) denotes the molar absorption coefficient, \(L\) denotes the path length, and \(C\) denotes the concentration of absorbing material. The relative sensitive (\(r\)) can be defined as (Biswas et al. 2019),
where \(n_{r}\) and \(n_{eff}\) denotes the RI of the sensing analyte and real value of effective mode index of the propagating modes, \(f\) is the fractional power which can be derived as (Biswas et al. 2019),
Here E and H denote the transverse Electric and Magnetic fields. The sensitivity of the PCF sensor relates to the parameter known as confinement loss; these are the leakage of optical power from the core confinement region. At any wavelength, the confinement loss is due to the imaginary part of the complex effective refractive index \(Im\left( {n_{eff} } \right)\). The confinement loss (Lc) of the fundamental mode is described by the following equation (Aoni et al. 2013),
Here K0 is the wavenumber and it is inversely proportional to the wavelength and \(Im\left( {n_{eff} } \right)\) denotes the imaginary value of the complex effective RI. The confinement loss can be controlled by the parameter variation like hole size, pitch, and design of geometry.
Another important parameter is the Effective mode area and it is generally known as the light carrying region. For the propagation of the fundamental mode, electric-field (E) distribution should be inside the core, and the effective mode area of a PCF can be expressed as (Ahmed et al. 2019),
Here E is the electric field distribution in x and y direction, for the sensing application, the EMA should be high.
Birefringence is an essential optical property of the PCFs. It is generated by some geometric asymmetry depending on the position of air holes and highly influential for polarization-maintaining fiber (PMF). The first ring of the PCF has highly structural asymmetry and produces higher order of birefringence and the structural symmetry of the PCF does not influence the birefringence. Mathematically it can be expressed as (De et al. 2017),
One more significant parameter is the Beat length. It is an extensive argument to determine the PCF birefringent. Beat length derives the light transmission length along with the fiber when the phase variations of two orthogonal polarization states vary 360 degrees or 2π radians and derive the periodic power exchange between two orthogonal components. This period is known as beat length and mathematically it can be derived as (Xin et al. 2016),
Here LB is the beat length, λ denotes the wavelength and B is the birefringence.
4 Results and discussion
For the simulation of the proposed PCF, the COMSOL Multiphysics software has been employed. This software is best for design and analysis the photonics or optical devices with less computational time and complexity. The optical behavior of the proposed model is analyzed by the FEM method (Bise and Trevor 2005). It is used to find the real world working of the model by meshing or breaking down the model into a number of finite elements. With equation no. 1–9, numerical analysis has investigated the effect of each finite element. The workflow of COMSOL software for the proposed PCF based sensor is first design the model structure of the PCF, add the material property of the design structure and define the electromagnetic physics as a waveguide. All the propagation modes are analyzed by the mode analysis.
The holes in the sensing ring can be easily filled with the sensing analytes for the direct interaction with light propagating through the fiber. This enhances the capability of sensing of the proposed sensor. Figure 2 shows the distribution of the electric field in the core of the proposed fiber. The light confinement when analytes are not infiltrated is shown in part (a), the polarization of light is shown in part (b), (c), (d) and (e) for the blood cells RBC, plasma, Glucose and WBC respectively. The light travels through the entire length of the PCF at the terahertz spectrum. The figure clearly differentiates the electric field of the polarized light for each components of the blood. The light confinement for the different blood component and air in the core shows the different behavior of light guided through the core. The main attraction of this paper is to attain high relative sensitivity and that leads to a very low confinement loss. Figure differentiate the electric field of the polarized light for each and every component of the blood which clearly shows the behavior of light in the proposed sensor.
Figure 3 shows the relative sensitivity variation with THz frequency ranging from1 to 4THz for distinct components of the human blood. The sensitivity is obtained with the help of Eqs. 4 and 5. It is directly proportional to the fractional power, higher the fractional power in the core or sensing region leads to the higher sensitivity. The sensitivity of all the blood components is higher in the lower range of the terahertz spectrum for the proposed design. The components like glucose, plasma, WBC and RBC shows that the sensitivity is higher for higher RI at one terahertz frequency, the sensitivity values are 84.55%, 85.09%, 85.62% and 87.68% respectively. The best part of this paper is that at 1THz frequency, the plot indicates the sensitivity of all the blood Components are more than 85%, which is more than the sensor has been reported in the literature review.
