Abstract
RF magnetron sputtering technique was employed to deposit Li-doped ZnO thin films onto quartz substrate at different substrate temperatures ranging from room temperature (RT) to 500 \(^{\circ }\hbox {C}\). X-ray diffraction analysis revealed that the deposited films had a hexagonal-wurtzite crystal structure with preferred orientation along the c-axis. Increasing the substrate temperature improved the crystallinity and caused a significant increase in the crystallite size (182 nm) for the film deposited at 500 \(^{\circ }\hbox {C}\). The energy band gap of the films deposited at RT, 350, 400, 450 and 500 \(^{\circ }\hbox {C}\) were found to be 3.292, 3.282, 3.281, 3.28 and 3.269 eV, respectively. All films exhibited a broad UV-violet emission band centered on 407 nm and attributed to the radiative recombination processes near the band edge. A Hall mobility of \(\sim \)33.3 \(\hbox {cm}^{2}\)/V s, concentration (\(n\)) of \(\sim \)7.6 \(\times 10^{18}\,\hbox {cm}^{-3}\) and resistivity of \(\sim \)39.7 \(\Omega \)-cm were obtained for the film deposited at 500 \(^{\circ }\hbox {C}\). The results show that the substrate temperature plays a crucial role in the structural, morphological, optical and electrical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently, ZnO has drawn significant attention in the semiconductor research field due to its extraordinary opto-electronic properties. Additionally, ZnO possesses a wide band gap \(\sim \)3.37 eV and large exciton binding energy \(\sim \)60 meV at room temperature. These properties of ZnO make it a suitable material to use in the fabrication of different devices including photo and gas sensors, laser diodes and field effect transistors (Liu et al. 2010; Bender et al. 2003; Chu et al. 2008; Fortunato et al. 2009). However, a production of ZnO-based optoelectronic devices is still difficult due to the lack of p-type ZnO (Ozgur et al. 2005). Nevertheless, recent research has proven that p-type conductivity in ZnO was almost possible by doping with Li, where a hole concentration of \(10^{17}\,\hbox {cm}^{-3}\) was obtained (Shinde et al. 2013). However, there are remain many challenges in this problem which require more extensive research. The difficulties preventing the attainment of p-type conductivity arises from the fact that the p-type dopants may compensate for low energy point defects such as \(\hbox {Zn}_{\mathrm{i}}\) and \(\hbox {V}_{\mathrm{O}}\), the lack of shallow acceptor level and the low solubility of p-type dopants into the ZnO lattice (Park et al. 2002).
P-type conductivity can be enhanced by doping with either group I elements (Li, K, and Na) where the dopants substitutes the Zn sites or some elements in Group V (N, P and As) where the dopants occupy O sites. In terms of shallow acceptor level, Li was found to be a shallow acceptor (90 meV above the valence band) (Ozgur et al. 2005; Shinde et al. 2013; Park et al. 2002; Wardle et al. 2005; Aghamalyan et al. 2003). However, theoretical studies showed that incorporation of H can enhance the solubility of Li dopants and that the subsequent elimination process of H can results in a low-resistivity p-type ZnO (Lee and Chang 2004). Negatively, Li doping can lead to the formation of Li interstitials and donors- acceptor complexes thus increasing resistivity (Aghamalyan et al. 2003). Previous research has shown that with optimal growth conditions and Li content of 0.6 % at a low resistivity of \(5.9\,\Omega \)-cm was achieved (Lu et al. 2006). Additionally, the electrical properties of Li-doped ZnO were found to be dependent on Li content with the carrier concentration decreasing when the Li content exceeds 0.8 % (Lu et al. 2006). Li-doped ZnO thin films can be deposited by a variety of techniques including PLD, sol–gel and sputtering (Ozgur et al. 2005). Recently, Li-doped ZnO thin films deposited with RF sputtering technique showed high crystalline quality and shallow acceptor level irrespective of the Li concentration (Zeng et al. 2006).
In this study, an attempt was made to investigate the structural, optical and electrical properties of Li-doped ZnO thin films deposited using RF magnetron sputtering at different substrate temperatures. The results showed that the deposited thin films were significantly affected by changes in the substrate temperature.
