Abstract
Anthocyanin (An) and chlorophyll (Chl) dyes have been extracted from black rice and fragrant screwpine (Pandanus amaryllifolius) leaves respectively using methanol as solvent. The anthocyanin and chlorophyll dyes were characterized using UV–Vis absorption spectroscopy. While the anthocyanin dye has a broader absorption peak at 541 nm, the chlorophyll dye has two prominent absorption peaks at 465 and 663 nm. The anthocyanin and chlorophyll dyes were deposited sequentially on the TiO2 electrode. The photoelectrode prepared by dipping in anthocyanin solution first and then in chlorophyll solution for the same duration showed the best efficiency of 0.81 % with J sc = 2.64 mA cm−2, V oc = 0.46 V and FF = 0.63 for DSSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Natural dyes extracted from natural sources have been used in various fields such as textile, coatings, food coloration, cosmetics, pharmaceuticals, pH indicators, non-linear optical applications and dye-sensitized solar cells (DSSCs) (Shahid et al. 2013; Kouissa et al. 2013; Bouchouit et al. 2010; Zongo et al. 2015a, b; Calogero and Marco 2008). Natural dyes which serve as sensitizers in DSSCs have the advantages of being readily available, environmental friendly and easy to extract compared to ruthenium-based dyes (Kimpa et al. 2012). Anthocyanin is a flavanoid compound present in many plant parts and is responsible for the red, violet and blue colors (Melo 2009; Konczak and Zhang 2004). Chlorophyll, on the other hand, is another attractive compound that absorbs in the visible region and reflects green color (Lim et al. 2015). Finding an efficient but cheap photo-sensitizer for good performance in DSSCs is a challenge.
In this study, anthocyanin and chlorophyll are extracted from black rice and pandan (fragrant screwpine) leaves respectively. Anthocyanin and chlorophyll have also been used as combined sensitizers. The TiO2 coated electrodes were successively dipped in chlorophyll and anthocyanin solutions for dye adsorption. Different combinations of dye soaking times were used to study the effect on the DSSC performance and to select the best combination. Two batches of photo-electrodes were prepared using two sequential dye deposition procedures either starting first with anthocyanin or chlorophyll.
2 Experimental
2.1 Materials
Tetrapropylammonium iodide (TPAI), polyethylene oxide (PEO), Triton X-100 a surfactant and ethylene carbonate (EC) were purchased from Sigma-Aldrich. Dimethyl-formamide (DMF) was purchased from Friedemann Schmidt Chemical. Iodine (I2) crystals were purchased from Amco-chemie-Humburg. Phthaloylchitosan was synthesized by the reaction of chitosan with phthalic anhydride, which was purchased from Merck-Germany. TiO2 particles of sizes 15 nm (P90) and 21 nm (P25) were purchased from Evonik Industries. Carbowax was purchased from Supelco, USA and methanol was purchased from John Kollin Corporation.
2.2 Preparation of TiO2 electrodes
A two layer TiO2 electrode were prepared and the porous TiO2 electrode was soaked in the natural pigment dye. The TiO2 paste for the first compact layer was prepared by adding 0.5 g of TiO2 (P90) powder with 2 mL of 0.1 mol nitric acid and ground for 30 min. The paste was spin-coated on fluorine doped tin oxide (FTO) glass and then sintered at 450 °C for 30 min. The paste for the second porous layer was prepared by grinding 0.5 g TiO2 (P25) powder with 2 mL of 0.1 mol nitric acid and added with 0.1 g carbowax. Then 2 drops of surfactant was added to get a homogeneous paste. The TiO2 paste was then coated on the top of the first compact layer using the doctor blade method and then sintered at 450 °C for 30 min.