Figure 4 shows the variation of confinement loss at different frequency values of the THz range. It is calculated by equation no. 6, very less leaky radiations are observed in this PCF based RI sensor. This work achieves very low values of confinement loss for glucose, plasma, WBC and RBC, mentioning7.92 × 10–09 dB/m, 6.66 × 10–09 dB/m, 3.14 × 10–09 dB/m and 1.86 × 10–09 dB/m respectively. The lower value of the confinement loss increases the sensitivity of the sensor. The very low value of confinement loss enhances the performance of this sensor. The figure also shows that for a low value of RI (1.34) the plot shows the maximum value of CL and for a high value of RI (1.40) the plot shows the minimum value of CL. The sensitivity is decreasing with frequency increases, while the confinement loss increases with frequency.
Figure 5 shows the effective mode index variation as a function of frequency from 1 to 4THz. The effective mode index of the fundamental modes slightly varies in accordance to the refractive index of the different blood components. The effective mode index also depends on the frequency of the fundamental modes, here it is calculated by equation no. 1. The \({n}_{eff}\) is inversely proportional to the frequency and the plot shows the mathematical behavior for the proposed sensor. The \({n}_{eff}\) have shown the average value near the respective RI of the different blood cells.
The effective area vs. frequency plot of the proposed PCF structure is shown in Fig. 6. It is numerically analyzed by equation no. 7 which describes the light carrying capacity of the fiber core. The maximum obtained value through simulation for the effective area is \(1.86\times {10}^{-7} {m}^{2}/1.86\times {10}^{5}{\mu m}^{2}\) and in design structure, the core area is around \(1.04\times {10}^{5}{\mu m}^{2}\), which is around 56% of the simulated effective area, it means the optimum design parameters have been opted to design the proposed sensor structure. The plot depicts that the maximum value of the effective area at 1THz frequency and slightly reduced as frequency varied to 4THz. The plot for sensitivity and effective area depicts that the sensitivity is high when effective area is maximum. The effective area should be large for sensing and data transmission and small for nonlinear applications. It has also shown the linear relation with RI of sensing analytes like RBC has maximum value and glucose has a minimum value of effective area at all the frequencies.
Birefringence is one of the crucial optical property of the PCF to evaluate the performance of the proposed blood sensor. Figure 7 shows the variation of birefringence with frequency and it is calculated by equation no. 8. The proposed PCF structure shows the controlled birefringence over the entire range of frequency. The cell RBC has the lowest birefringence as their RI is maximum and glucose has the highest value as their RI is minimum among all blood components. It describes the polarization changes of the transmitted light in the x and y-direction. Another important parameter is Beat length. The beat length is a birefringence dependent parameter the variation of beat length with terahertz frequency is shown in Fig. 8. The beat length shows the highest peak at 1 THz and then it reduces linearly with frequency reaching 4 THz. RBC shows the high value of beat length and the blood substances like WBC, plasma, glucose have almost same values from this discussion it is cleared that the beat length follows the RI of sensing analytes.
The sensitivity variation with radius of air hole is optimized in Table 1. The performance of the proposed sensor in terms of sensitivity and confinement loss, effective area, birefringence and beat length at 1THz frequency is presented in Table 2. The performance comparison of this paper with the previous work is tabulated in Table 3.
5 Conclusion
In this article, a new design of the PCF sensor for sensing blood components has been proposed and analyzed. The sensing ring holes are used as a blood cell cavity, which is filled by the blood cells. When the optical power is transmitted through the fiber core, the intensity of light is modulated by the refractive index of the blood cells. The sensitivity obtained for the various components of the blood is as follows: RBC-87.68%, WBC-85.62%, Glucose-84.55% and Plasma-85.09%. To the best of our knowledge, to date, no other sensor has been able to obtain such a high value of sensitivity. The small value of birefringence and large effective area adds to the advantages of this sensor. The high sensitivity, a low value of the confinement loss and simple geometry makes this sensor unique for biomedical real time sensing applications.
6 Declaration
The authors confirm that this proposed research did not get any funding grant from the public and private agencies. We confirm that the proposed manuscript has been read and approved by all the listed authors. The authors confirm that we have followed all the regulations of the institution concerning intellectual property associated with this work. The authors also declare that they have no known or personal relationships that could have seemed to affect the proposed reported work.