2 Experimental methodology
Before the sputtering process, a surface cleaning process was completed by immersing the quartz substrates successively into acetone, ethanol and deionized water for 10 min and then drying in air. Then, Li-doped ZnO thin films were deposited onto quartz substrate by RF magnetron sputtering using ceramic \(\hbox {Zn}_{1-\mathrm{x}}\hbox {Li}_{\mathrm{x}}\hbox {O}\) as sputtering target (x = 0.2 at.%). The substrate-target distance was 7 cm. Flow mass meters were used to adjust the flow rate ratio of argon to oxygen to 10:1 sccm, respectively. The backup reference pressure was maintained at \(2\times 10^{-4}\) pa while the deposition pressure was 1 pa. The sputtering power (100 W) was maintained constant during the sputtering. Pre-sputtering for 10 min was done to remove contaminants from the sputtering target surface. Five samples were prepared at different substrate temperatures. For simplicity, the samples deposited at RT, 350, 400, 450 and 500 \(^{\circ }\hbox {C}\) are denoted as \(a, b, c, d\) and \(e\), respectively.
X-ray diffraction measurements were done using a MiniFlex-D/MAX-rb with Cu \(\hbox {k}{\upalpha }\) radiation. Surface roughness and morphology were investigated using an atomic force microscope (AFM). Absorption spectra were acquired using a UV–Vis spectrophotometer (Phenix-1700 PC). Photoluminescence (PL) measurements were carried out using He–Cd laser using an excitation wavelength of 325 nm. Resistivity, carrier concentration (\(n)\) and the Hall mobility of the Li-doped ZnO thin films were measured in Van Der Pauw structure using a four-point probe and Hall setup system (Swin Hall8800) at room temperature and constant magnetic field of 5.3 KG.
3 Results
3.1 Structure and morphology
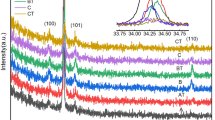
Figure 1 shows the XRD patterns for Li-doped ZnO thin films deposited at different substrate temperatures. It is clear that, all the films exhibited hexagonal-wurtzite crystal structure belonging to the space group p63mc with preferred growth orientation along the c-axis. The crystallinity of the films was evaluated using MDI Jade software. As the substrate temperature increases, the crystallinity along the c-axis improved reaching up to 94 % for the film deposited at 500 \(^{\circ }\hbox {C}\) (Table 1). All films showed a dominant peak around 34.5 \(^{\circ }\) indexed (002) along with another weak peak at 73.3 \(^{\circ }\) indexed (004). Both peaks were assigned to ZnO. We did not observe either Li nor Li–O phases which indicate that the Li dopants were dissolved completely in the host lattice. It can be seen that when the film was deposited at room temperature (sample a), the diffraction intensity from the (002) plane is very weak, indicating the poor crystalline quality of sample a.
As the substrate temperature of the films increased the diffraction intensity from the (002) plane continued to increase reaching a maximum for the film deposited at 500 \(^{\circ }\hbox {C}\). The enhancement in the diffraction intensity from the (002) plane can be attributed to the diffusivity enhancement since at a higher substrate temperature the atoms move to find the most active sites, while at a low substrate temperature, the surface diffusion is limited because the atoms remain where they land. The position of the (002) peak showed a slight shift toward a higher angle relative to the standard ZnO (JCPDS: 36e1451). The shift continued to increase as the substrate temperature increased (inset in Fig. 1). This result has confirmed the substitution of \(\hbox {Zn}^{+2}\) by \(\hbox {Li}^{+}\) suggesting that at high substrate temperature the dopants solubility will be enhanced (OnoderA et al. 1996).
The average crystallite size on the (002) plane was investigated using the famous Scherrer’s equation (Sanon et al. 1989) and found to increase with increasing substrate temperature (65–128 nm) (Fig. 2a). The lattice parameter (c) of the films along the (002) plane have been determined from Eqs. (1, 2).
where \(\hbox {n}, \lambda , \hbox {d}\) and \({\uptheta }\) are the diffraction order, the X-ray wavelength, the plane distance and the diffraction angle, respectively.
