Abstract
Influence of the solvent environments, pumping pulse energies and concentrations on the spectral properties of 1,2,3,8-tetrahydro-1,2,3,3,5-pentamethyl-7H-pyrrolo[3,2-g]quinolin-7-one (LD-423) have been investigated. The photophysical characteristics such as absorption, fluorescence spectra, Stokes’ shift, fluorescence quantum yield, absorption, emission cross sections, extinction coefficient and amplified spontaneous emission (ASE) were measured. Here, LD-423 showed two ASE in a certain organic solution under pulsed laser excitation (Nd: YAG λex = 355 nm). One of these peaks corresponds to the fluorescence, while the other peak is an anomalous peak which does not have a corresponding fluorescence peak. This abnormal ASE peak can be ascribed to the fact that the excited molecules are connected in the excited state and the solvent acts as a link between them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Laser dyes such as coumarin and rhodamines are well known because they have an excellent quantum yield of fluorescence. LD derivatives are extensively used to generate lasers in the blue and green regions of the spectrum. The photophysical properties of these molecular species such as absorption, fluorescence, laser, quantum yield, fluorescence decay time, spectral shift have been studied for a long time (del Valle et al. 1997; Senthilkumar et al. 2004; Al-Shamiri et al. 2011; Deepa et al. 2013). Under pulsed laser excitation, the molecules absorb the photon and generate laser or amplified spontaneous emission (ASE) with new properties (Ibnaouf et al. 2012; Zhang et al. 2006; Sastikumar and Masilamani 1997; Somasundaram and Ramalingam 2000). ASE is a type of semi-coherent lasers, where the mirrors are not used between the cuvette for the feedback process. Consequently, the ASE totally depends on the dye medium. However, any medium produces an ASE, has an ability to produce laser, the reverse is incorrect (Ibnaouf et al. 2005, 2012; Ibnaouf 2015). The dimer formation occurs when two similar molecules of the substance combine in the ground state, while the exciplex occurs when one molecule of the material combines with the solvent molecule in the excited state. Dimer formation takes place when two molecules combined together in the ground state. In another side, excimer formation occurs when an excited molecule combines with another molecule in the ground state. In addition, when an excited dye molecule interacts with an unexcited solvent molecule, then and exciplex is obtained. Finally, when two excited molecules combine together and the solvent molecule between them, this is called superexciplex (Ibnaouf et al. 2012; Sastikumar and Masilamani 1996; Masilamani and Aldwayyan 2002).
In the present study, the optical and amplified spontaneous emission (ASE) spectra of 1,2,3,8-tetrahydro-1,2,3,3,5-pentamethyl-7H-pyrrolo[3,2-g]quinolin-7-one (LD-423) in different organic solvents, concentrations and pump pulse energies were investigated. In non-polar solvents and under pulsed excitation, LD-423 showed two ASE peaks at 413 and 427 nm. These ASE peaks could be endorsed from the monomer and superexciplex states, respectively. The peak around 413 nm has coincided with the maximum of the fluorescence spectrum. In contrast, the peak around 427 nm has no fluorescence band at this concentration.
2 Experimental
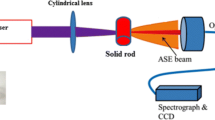
A laser dye of LD-423 was purchased from Exciton Co. (USA) and used as received. The molecular weight (MW) was 256.34 and its molecular structure is given in Fig. 1. A set of solutions was prepared by dissolving LD-423 in different solvents with different concentrations. The absorption spectra were recorded using a Perkin-Elmer lambda 950 spectrophotometers over the range from 100 to 1100 nm. The fluorescence spectra were recorded using a Perkin Elmer LS 55spectrofluorometer with a scan range of 200–1000 mm at room temperature. The excitation wavelength was 355 nm. For the ASE experiments, LD-423 was dissolved in various organic solvents, the solutions were placed in a quartz cuvette its dimensions was (10 mm × 10 mm × 40 mm). The Nd: YAG laser of 355 nm was used as an excitation source with pulse duration and repetition rate of 6 ns and 1–10 Hz, respectively. A quartz cylindrical lens with a focal length of 5 cm was used to focus the pump beam on the cuvette which was tilted to avoid the feedback (Masilamani et al. 2007). The ASE spectrum was collected from the sides of the cuvette by a small slit of the spectrograph, which was connected to a CCD camera (Andor).
