Abstract
We report the growth of CuO nanowires from copper foils oxidized in air at temperatures between 400 and 800 \(^{\circ }\hbox {C}\). The synthesized nanowires have two different morphologies, straight and bent, with diameters of 50–400 nm and lengths of 1–55 \(\upmu \hbox {m}\). Here, we demonstrate, highly dense, regular arrays of nanowires with high uniformity and few composite particles of CuO and \(\hbox {Cu}_{2}\hbox {O}\) by a simple contact printing process, in assistant with the viscosity and lubrication of the silicone oil. The average length of nanowires is approximately 15 \(\upmu \hbox {m}\) and the longest NW’s length is around 55 \(\upmu \hbox {m}\). The distribution is uniform, basically in a direction and the density is very large. The nanowires are rarely damaged. Compare to the conventional way, the contact printing used in this paper is an easy, efficient method of the nanowires transferred from one substrate to another substrate and significantly reduced the experiment cost of the nanowire transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanostructure materials have many special physical and chemical properties compared to traditional micron scale materials. For instance, nanomaterials with quantum confined effect have unique electrical, optical, magnetic and thermal properties (Manmeet et al. 2006). Potential applications of nanomaterials have attracted extensive concern of scientific research. In recent years, human beings are extremely interested in one dimensional nanostructure materials such as nanowires, nanotubes and nanorods owing to their potential applications in fabrication of nanodevices (Xia et al. 2003; Liu et al. 2014a, b; Miao et al. 2014). Cupric oxide (CuO) is a p-type semiconductor with a narrow band gap of about 1.2 eV (Rakhshani 1986) and is expected to be used as high temperature superconductors (MacDonald 2001), giant magnetoresistance materials (Zheng et al. 2000), promising solar cells materials (Maruyama 1998), lithium ion battery material (Gao et al. 2004), gas sensor (Liao et al. 2009) and electron field emission source (Zhu et al. 2005).
There are many ways to synthesize copper oxide nanowires including liquid-liquid interface technique (Saravanan et al. 2005) and catalytic synthesis (Tsai et al. 2009) etc. Jiang et al. (2002) has proposed a simple method of preparing CuO nanowires by directly heating of copper foils and copper film deposited on silicon substrate in air. Zhang et al. (2007) and Hsueh et al. (2011) have also prepared CuO nanowires on silicon and glass substrate, which was expected to offer CuO nanowires on different substrates for nano-devices. However, the thermal oxidized product is usually a composite of \(\hbox {Cu}_{2}\hbox {O}\) particles and CuO nanowires. Moreover, due to the thermal expansion difference between CuO nanowires and the substrate, the as produced CuO usually easy to crack and peel off from substrates, which largely limit the application of CuO nanowires. One of the solutions is detaching nanowires from substrates and transfer to specified substrates for nanodevices, which has been well applied in silicon nanowires (Fan et al. 2008), while there has been rarely any report about detaching CuO nanowires for nanodevices.
In this paper, CuO nanowires arrays have been synthesized by thermal oxidation with diameters between 50 and 400 nm and lengths between 1 and 55 \(\upmu \hbox {m}\). The nanowires were detached from Cu substrates using a simple contact printing method which is a physical method without destroying the nanowires and at low cost. Silicon oil was used with its viscosity and lubrication in the contact printing method and then removed by Chlorobenzene. Dense and regular arrays of nanowires with high uniformity and purity could be obtained through this simple contact printing method.
2 Experimental details
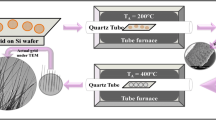
Commercial Cu foils with 0.2 mm (purity: 99.9 % Cu) thickness were used in the present experiments. Cu foils were cleaned with acetone, ethanol and deionized water under ultrasonic bath for 20 min successively. The foils were then dried and put into a muffle furnace. The temperature was raised at a rate of \(20\,\,^{\circ }\hbox {C}/\hbox {min}\) and held for 10 h at 400, 500, 600, 700, 800 \(^{\circ }\hbox {C}\).
For the contact printing process, the \(700\,\,^{\circ }\hbox {C}\) annealed Cu foil was pasted onto glass substrate with double faced adhesive tape and named as donor substrate. A receiver substrate was formed by coating a thin layer of silicon oil to Si, glass or silica surface. The donor substrate was slide on top of the receiver substrate with proper pressure to print the CuO nanowires onto the receiver substrate. After printing, the receiver substrate was cleaned with chlorobenzene. The detailed process is as follows: the receiver substrate was laid on a petri dish with CuO nanowires upward, chlorobenzene was then added into the petri dish dropply without flushing the nanowires until the receiver substrate was totally rinsed. After soaking for 5 min, the chlorobenzene was sucked up carefully to avoid detaching of CuO nanowires. The cleaning process was repeated for three times and then the receiver substrate was taken out from chlorobenzene and dried naturally in glove box.