Lattice constant c values were substituted in Eq. (3) to determine the lattice strain (\({\upvarepsilon }\) %) (Salman et al. 2012).
Figure 2b shows the lattice strain of the Li-doped ZnO thin films as a function of the substrate temperature. All films showed compressive strain. The compressive strain increases with an increase in the substrate temperature to reach its maximum (\(-\)0.327 %) at a substrate temperature of 500 \(^{\circ }\hbox {C}\). The reason for this is that, the Li-doped ZnO film has a higher thermal expansion coefficient than the quartz substrate. Therefore, the films will be highly bonded to the substrate, which could suppress film expansion during the cooling down process (Zahedi et al. 2013).
Figure 3a–e Shows AFM images of the Li-doped ZnO thin films deposited at different substrate temperatures. The images were acquired using contact mode in the scanning area of \(2\times 2\, {\upmu }\hbox {m}^{2}\). The root mean square of the surface roughness (\(\hbox {R}_{\mathrm{rms}})\) values increases slightly when the substrate temperature increased to 350 and 400 \(^{\circ }\hbox {C}\) (samples a and b) and then decreased significantly for the films deposited at 450 and 500 \(^{\circ }\hbox {C}\). The root mean squares of the surface roughness (\(\hbox {R}_{\mathrm{rms}})\) are shown in Table 1. It can be observed that the surface of the film deposited at RT (sample a) shows dome-like structures with different diameters. This type of structure has appeared either due to an increase in the coarseness of the nucleation or due to low surface diffusion, since the substrate temperature was much lower than the melting temperature of ZnO. For the films deposited at 350 and 400 \(^{\circ }\hbox {C}\) (samples b and c), both films showed distributed columns with voids due to a self-shadowing effect. The columns tended to be thinner and denser with thin voids in the film deposited at 400 \(^{\circ }\hbox {C}\). This may suggest that the Li atoms took proper places in the host lattice owing to the slightly small ionic radii of \(\hbox {Li}^{+}\) (0.06 nm) compared to \(\hbox {Zn}^{+2}\) (0.074 nm), which may in turn serve surface diffusion of Li dopants in the host lattice (Ruankham et al. 2011). When the substrate temperature increased to 450 \(^{\circ }\hbox {C}\), the surface of the film showed clear vertical column growth with thin diameter and small voids. The columns diameter decreased slightly for the film deposited at 500 \(^{\circ }\hbox {C}\), while the voids slightly increased. For samples d and e the columns’ surface did not flatten, which indicates differences in the surface diffusion within the scanned area. The improvements in the morphology of the film deposited at 450 \(^{\circ }\hbox {C}\) could be attributed to a reduction in the surface roughness and voids due to the enhancement of surface diffusion by increasing the substrate temperature.
3.2 Optical properties
Figure 4 shows the absorption spectra of the Li-doped ZnO thin films deposited at different substrate temperatures. Basically, the absorbance coefficient is highly affected by various factors such as the presence of point defects, dislocations, dopants and the size of the grain boundaries (Srikant and Clarke 1997). It can be observed that, the absorbance is high in the short wavelength region with the absorption edge centered on 365 nm and decreasing sharply beyond the edge to become approximately zero in the long-wavelength region. The films deposited at 350, 400 and 450 \(^{\circ }\hbox {C}\) (b, c and d) showed almost equal absorbance in the short-wavelength region. Conversely, for the film deposited at RT the absorbance in the short wavelength region decreased significantly. This could be related to the point defects.
From Fig. 5, the energy band gaps of the films were extracted from the parabolic function (Eq. 4) of the absorption coefficient versus the incident photon energy near the band edge region.
where \(\alpha ,\,\hbox {h}\nu ,\,\hbox {A}^{*}\) and \(E_{g}\) are the absorption coefficient, the incident photon energy, constant and the energy gap, respectively.