3 Results and discussion
The main purpose of this paper is to study the effect of the solvent polarities on the spectral and dynamic transient states of the ASE spectra.
3.1 Absorption and fluorescence spectra
The steady-state of the luminescent LD-423 in different solvents, that having different dielectric constants, were studied. Figure 2 shows the absorption spectra of LD-423 in acetone for different concentrations ranging from 10−4 to 10−2 M. It shows that there were two peaks; one at 350 nm and another at 365 nm with a high absorbance in the UV region. When the concentration was increased, the optical density increased, and the shape of the spectra remained constant irrespective of concentration and optical density. This indicates the absence of aggregation in the ground state for these dye solutions over a wide range of concentration used. Note that the absorbance was not to the scale.
The fluorescence spectra of LD-423 in acetone for different concentrations (as mentioned above) were recorded as shown in Fig. 3. The results revealed that the fluorescence has only one peak at 420 nm. The spectral profile did not change regardless of concentration. This indicates the absence of excimer or exciplex for these dye solutions.
Influences of the solvents on the absorption and fluorescence spectra for LD-423 in different solvents (10−4 mM) were investigated. It could be seen that the shape of the absorption spectra did not change. Nevertheless, a slight shift in the position of the bands due to solvent polarities had observed, whereas the red shift increases with increasing the polarity of the solvents in particular in the fluorescence spectra as displayed in Table 1.
3.2 Stokes shift
The absorption and fluorescence spectra of LD-423 in various organic solvents were recorded. The concentration was kept at 10−4 mM for each solution to avoid the reabsorption process. The obtained results exhibited small variations in the absorption and fluorescence spectra peaks due to the solvent’s polarity, which is in good agreement with previous studies (Deepa et al. 2013). Metaga and Tsuno demonstrated that the relationship between Stokes’ variation and dipole factor of the solvent is a linear (Mataga and Tsuno 1957). Figure 4 shows that LD-423 undergoes significant changes in the electron delocalization and becomes more polar in the excited state than in the ground state. The Stokes’ shift has a linear variation with the dipole factor, given within square brackets in the expression:
where νa and νf are the wavenumbers maxima in cm−1 for the absorption and fluorescence, respectively, µe and µg are the dipole moment of the solute in the excited and ground states, respectively. ‘a’ is the radius of the cavity in which the solute resides and ∆f is the orientation polarizability
where ε is dielectric constant, n is the solvents refractive index. It could be seen that LD-423 is polar in the excited state.
3.3 Quantum yield of fluorescence
The absorption and fluorescence spectra of LD-423 in different organic solvents were obtained. The concentration was kept at 10−4 mM for each solution. The fluorescence spectra were recorded at the excitation wavelength of 355 nm. The quantum yield of these solutions was calculated as shown in Table 1 (Ibnaouf 2013). The other photophysical properties were calculated as shown in Table 1. According to Beer–Lambert law, the extinction coefficient \(\left(\epsilon \right)\) of LD-423 in different solvents was calculated. The extinction coefficient represents the π–π* transition via a singlet–singlet transition. The absorption cross section (σa) and emission cross sections (σe) were calculated according to formula (Moulton 1986). The results have shown that the σa and σe strongly depend on the solvent nature (Table 1)
where I(λ) is the relative fluorescence intensity at a wavelength of λ.
3.4 Amplified spontaneous emission (ASE)
The ASE spectra of LD-423 were recorded for three types of solvents under a concentration of 1.2 mM. Figure 5a displays the ASE spectra of LD-423 in polar solvents like ethanol and acetone. These solutions were transversely excited with a UV laser at (λex = 355 nm) and the pump power energy kept at 3 mJ. The results showed a single ASE peak around 425 nm with a full width at half maximum (FWHM) of 5 nm. This peak corresponds to the fluorescence peak at this concentration. When the concentration and the pump pulse energy were increased, the shape did not change regardless the intensity. For intermediate polar solvents like tetrahydrofuran (THF) and n-butyl acetate (n-BA), as shown in Fig. 5b, the ASE peak was located around 420 nm. This peak also coincides the fluorescence peak at this concentration (FWHM = 6). The spectral shape remained constant with increased the concentration and pumping power.