2.3 Preparation of sensitizers
The chlorophyll dye was prepared from fragrant screwpine (Pandanus amaryllifolius) or locally known as pandan leaves. The leaves were washed with distilled water. 200 g of pandan leaves were cut into small pieces and then immersed in 200 mL methanol adjusted to pH 1 using hydrochloric acid (HCl) and kept in a fridge for 24 h. Anthocyanin was extracted from black rice. 200 g of black rice was immersed in 200 mL methanol adjusted to pH 1 using HCl in a glass beaker covered with aluminum foil to prevent evaporation and kept for 24 h in the fridge. After filtration, the anthocyanin and chlorophyll dyes were characterized by UV–Vis spectroscopy using the UV-3101 PC Shimadzu spectrophotometer. The photo-electrodes were prepared in two batches A and B. Batch A electrodes were dipped in the anthocyanin (An) solution first and for batch B, first dipping was in chlorophyll (Chl) solution. The TiO2 electrodes were sequentially dipped in anthocyanin and chlorophyll dye solutions for a total duration of 48 h. The dipping times used for dye adsorption for batches A and B are listed in Table 1.
2.4 Cell assembly and characterization
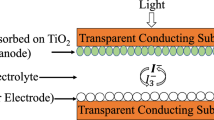
An optimized phthaloylchitosan (PhCh) based gel polymer electrolyte (GPE) having the composition 5.04 wt% PhCh-1.26 wt% PEO-31.51 wt% DMF-37.81 wt% EC-24.38 wt% TPAI(+I2) was used to fabricate DSSCs with the configuration FTO/TiO2-dyes/GPE/Pt/FTO (Noor et al. 2011). The electrolyte was sandwiched between photoanode and platinum (Pt) counter electrode. The photocurrent density–voltage (J–V) characteristics were measured with a Keithley 2400 electrometer at 1000 W m−2 illumination. The active area of the solar cell is 0.2 cm2. The fill factor (FF) and efficiency were calculated using equations obtained elsewhere (Noor et al. 2011).
2.5 Electrochemical impedance spectroscopy (EIS)
EIS measurements were carried out with an AUT 85988 advanced electrochemical system (Metrohm Autolab B.V. PGSTAT 128N Netherlands) from 10 mHz to 100 kHz at room temperature. All the measurements were carried out with applied bias of open circuit voltage (Voc) under AM 1.5 global illuminations.
3 Results and discussion
3.1 UV–Vis spectrum of sensitizer
The UV–Vis spectra of anthocyanin and chlorophyll are shown in Fig. 1.
The anthocyanin spectrum shows a broad absorption peak at 541 nm and small peak at 663 nm. The Chl dye has two prominent absorption peaks at 465 and 663 nm as well as two small peaks at 545 nm and 613 nm. These absorption peak positions are comparable with those reported results (Noor et al. 2011).
3.2 J–V Characteristic of DSSCs
Figure 2 shows the J–V curves of the DSSCs fabricated with all four photoelectrodes. The corresponding performance paramet-ers are summarized in Table 2.
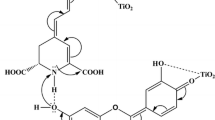
It can be observed that the DSSC using A0 electrode exhibited a higher current density of 1.83 mA cm−2 and a higher efficiency of 0.59 % compared to the DSSC with B0 electrode that exhibited 1.19 mA cm−2 and efficiency 0.39 %. These values are comparable with those obtained from the DSSCs as reported by Hao et al. (2006). The higher current density obtained for the DSSC with A0 photoanode may be due to carbonyl and hydroxyl groups present in the An molecule can bind with the surface of TiO2 porous film thus enabling the An to transfer electrons to the CB of the TiO2 efficiently (Garcia et al. 2003) as shown in Fig. 3. On the other hand, Chl molecules do not have hydroxyl group in the structure. Thus, the ability of the An to adsorb to the TiO2 surface is higher than Chl.
The combination of An and Chl sensitizers exhibits better electricity conversion efficiency as shown in the Table 2. The cell having the photoelectrode sensitized with An first and then Chl dyes (A1) exhibits higher current density, J sc of 2.64 mA cm−2 and efficiency of 0.81 % compared to the cell having the photoelectrode sensitized with only An dye (A0). While the J sc increased by 44.3 %, the efficiency is increased by 37.3 %. Also, the J sc and efficiency of the cell having B1 photoelectrode that was dipped first in Chl and then An showed an increment in J sc and efficiency of 37.8 and 43.6 % respectively compared to the cell having B0 photoelectrode. The en-hancement of the performance may be due to the ability to absorb in a wider wavelength range. Therefore, a better DSSC performance could be expected from DSSCs sensitized with mixed dyes. The An–Chl (Noor et al. 2011) and Chl–xanthophyll (Kartini et al. 2015) mixed dyes have been reported to enhance of DSSC performance.