The calculated energy gaps are summarized in Table 1. It is evident that when the substrate temperature was increased gradually to 500 \(^{\circ }\hbox {C}\), a slight decrease in the energy gap of the film occurred. It is important to mention that at elevated substrate temperatures the dopant’s diffusion process will be enhanced giving a greater opportunity for \(\hbox {Li}^{+}\) to substitute \(\hbox {Zn}^{+2}\) sites. This can result in a shift in the band gap due to changes in the periodic potential well since the atomic radius values of \(\hbox {Li}^{+}\) (0.06 nm) and \(\hbox {Zn}^{+2}\) (0.074) are different which may also lead to a change in the lattice strain (Zeng et al. 2006). Additionally, increasing the substrate temperature to 400 and 450 \(^{\circ }\hbox {C}\) (samples c and d) the energy band gap remained almost equal to the energy gap of sample b, which was deposited at 350 \(^{\circ }\hbox {C}\). For the films deposited at 500 \(^{\circ }\hbox {C}\) (sample e), there was a clear decrease in the band gap compared to sample a. This observation can be correlated with the change in the lattice strain in Fig. 2b.
Room temperature photoluminescence (PL) of the Li-doped ZnO thin films deposited at different substrate temperatures are shown in Fig. 6. All films showed a broad emission peak increasing sharply in the UV-violet region to reach a maximum intensity at a wavelength of 407 nm and then decreasing gradually and vanishing in the long wavelength region. The broadness of this peak may indicate that the prepared samples contained high concentrations of defect states within the band gap since ZnO is inherently rich with point defects (Tan et al. 2005; Willander et al. 2010). In addition, the broadness of the emission band refers to the fact that an overlap of various deep level emission bands occurred. It is noted that, when the substrate temperature increased from RT to 350 \(^{\circ }\hbox {C}\), the UV-violet luminescence intensity increased slightly and continued to increase for the films deposited at 400, 450 \(^{\circ }\hbox {C}\) to reach a maximum for the film deposited at 500 \(^{\circ }\hbox {C}\). The origin of the violet emission in ZnO is commonly attributed to transitions from \(\hbox {Zn}_{\mathrm{i}}\) states beneath the conduction band to the photo-excited holes in the valence band maxima. The enhancement of the UV-violet emission for the film deposited at 500 \(^{\circ }\hbox {C}\) is attributed to its high crystalline quality. Furthermore, there are two weak peak shoulders at \(\sim \)440 and 476 nm. The origin of these emissions could be assigned to the transition from zinc interstitials (\(\hbox {Zn}_{\mathrm{i}}\)) to zinc vacancies (\(\hbox {V}_{\mathrm{Zn}}\)) (Zhang et al. 2002).
It is well known that, the doping of ZnO with Li could results in a yellow emission band at 2.2 eV which is probably due to the recombination between donors and deep acceptors above the valence band (Wardle et al. 2005). However, in this study we did not observe a clear yellow luminescence band, which implies the lack of deep acceptor states within the forbidden band gap.
3.3 Electrical properties
Figure 7 shows the variations of the electrical properties resistivity (\(\rho \)) and mobility (\(\mu \)) of Li-doped ZnO thin films as a function of substrate temperature. The resistivity (\(\rho \)) of Li-doped ZnO thin films decreased remarkably to \(65\,\Omega \)-cm at 350 \(^{\circ }\hbox {C}\) substrate temperature. It then continued to decreases gradually with an increase in the substrate temperature to reach its minimum (\(39.7\,\Omega \)-cm) at 500 \(^{\circ }\hbox {C}\). The resistivity \(\rho \) of the films is highly related to dopants and free carrier concentration, point and surface defects, and different scattering centers (impurity scattering and lattice scattering). At low substrate temperature (RT) the Li ions do not occupy substitutional sites, but incorporate interstitially and/or segregate in the grain boundaries, providing more scattering centers and thus leading to a high resistivity (\(150\,\Omega \)-cm) and low mobility (\(6.24\,\hbox {cm}^{2}\)/V s).When the substrate temperature is \(\ge \)350 \(^{\circ }\hbox {C}\) the \(\hbox {Li}^{+}\) can substitute \(\hbox {Zn}^{+2}\), providing more free carriers (\(n)\) and less scattering centers, which in turn reduces resistivity (\(\rho \)) and increases mobility (\({\upmu }\)) (Bertazzi et al. 2009). A maximum carrier concentration (n) of \(5.9\times 10^{19}\,\hbox {cm}^{-3}\) was obtained for the film deposited at room temperature. The \(n\) decreases further with the increase in the substrate temperature to reach a minimum of \(7.1\times 10^{18}\,\hbox {cm}^{-3}\) for the film deposited at 500 \(^{\circ }\hbox {C}\). The high n concentration is related to the donors, O vacancies and Zn interstitials concentration. Thus, when the substrate temperature is \(\ge \)350 \(^{\circ }\hbox {C}\) the Li ions substitute Zn ions and thus reduce n. The carrier concentrations (n) of the film are shown in Table 1. As mentioned above, as the substrate temperature of the films increases, the Hall mobility increases. This is commonly attributed to a reduction in the scattering centers.