In non-polar solvents like benzene and toluene, under the same operational conditions, dual ASE peaks were observed in two narrow spectral regions, around 412 and 425 nm as shorter (SW) and longer (LW) wavelengths with FWHM of 6 nm each peak as depicted in Fig. 5c. It is important to note that corresponding to ASE peak around 412 nm has a fluorescence peak around 411 nm whereas there is no fluorescence peak around 425 nm corresponding to the LW of ASE peak. To the best of our knowledge, the LW of ASE could be attributed to the existence of two distinct molecular species in the excited state. Hence, the LW ASE may be due to two excited molecules coming together with the solvent acting as a bridge (superexciplex). When the concentration was increased to 3 mM, the LW (425 nm) grows faster than the SW (412 nm) as shown in Fig. 5. In addition, the intensity of the ASE peak at 425 nm also grew non-linearly when the pump pulse energy was increased as shown in Fig. 6 and inset Fig. 6.
Our explanation for this phenomenon is due to the dipole–dipole interaction between solvents and solute species in the excited state. In polar solvents, the interactions (due to dipole–dipole) in the excited state are very strong. Therefore, all of the solvent species surrounded the LD-423 and trapped it as in a cage. Therefore, this interaction leads to shifting the wavelength to the red region whether in a steady state or under intense pulsed laser excitation. In contrast, In non-polar solvents, there is a large number of LD-423 molecules did not interact with the solvent’s molecules, therefore this gives a peak corresponding to the fluorescence peak (SW), while the molecules that interacted with the solvents led to the emergence of long wavelength (LW). The natural logarithm of the intensities of ASE of LD-423 in non-polar solvents (benzene, 1.2 mM) as a function of pump pulse energies (1–12 mJ) was measured. It can be seen that the LW peak (425 nm) grows faster non-linearly than the SW (412 nm). The natural logarithm of ASE intensities of the two peaks shows rates of increase. As shown in Fig. 7, the ratio of \(\frac{{slope\,Ln \left( {LW} \right)}}{{slope\,Ln\left( {SW} \right)}} = 1.56\).
From the fluorescence spectrum (Fig. 3), the intensity ratio of LD-423 in benzene is \(\frac{{{\text{I}}\left( {425} \right)}}{{{\text{I}}\left( {412} \right)}}\) = 0.69. Therefore, the stimulated emission cross section related to the quantum yield of fluorescence \(\left( {\Phi _{f } } \right)\) and life-time (τf) is \(\sigma_{SE} = \frac{{\lambda_{e }^{4} \varPhi_{f } I\left( \lambda \right)}}{{8\pi cn^{2} \tau_{f} }}\), where n is the refractive index and C is the light speed. Thus, the stimulated emission cross ratio for the dual ASE peaks was calculated and found to be \(\frac{{\upsigma_{\text{SE}}^{425} }}{{\upsigma_{\text{SE}}^{412} }} = \frac{{\left( {425} \right)^{4} \times {\text{I}}\left( {425} \right)}}{{\left( {412} \right)^{4} \times {\text{I}}\left( {412} \right)}}\) = 0.78. Since ΔN is the same for both ASE peaks, so the optical gain γ is \(\left( {\frac{{\upgamma^{425} }}{{ \upgamma^{412} }}} \right) = 0.78\). So, \(\frac{{Ln\left( {I_{ASE}^{425} } \right)}}{{Ln\left( {I_{ASE}^{412} } \right)}} = \frac{{\sigma_{SE}^{425} }}{{\sigma_{SE}^{412} }} = 0.78\). Now, suppose the ASE peak at 425 nm is due of two excited molecules, then \(\frac{{\upsigma_{\text{SE}}^{425} }}{{\upsigma_{\text{SE}}^{412} }} \times \frac{2}{1} = 0.78 \times 2 = 1.56\), which is in good agreement with Fig. 7. These results confirmed beyond any doubt that LW of ASE is due to superexciplex.