References
Abdur Razzak, S.M., Namihira, Y., Khan, A.G., Begum, F., Kaijage, S.: Guiding properties of a decagonal photonic crystal fiber. J. Microw. Optoelectron. Electromagn. Appl. 6(1), 44–49 (2007)
Ademgil, H.: Highly sensitive octagonal photonic crystal fiber-based sensor. Optik 125(20), 6274–6278 (2014)
Ademgil, H., Haxha, S.: PCF based sensor with high sensitivity, high birefringence and low confinement losses for liquid analyte sensing applications. Sensors 15(12), 31833–31842 (2015)
Ahmed, K., Ahmed, F., Roy, S., Paul, B.K., Aktar, M.N., Vigneswaran, D.: Refractive Index Based Blood Components Sensing in Terahertz Spectrum. IEEE Sensor Journal 19(9), 3368–3375 (2019)
Al-Mamun Bulbul, A., Imam, F., Awal, M.A., Parvez Mahmud, M.A.: A novel ultra-low loss rectangle-based porous-core PCF for efficient THz waveguidance: design and numerical analysis. Sensors 20, 6500 (2020)
Aoni, R.A., Ahmed, R., Alam, M.M., Razzak, S.A.: Optimum design of a nearly zero ultra-flattened dispersion with lower confinement loss photonic crystal fibers for communication systems. Int. J. Sci. Eng. Res. 4, 1–4 (2013)
Awadah, N., Binti, N., Kabir, S.I., Emeroylariffion, Y., Kaijage, A.S., Begum, F.: Modelling and simulation of novel liquid-infiltrated PCF biosensor in Terahertz frequencies. IET Optoelectron 14, 411–416 (2020)
Bise, R.T., Trevor, D.J.: Sol–gel derived microstructured fiber: fabrication and characterization, In Tech. Dig. Opt. Fiber Commun. Conf. (OFC/NFOEC), p. 3 (2005)
Biswas, B., Ahmed, K., Paul, B.K., Khalek, M.A., Uddin, M.S.: Numerical evaluation of the performance of different materials in nonlinear optical applications. Results Phys., 2211–3797, Published by Elsevier (2019)
Chaudhary, V.S., Kumar, D.: TOPAS based porous core photonic crystal fiber for terahertz chemical sensor. Optik 223, 165562 (2020)
Cheng, R., Xu, L., Yu, X., Zou, L., Shen, Y., Deng, X.: High-sensitivity biosensor for identification of protein based on terahertz Fano resonance metasurfaces. Opt. Commun. 473, 125850 (2020)
Chopra, H., Kaler, R.S., Painam, B.: Photonic crystal waveguide-based biosensor for detection of diseases. J. Nanophotonics 10(3), 036011 (2016)
De, M., Gangwar, R.K., Singh, V.K.: Designing of highly birefringence, dispersion shifted decagonal photonic crystal fiber with low confinement loss. Photonics Nanostruct. Fundam. Appl. 26, 15–23 (2017)
Ebendorff-Heidepriem, H., Schuppich, J., Dowler, A., Lima-Marques, L., Monro, T.M.: 3D-printed extrusion dies: a versatile approach to optical material processing. Opt. Mater. Exp. 4(8), 1494–1504 (2014)
Fischer, B.M., Walther, M., Jepsen, P.U.: Far-infrared vibrational modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 47(21), 3807–3814 (2002)
Ghazanfari, A., Li, W., Leu, M.C., Hilmas, G.E.: A novel freeform extrusion fabrication process for producing solid ceramic components with uniform layered radiation drying. Addit. Manuf. 15, 102–112 (2017)
Hou, Y., Fan, F., Jiang, Z.-W., Wang, X.-H., Chang, S.-J.: Highly birefringent polymer terahertz fiber with honeycomb cladding. Optik 124(17), 3095–3098 (2013)
Jin, Y.L., Chen, J.Y., Xu, L., Wang, P.N.: Refractive index measurement for biomaterial samples by total internal reflection. Phys. Med. Biol. 51, N371–N379 (2006)
Kaur, V., Singh, S.: Design approach of solid-core photonic crystal fiber sensor with sensing ring for blood component detection. J. Nanophoton. 13(2), 026011 (2019)
Knight, J.C.: Photonic crystal fiber. Nature 424, 847–851 (2003)
Knight, J.C., Birks, T.A., Russell, P.S.J., Atkin, D.M.: All-silica single-mode optical fiber with photonic crystal cladding. Opt. Lett. 21(19), 1547–1549 (1996)