The phase composition were identified by X-ray diffraction (XRD, Bruker D8A25) using Cu \(\hbox {K}\upalpha \) radiation \((\uplambda =1.5418{\AA })\). The morphology of CuO nanowires was characterized by field emission scanning electron microscope (FESEM JEOL JSM-7001). The crystal structure was investigated by transmission electron microscopy (TEM FEI TecnaiG20) and Raman spectroscopy (Renishaw in Via Plus).
3 Results and discussion
Figure 1 shows the x-ray diffraction (XRD) patterns of the as-grown nanowires. We can see that the product obtained at \(400\,^{\circ }\hbox {C}\) was mainly \(\hbox {Cu}_{2}\hbox {O}\) with trace CuO and Cu from substrate. With the increase of temperature, relative content of CuO to \(\hbox {Cu}_{2}\hbox {O}\) increased, and CuO turned to be the main phase when annealing temperature was \(800\,^{\circ }\hbox {C}\). The results agreed very well with previous literature report (Yu et al. 2004; Jiang et al. 2002). The XRD results suggest that higher temperature favors the formation of CuO crystals.
Morphology of the annealed Cu foil surfaces were examined by SEM and the results are shown in Fig. 2. It was found that grains with diameters of about 500 nm was produced by annealing Cu foil at \(400\,^{\circ }\hbox {C}\) as shown in Fig. 2a, no nanowires was observed. Dense and uniform nanowires were produced with annealing temperature up to \(600\,^{\circ }\hbox {C}\) as shown in Fig. 2b. Diameter of the nanowires is in the range of 50–200 nm and length of 2–20 \(\upmu \hbox {m}\). With length more than \(10\,\upmu \hbox {m}\), the nanowires tend to bent due to gravity. Nanowires formed at \(700\,^{\circ }\hbox {C}\) was shown in Fig. 2c, they are straight with diameters in the range of 50–400 nm and length of 1–15 \(\upmu \hbox {m}\) observed from SEM picture, and density of which is smaller than that produced at \(600\,^{\circ }\hbox {C}\). The nanowires have bigger diameter at root and smaller diameter at tip, like nanoneedles. With temperature raised to \(800\,^{\circ }\hbox {C}\), only a few nanowires could be found, they have uniform diameter of about 50 nm and length about 1–2 \(\upmu \hbox {m}\), in a column structure. The main product is crystal grains on Cu substrate as shown in Fig. 2d. From the SEM morphology, we found that temperature as high as \(800\,^{\circ }\hbox {C}\) is not the best for nanowire growth though the Cu foil was the most thoroughly oxidized as indicated by XRD. 600 and \(700\,^{\circ }\hbox {C}\) are appropriate temperature for CuO nanowire growth. Except for morphology difference, adhesion of Cu nanowires to substrate also changed with the increase of annealing temperature. The sample prepared at \(700\,^{\circ }\hbox {C}\) has the strongest adhesion to the substrate, while the ones prepared at 600 and \(800\,^{\circ }\hbox {C}\) very easily cracked and peeled off. Thus in the following detaching experiment, samples prepared at \(700\,^{\circ }\hbox {C}\) were used.
Many growth mechanisms for thermal oxidation of CuO nanowires have been put forward, such as stress driven mechanism (Kumar et al. 2004) and vapor-solid growth mechanism (Leonardo et al. 2008) etc. At proper oxidation temperature, the diffusion of atoms or ions may take place within the lattice, on grain boundaries, or at dislocations in the oxide layer (Xu et al. 2004). At low temperature, the main driven force of oxidation is short-circuit diffusion whose path can be through the oxide grain boundaries or sub-boundaries (dislocations) in the oxide layer. While at high temperature, metal is oxidized through lattice diffusion of reacting atoms or ions. According to Wagner’s theory (Wagner 1933), the growth of CuO nanowires is the result of lattice diffusion of reacting Cu atoms or Cu ions along \(\hbox {Cu}_{2}\hbox {O}\) grain boundaries and reacted with oxygen. Firstly, \(\hbox {Cu}_{2}\hbox {O}\) forms at the interface of Cu foil and air, Cu atoms diffused to the interface through two paths including grain boundaries and crystal lattice. At oxidation temperature lower than \(400\,^{\circ }\hbox {C}\), grain boundary diffusion is the main path, Cu was oxidized to be \(\hbox {Cu}_{2}\hbox {O}\) at the grain boundaries. The low steam pressure could not promote CuO nanowires growth at low temperature, which could explain that there was no nanowires as shown in Fig. 2a. As temperature increased to \(500\,^{\circ }\hbox {C}\), lots of grains with small diameters and defects were produced, Cu atoms diffused to the surface through dislocation faces and reacted with oxygen. In temperature range of 500–700 \(^{\circ }\hbox {C}\), the reaction activity of Cu and \(\hbox {O}_{2}\) is low enough to be oxidized completely and grew into highly dense CuO nanowires. At temperature above \(800\,^{\circ }\hbox {C},\, \hbox {Cu}_{2}\hbox {O}\) grains grew into bigger crystals and diffusion of Cu atoms along grain boundaries was reduced, oxidation of copper is dominated by lattice diffusion which has very low speed. Therefore growth of nanowires was slow down and the decreased growth speed results in the uniform column structure of nanowires instead of nanoneedles.