A 1.2 mM of LD-423 in ethanol was prepared and excited by Nd: YAG laser pulses 3 mJ pulse energy. The optical gain coefficient was measured using measuring the intensity of ASE intensity for two lengths of excitation \(I_{l} \,{\text{cm }}\) and \(I_{l/2} \,{\text{cm}}\) according to the following relation (del Valle et al. 1997):
Figure 8 shows that as the pump pulse energy was increased, the optical gain increased. In addition, the relationship between the optical gain and the concentration was studied under pump pulse excitation of 6 mJ. It can be seen that the optimum gain was found 3.67 cm−1 at a concentration of 4 mM as shown in Fig. 8 inset (a).
4 Conclusion
To sum up, the spectral and ASE properties of LD-423 in different solvents and concentrations were studied. The results showed that dual ASE from LD-423 is due to superexciplex and which depends on the solvent type and concentration. Such a superexciplex is an entirely new molecular species, which is a combination of two excited molecules and the solvent between them.
References
Al-Shamiri, H.A.S., Badr, Y., Kana, M.T.A.: Optical, photo-physical properties and photostability of laser dyes impregnated in sol–gel matrix. In: Electronics, Communications and Photonics Conference (SIECPC), 2011 Saudi International. IEEE (2011)
Deepa, H., et al.: Solvatochromic shift studies in LD-425 and LD-423: estimation of ground and excited state dipole moments. J. Mol. Liq. 181, 82–88 (2013)
del Valle, J.C., Kasha, M., Catalán, J.: Spectroscopy of amplified spontaneous emission laser spikes in phenyloxazoles. Prototype classes. J. Phys. Chem. A 101(18), 3260–3272 (1997)
Ibnaouf, K.: Dual ASE from the monomeric and excimeric states of a conjugated-polymer (PDHF) in solution. Opt. Laser Technol. 54, 309–314 (2013)
Ibnaouf, K.: Effects of the solvent environments on the ASE from coumarin 503. Opt. Int. J. Light Electron Opt. 126(24), 5057–5060 (2015)
Ibnaouf, K., et al.: Dual ASE spectra from” Superexciplex” TICT states of dye molecules. Laser Phys. Lawrence 15(11), 1536–1542 (2005)
Ibnaouf, K., et al.: Amplified spontaneous emission spectra from the superexciplex of coumarin 138. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 97, 1145–1151 (2012)
Masilamani, V., Aldwayyan, A.: Evidence of superexciplex in dye molecules. Jpn. J. Appl. Phys. 41(9R), 5801–5804 (2002)
Masilamani, V., et al.: Laser properties of a conjugate polymer (MEH-PPV) in the liquid-excimeric state. Laser Phys. 17(12), 1367–1373 (2007)
Mataga, N., Tsuno, S.: Hydrogen bonding effect on the fluorescence of some nitrogen heterocycles. I. Bull. Chem. Soc. Jpn. 30(4), 368–374 (1957)
Moulton, P.F.: Spectroscopic and laser characteristics of Ti: Al2O3. JOSA B. 3(1), 125–133 (1986)
Sastikumar, D., Masilamani, V.: Influence of solvents on amplified spontaneous emission characteristics of 7-diethylamino-4-methylcoumarin. In: Proceedings of the Indian Academy of Sciences-Chemical Sciences. Springer (1997)
Sastikumar, D., Masilamani, V.: Dual amplified spontaneous emission from 7-ethylamino-6-methyl-4-trifluoromethyl coumarin (coumarin 307). Indian J. Pure Appl. Phys. 34(10), 827–829 (1996)
Senthilkumar, S., Nath, S., Pal, H.: Photophysical properties of coumarin-30 dye in aprotic and protic solvents of varying polarities. Photochem. Photobiol. 80(1), 104–111 (2004)
Somasundaram, G., Ramalingam, A.: Gain studies of Coumarin 1 dye-doped polymer laser. J. Lumin. 90(1), 1–5 (2000)
Zhang, D., et al.: Low threshold amplified spontaneous emission based on coumarin 151 encapsulated in mesoporous SBA-15. Appl. Phys. Lett. (2006). https://doi.org/10.1063/1.2402908
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibnaouf, K.H., Alhathlool, R. Influence of solvents on the amplified spontaneous emission characteristics of LD-423. Opt Quant Electron 50, 289 (2018). https://doi.org/10.1007/s11082-018-1551-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-018-1551-8