In another study, the mobility showed linear dependency on the crystallite size (Fortunato et al. 2008). In this research, mobility as a function of the crystallite size increased sharply and then increased linearly with the crystallite size (Fig. 8). This result is consistent with the XRD results, where the crystallinity of the films was found to improve with an increase in the substrate temperature.
4 Conclusion
This research investigated the effect of substrate temperature (RT to 500 \(^{\circ }\hbox {C}\)) on the structural, morphological, optical and electrical properties of the Li-doped ZnO thin films deposited by a radio frequency magnetron sputtering technique. XRD studies revealed that all films exhibited a hexagonal-wurtzite crystal structure with preferred crystal growth orientation along the c-axis perpendicular to the substrate. We also found that the crystallite sizes varied from 65 to 128 nm. The measured energy band gaps of the films deposited at RT, 350, 400, 450 and 500 \(^{\circ }\hbox {C}\) were found to be 3.292, 3.282, 3.281, 3.28 and 3.269 eV, respectively. The shift in the energy band gap was attributed to the deformation in the potential well due to induced strains by the substrate temperature and/or by the incorporation of Li dopants into the host lattice. The electrical properties showed remarkable dependency on the substrate temperature, where the highest hall mobility value of \(\sim \)33.3 \(\hbox {cm}^{2}\)/V s was observed for the film deposited at 500 \(^{\circ }\hbox {C}\). This result has been attributed to a reduction in the scattering centers due to increases in the substrate temperature.
References
Aghamalyan, N., Goulanian, E.K., Hovsepyan, R., Vardanyan, E., Zerrouk, A.: Effect of lithium impurity on the opto-electrical properties of zinc oxide films. phys. Status Solidi A 199, 425–430 (2003)
Bender, M., Fortunato, E., Nunes, P., Ferreira, I., Marques, A., Martins, R., Katsarakis, N., Cimalla, V., Kiriakidis, G.: Generation of optical bistability in a fiber Fabry–Perot resonator using mode-locked picosecond pulses. Jpn. J. Appl. Phys. 42, 435–437 (2003)
Bertazzi, F., Bellotti, E., Furno, E., Goano, M.: Experimental electron mobility in ZnO: a reassessment through Monte Carlo simulation. J. Mater. Sci Mater. Electron. 38, 1677–1683 (2009)
Chu, S., Olmedo, M., Yang, Z., Kong, J., Liu, J.L.: Electrically pumped ultraviolet ZnO diode lasers on Si. Appl. Phys. Lett. 93, 181106 (2008)
Fortunato, E., Raniero, L., Silva, L., Gonçalves, A., Pimentel, A., Barquinha, P., Aguas, H., Pereira, L., Gonçalves, G., Ferreira, I.: Highly stable transparent and conducting gallium-doped zinc oxide thin films for photovoltaic applications. Sol. Energy Mater. Sol. Cells 92, 1605–1610 (2008)
Fortunato, E., Goncalves, A., Pimentel, A., Barquinha, P., Goncalves, G., Pereira, L., Ferreira, I., Martins, R.: Zinc oxide, a multifunctional material: from material to device applications. Appl. Phys. A 96, 197–205 (2009)
Lee, E.-C., Chang, K.: Possible p-type doping with group-I elements in ZnO. Phys. Rev. B 70, 115210–115214 (2004)
Liu, K., Sakurai, M., Aono, M.: ZnO-based ultraviolet photodetectors. Sensors 10, 8604–8634 (2010)