Figure 3a shows SEM pictures of nanowires detached by applying the contact printing method once, from which we could see that aligned and clean nanowires were obtained. The average length of nanowires is approximately \(15\,\upmu \hbox {m}\) which is very similar to the as prepared nanowires shown in Fig. 2c. And the longest nanowire was found to be around \(55\,\upmu \hbox {m}\) as shown in Fig. 3b, which means that the contact printing method is damage free. Ultrasonic method was also adopted for comparison, the annealed Cu foil was put into ethanol and ultrasonic for 5 min, the obtained precipitate fell off from Cu substrate is shown in Fig. 3c. We can see that small particles as well as short wires with length of about \(5\,\upmu \hbox {m}\) were obtained, the length of the detached nanowires was only one third of the originally produced nanowires. Moreover, no orientation of nanowires was achieved. It suggests that though ultrasonic method could also detach nanowire from Cu foil, the obtained products is neither clean nor aligned, and the nanowires were damaged badly by the high energy ultrasonication. By applying the contact printing method for three times, density of the aligned nanowires could be enhanced as shown in Fig. 3d. We can get dense and well aligned nanowires with highly uniformity and purity by this simple contact printing method.
In order to identify the structure and component of the detached nanowires, selected area electron diffraction (SAED) pattern and Raman spectra were adopted. Transmission electron microscopy (TEM) image and SAED patterns of a single nanowire is shown in Fig. 4. The sharp diffraction spots demonstrated that the as prepared nanowire is single crystalline CuO corresponding to monoclinic structure CuO with lattice constants a \(=\) 0.469 nm, b \(=\) 0.343 nm, c \(=\) 0.513 nm, and \(\upbeta = 99.55\,^{\circ }\hbox {C}\).
Figure 5 Displays the Raman spectrum of a single CuO nanowire detached by contact printing method on silicon substrate. Table 1 lists the position and full-width at half maximum (FWHM) of the Raman peaks. It can be seen that there are three Raman peaks located at 301, 335, and 625 \(\hbox {cm}^{-1}\) which is a little bigger than the reported CuO crystals \((288,330,621\,\hbox {cm}^{-1})\) (Xu et al. 1999). CuO belongs to \(\hbox {C}^{6}_\mathrm{2h}\) space group with two molecules per primitive cell, and there are nine zone-center optical phonon modes with symmetries 4Au + 5Bu + Ag + 2Bg for CuO powder (Reichardt et al. 1990) and single crystals (Goldstein et al. 1990), while only three Ag + 2Bg modes could be observed by Raman detection (Chrzanowske and Irwin 1989). The peak position and vibrational intensity are determined by grain size of crystals. Stronger and sharper Raman peaks are observed with bigger crystal size, and peaks shifted to longer wavenumbers with decreasing grain size, that’s why the wavenumber of CuO nanowire is slightly bigger than that of CuO bulk materials and the peaks are slightly broaden.
Combining the characterization results that the nanowires are single crystal CuO identified by SAED and Raman, the as annealed product is a mixture of \(\hbox {Cu}_{2}\hbox {O}\) and CuO examined by XRD results and the morphology of the sample obtained by ultrasonic method is a mixture of particles and nanowires, we could conclude that the nanowires are single crystal CuO, and the particles are \(\hbox {Cu}_{2}\hbox {O}\), which agrees well with the proposed growth mechanism. Since the as prepared product on Cu foil is a mixture of CuO and \(\hbox {Cu}_{2}\hbox {O}\), it is hard to use it directly in nanodevices, and detaching of CuO nanowires is absolutely necessary for nanomanufacturing. The contact printing method could offer convenient way for detaching pure CuO nanowires and further applied in nanodevices fabrication.