Li, W., Cheng, H., Xia, M., Yang, K.: An experimental study of pH optical sensor using a section of no-core fiber. Sens. Actuat. A 199, 260–264 (2013)
Liu, Y., Salemink, H.: All-optical on-chip sensor for high refractive index sensing in photonic crystals. EPL (Europhys. Lett.) 107, 34008 (2014)
Luo, B., Yan, Z., Sun, Z., Li, J., Zhang, L.: Novel glucose sensor based on enzyme-immobilized 81 tilted fiber grating. Opt. Express 22, 30571–30578 (2014)
Mobasser, S., Khanjari, S.P., Bazgir, M., Zarrabi, F.B.: Highly sensitive reconfigurable plasmonic metasurface with dual-band response for optical sensing and switching in the mid-infrared spectrum. J. Electron. Mater. 50, 120–128 (2020)
Natesan, A., Govindasamy, K.P., Raja, G.T., Dhasarathan, V., Aly, A.H.: Tricore photonic crystal fibre based refractive index sensor for glucose detection. IET Optoelectron. 13(3), 118–123 (2019)
Obayya, S., Hameed, M.F.O., Areed, N.F.F.: Computational Liquid Crystal Photonics Fundamentals Modelling and Applications. Wiley, Chichester (2016)
Paul, B.K., Ahmed, K., Vigneswaran, D., Ahmed, F., Roy, S., Abbott, D. (2018) Quasi photonic crystal fiber based spectroscopic chemical sensor in the terahertz spectrum: design and analysis, IEEE Sens. J., 1558–1748 (2018)
Podder, E., Jibon, R.H., Kabir, M.A.: Alcohol sensing through photonic crystal fiber at different temperature. Optic Photonic J. 08(10), 309–216 (2018)
Prabhakar, G.: Finite Element Analysis of Solid-Core Photonic Crystal Fiber, IEEE, (2012)
Rahman, A., Rahman, A.K., Rao, B.: Early detection of skin cancer via terahertz spectral profiling and 3D imaging. Biosens. Bioelectron. 82(15), 64–70 (2013)
Sen, S., Abdullah-Al-Shafi, M., Kabir, M.A.: Hexagonal photonic crystal Fiber (H-PCF) based optical sensor with high relative sensitivity and low confinement loss for terahertz (THz) regime. Sensing and Bio-Sensing Research 30, 100377 (2020)
Sharma, P., Sharan, P.: Design of photonic crystal-based ring resonator for detection of different blood constituents. Opt. Commun. 348, 19–23 (2015)
Soylemez, S., Udum, Y.A., Kesik, M., Hızlıateş, C.G., Ergun, Y., Toppare, L.: Electrochemical and optical properties of a conducting polymer and its use in a novel biosensor for the detection of cholesterol. Sens. Actuators B Chem. 212, 425–433 (2015)
Sun, D., Guo, T., Ran, Y., Huang, Y., Guan, B.-O.: In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 61, 541–546 (2014)
Vigneswaran, D., Ayyanar, N., Sharma, M., Sumathi, M., Rajan, M., Porsezian, K.: Salinity sensor using photonic crystal fiber. Sens. Act. A Phys. 269, 22–28 (2018)
Xin, L., Ying, L., Guo, X.: Modular interference characteristics and beat length of a two-hole photonic crystal fiber. International Symposiumon Optoelectronic Technology and Application, pp. 101550T-101550T-7, (2016)
Zhang, T., Zheng, Y., Wang, C., Mu, Z., Liu, Y., Lin, J.: A review of photonic crystal fiber sensor applications for different physical quantities. Appl. Spectrosc. Rev. Taylor Francis 53(6), 486–502 (2017)
Zhao, C.-L., Wang, D.N., Xiao, L.: Filling technology of PCFs and their applications. In: Peng, G.-D. (ed.) Handbook of Optical Fibers, pp. 1–62. Springer, Singapore (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Verma, P. & Jindal, P. Decagonal solid core PCF based refractive index sensor for blood cells detection in terahertz regime. Opt Quant Electron 53, 165 (2021). https://doi.org/10.1007/s11082-021-02818-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-021-02818-x