In the present study, the DSSC with A1 photoelectrodes has produced the highest efficiency of 0.81 %. On comparing A1 and B1 photoelectrodes, where the durations of soaking time in the An and Chl dyes are the same, it is clear that dipping in An dye first and then in Chl produced a higher photocurrent and increase of 44 % in efficiency compare to the DSSC with B1 photoelectrodes. Figure 4 shows the optical absorption spectra of A0, B0, A1 and B1 photoelectrodes. As can be seen in Fig. 4, the A1 and B1 electrodes showed better absorption range than the A0 and B0 photoelectrodes.
It is expected that when the TiO2 layer were soaked first in An and then Chl, electrons released by the dye molecules are injected more efficiently into the semiconductor through the carbonyl and hydroxyl groups of An compared to when dipped first in Chl. Thus, easy transfer of electron is facilitated through A1 photo-anode giving rise to higher currents. Noor et al. (2011) have used the same dye ratio i.e. 1:1 and reported an efficiency of 0.42 % for a DSSC with a PVdF-HFP based GPE. The present work however shows that the sequential deposition of the two dyes is a useful way to improve performance of DSSC using natural dyes. DSSC with natural dyes exhibits lower efficiency compared to DSSC using Ru-based dyes because the natural dyes molecules mostly have OH and O ligands and lack –COOH ligands possessed by ruthenium dye which combines with the hydroxyl group on the surface of TiO2.
3.3 The EIS analysis
EIS measurement is an effective method to analyze the electron transport/transfer process between the interfaces in DSSCs (Fabregat-S et al. 2008). Figure 5 shows the Nyquist plots for all cells studied. It is obvious that the size of the second semicircle at the mid-frequency region which represents the charge-transfer resistances at the TiO2/dye/electrolyte interface (Fabregat-S et al. 2008) for the [B0] DSSC is larger than that of the [A0] DSSC. Also, the second semicircle for [B1] is larger than [A1].
The larger semicircle implies the higher resistance. The higher resistances at the TiO2/dye/electrolyte interface identically lower electron recombination process. The lower electron recombination process in [B0] cell may be due to lesser electron density in CB of TiO2 which results from lower electron injection rate from dyes to CB of TiO2. According to Suryanarayanan et al. (2009), the conversion efficiency η (%) of DSSCs depends on the R2 values (second semicircle in the Nyquist plot). If the R2 value decreased, the Jsc value increased thus cell efficiency increased. The R2 values of the DSSCs in this work increase on according to A1 < A0 < B1 < B0 as shown in the Table 3.
4 Conclusions
A series of composite TiO2 photoanodes with An and Chl sensitizers deposited sequentially were prepared and tested in DSSCs in order to select the best combination. The PhCh gel polymer electrolyte based DSSCs shows best performance with the photoelectrode prepared with equal dipping durations but anthocyanin deposited first. This cell showed the highest efficiency of 0.81 % with J sc = 2.64 mA cm−2, V oc of 0.46 V, and FF of 0.63. This work shows the effectiveness of sequential deposition of dyes on the photoanode as well as cell performance rather than using individual or mixture of dyes. The better performance is attributed to the better anchoring of anthocyanin to the TiO2 surface and better light absorption by chlorophyll.