Lu, J.G., Zhang, Y.Z., Ye, Z.Z., Zeng, Y.J., He, H.P., Zhu, L.P., Huang, J.Y., Wang, L., Yuan, J., Zhao, B.H., Li, X.H.: Control of p- and n-type conductivities in Li-doped ZnO thin films. Appl. Phys. Lett. 89, 112113 (2006)
Onodera, A., Tamaki, N., Kawamura, Y., Yamashita, H.: Dielectric activity and ferroelectricity in piezoelectric semiconductor Li-doped ZnO. Jpn. J. Appl. Phys. 35, 5160–5162 (1996)
Özgür, Ü., Alivov, Y.I., Liu, C., Teke, A., Reshchikov, M.A., Doǧan, S., Avrutin, V., Cho, S.J., Morkoç, H.: A comprehensive review of ZnO materials and devices. Appl. Phys. 98, 041301 (2005)
Park, C., Zhang, S., Wei, S.: Origin of p-type doping difficulty in ZnO: the impurity perspective. Phys. Rev. B 66, 073202–073203 (2002)
Ruankham, P., Sagawa, T., Sakaguchi, H., Yoshikawa, S.: Vertically-aligned ZnO nanorods doped with lithium for polymer solar cells: defect related photovoltaic properties. J. Mater. Chem. 21, 9710–9715 (2011)
Salman, K.A., Omar, K., Hassan, Z.: Nanocrystalline ZnO film grown on porous silicon layer by radio frequency sputtering system. Mater. Lett. 68, 51–53 (2012)
Sanon, G., Rup, R., Mansingh, A.: Growth and characterization of tin oxide films prepared by chemical vapour deposition. Thin Solid Films 190, 287–301 (1989)
Shinde, S.S., Bhosale, C.H., Rajpure, K.Y., Photoch, J.: Photoelectrochemical properties of highly mobilized Li-doped ZnO thin films. J. Photochem. Photobiol. B 120, 1–9 (2013)
Srikant, V., Clarke, D.R.: Optical absorption edge of ZnO thin films: the effect of substrate. J. Appl. Phys. 81, 6357–6364 (1997)
Tan, S.T., Chen, B.J., Sun, X.W., Fan, W.J.: Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. 98, 013505 (2005)
Wardle, M.G., Goss, J.P., Briddon, P.R.: Theory of Li in ZnO: a limitation for Li-based p-type doping. Phys. Rev. B 71, 155205 (2005)
Willander, M., Nur, O., Sadaf, J.R., Qadir, M.I., Zaman, S., Zainelabdin, A., Bano, N., Hussain, I.: Luminescence from zinc oxide nanostructures and polymers and their hybrid devices. Materials 3, 2643–2667 (2010)
Zahedi, F., Dariani, R.S., Rozati, S.M.: Effect of substrate temperature on the properties of ZnO thin films prepared by spray pyrolysis. Mater. Sci. Semicond. Process. 16, 245–249 (2013)
Zeng, Y.J., Ye, Z.Z., Lu, J.G., Xu, W.Z., Zhu, L.P., Zhao, B.H., Limpijumnong, S.: Identification of acceptor states in Li-doped p-type ZnO thin films. Appl. Phys. Lett. 89, 1–3(2006)
Zhang, D.H., Xue, Z.Y., Wang, Q.P.: The mechanisms of blue emission from ZnO films deposited on glass substrate by r.f. magnetron sputtering. J. Phys. D Appl. Phys. 35, 2837–2840 (2002)
Acknowledgments
This work has been partly supported by Program for New Century Excellent Talents in University (NCET-10-0066), 863 project Grants (2013AA031502) and the project of 2011RFLXG006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babikier, M., Li, Q., Wang, J. et al. Li doped ZnO thin film: effect of substrate temperature on structure, optical and electrical properties. Opt Quant Electron 47, 3655–3665 (2015). https://doi.org/10.1007/s11082-015-0256-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11082-015-0256-5