References
Bouchouit, K., Derkowska, B., Migalska-Zalas, A., Abed, S., Benali-cherif, N., Sahraoui, B.: Nonlinear optical properties of selected natural pigments extracted from spinach: carotenoids. Dyes Pigments 86, 161–165 (2010)
Calogero, G., Marco, G.D.: Red Sicilian orange and purple eggplant fruits as natural sensitizers for dyesensitized solar cells. Sol. Energy Mater. Sol. Cells 92, 1341–1346 (2008)
Fabregat-S, F., Barea, E.M., Bisquert, J., Mor, G.K., Shankar, K., Grimes, C.A.: High carrier density and capacitance in TiO2 nanotube arrays induced by electrochemical doping. J. Am. Chem. Soc. 130, 11312–11316 (2008)
Garcia, C.G., Polo, A.S., Murakam, I.N.Y.: Fruit extracts and ruthenium polypyridinic dyes for sensitization of TiO2 in photoelectrochemical solar cells. J. Photochem. Photobiol. A 160, 87–91 (2003)
Hao, S., Wu, J., Huang, Y., Lin, J.: Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy 80, 209–214 (2006)
Kartini, I., Dwitasari, L., Wahyuningsih, T.D., Chotimah, C., Wang, L.: The sensitization of xanthophylls–chlorophyllin mixtures on titania solar cells. Int. J. Sci. Eng. 8, 109–114 (2015)
Kimpa, M.I., Momoh, M., Isah, K.U., Yahya, H.N., Ndamitso, M.M.: Photoelectric characterization of dye sensitized solar cells using natural dye from pawpaw leaf and flame tree flower as sensitizers. Mater. Sci. Appl. 3, 281–286 (2012)
Konczak, I., Zhang, W.: Anthocyanins—more than nature’s colours. J. Biomed. Biotechnol. 5, 239–240 (2004)
Kouissa, B., Bouchouit, K., Abed, S., Essaidi, Z., Derkowska, B., Sahraoui, B.: Investigation study on the non-linear optical properties of natural dyes: chlorophyll a and b. Opt. Commun. 293, 75–79 (2013)
Lim, A., Manaf, N.H., Tennakoon, K., Chandrakanthi, R.L.N., Lim, L.B.L., Bandara, J.M.R.S., Ekanayake, P.: Higher performance of DSSC with dyes from Cladophora sp. as mixed cosensitizer through synergistic effect. J. Biophys. (2015). doi:10.1155/2015/510467
Melo, M.J.: History of natural dyes in the ancient mediterranean world. In: Bechtold, T., Mussak, R. (eds.) Handbook of Natural Colorants, pp. 3–20. Wiley, Chichester (2009)
Noor, M.M., Buraidah, M.H., Yusuf, S.N.F., Careem, M.A., Majid, S.R., Arof, A.K.: Performance of dye-sensitized solar cells with (PVDF-HFP)-KI-EC-PC electrolyte and different dye materials. Int. J. Photoenergy 2011, 6–11 (2011)
Shahid, M., Islam, S.-.U., Mohammad, F.: Recent advancements in natural dye applications: a review. J. Clean. Prod. 53, 310–331 (2013)
Suryanarayanan, V., Lee, K.M., Chen, J.G., Ho, K.C.: High performance dye-sensitized solar cells containing 1-methyl-3-propyl imidazolinium iodide-effect of additives and solvents. J. Electroanal. Chem. 633, 146–152 (2009)
Zongo, S., Sanusi, K., Britton, J., Mthunzi, P., Nyokong, T., Maaza, M., Sahraoui, B.: Nonlinear optical properties of natural laccaic acid dye studied using Z-scan technique. Opt. Mater. 46, 270–275 (2015a)
Zongo, S., Dhlamini, M.S., Kerasidou, A.P., Beukes, P., Sahraoui, B., Maaza, M.: Linear and nonlinear optical absorption characterization of natural laccaic acid dye. Appl. Phys. B. 120, 389–396 (2015b)
Acknowledgments
We thank University of Malaya and Ministry of Higher Education for financial support (Grants Nos. PG112-2013A and ER001-2013A).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Advanced Materials for photonics and electronics.
Guest Edited by Bouchta Sahraoui, Yahia Boughaleb, Kariem Arof, Anna Zawadzka.
Rights and permissions
About this article
Cite this article
Shah, S., Buraidah, M.H., Teo, L.P. et al. Dye-sensitized solar cells with sequentially deposited anthocyanin and chlorophyll dye as sensitizers. Opt Quant Electron 48, 219 (2016). https://doi.org/10.1007/s11082-016-0492-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0492-3