4 Conclusion
CuO nanowires with diameters of 50–400 nm and length of 1–55 \(\upmu \hbox {m}\) were prepared at temperatures between 400 and 800 \(^{\circ }\hbox {C}\). The nanowires were successfully detached from the CuO and \(\hbox {Cu}_{2}\hbox {O}\) mixture by a very simple contact printing method. Highly ordered, dense, and regular arrays of CuO nanowires with high uniformity and purity was obtained. This contact printing method has very promising application in the fabrication of nanodevices.
References
Chrzanowske, J., Irwin, J.C.: Raman scattering from cupric oxide. Solid State Commun. 70, 11–14 (1989)
Fan, Z.Y., Ho, Johnny C., Jacobson, Zachery A., Yerushalmi, Roie, Alley, Robert L., Razavi, Haleh, Javey, Alii: Wafer-scale assembly of highly ordered semiconductor nanowire arrays by contact printing. Nano Lett. 8, 20–25 (2008)
Gao, X.P., Bao, J.L., Pan, G.L., Zhu, H.Y., Huang, P.X., Wu, F.: Preparation and electrochemical performance of polycrystalline and single crystalline CuO nanorods as anode materials for Li ion battery. J. Phys. Chem B. 108, 5547–5551 (2004)
Goldstein, H.F., Kim, D., Yu, P.Y., Bourne, L.C., Chaminade, J.P., Nganga, L.: Raman study of CuO single crystals. Phys. Rev. B 41, 7192–7194 (1990)
Hsueh, H.T., Hsueh, T.J., Chang, S.J.: CuO nanowire-based humidity sensors prepared on glass substrate. Sens. Actuators B 156, 906–911 (2011)
Jiang, X.C., Herricks, T., Xia, Y.N.: CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2, 1333–1338 (2002)
Kumar, A., Srivastava, A.K., Tiwari, P.: The effect of growth parameters on the aspect ratio and number density of CuO nanorods. J. Phys. Condens. Matter 16, 8531–8543 (2004)
Leonardo, C., Campos, L.C., Tonezzer, M.: Vapor–solid–solid growth mechanism driven by epitaxial match between solid AuZn alloy catalyst particles and ZnO nanowires at low temperatures. Adv. Mater. 20, 1499–1504 (2008)
Liao, L., Zhang, Z., Yan, B., Zheng, Z., Bao, Q.L., Wu, T., Li, C.M., Shen, Z.X., Zhang, J.X., Gong, H., Li, J.C., Yu, T.: Multifunctional CuO nanowire devices: p-type field effect transistors and CO gas sensors. Nanotechnology 20, 08520 (2009)
Liu, X., Jiang, L., Zou, X., Xiao, X., Guo, S., Jiang, C., Liu, X., Fan, Z., Hu, W.D., Chen, X., Lu, W., Hu, W., Liao, L.: Scalable integration of indium zinc oxide/photosensitive nanowires composite thin film transistors for transparent multicolor photodetectors array on glass. Adv. Mater. 26, 2919–2924 (2014a)
Liu, X.Q., Miao, J.S., Liao, L., Hu, W.D.: High-mobility transparent amorphous metal oxide/nanostructure composite thin film transistors with enhanced-current paths for potential high-speed flexible electronics. J. Mater. Chem. C 2, 1201–1208 (2014b)
MacDonald, A.H.: Superconductivity: copper oxides get charged up. Nature 414, 409–410 (2001)
Manmeet, K., Muthe, K.P., Despande, S.K.: Growth and branching of CuO nanowires by thermal oxidation of copper. J. Cryst. Growth 289, 670–675 (2006)
Maruyama, T.: Preparation of copper oxide thin film by the sol-gel-like dip technique and study of their structural and optical properties. Sol. Energy Mater. Sol. Cells 56, 85–92 (1998)
Miao, J.S., Hu, W.D., Nan, G., Lu, Z.Y., Zou, X.M., Liao, L., Shi, S.X., Chen, P.P., Fan, Z.Y., Ho, Johnny C., Li, T.X., Chen, X.S., Lu, W.: Single InAs nanowire room-temperature near-infrared photodetectors. ACS Nano 8, 3628–3635 (2014)
Rakhshani, A.E.: Preparation, characteristics and photovoltaic properties of cuprous oxide—a review. Solid-State Electron. 29, 7–17 (1986)
Reichardt, W., Gompf, F., Aïn, M., Wanklyn, B.M.: Lattice dynamics of cupric oxide. Zeitschrift für Physik B 81, 19–24 (1990)
Saravanan, P., Alam, S., Mathur, G.N.: A liquid–liquid interface technique to form films of CuO nanowhiskers. Thin Solid Films 491, 168–172 (2005)
Tsai, C.M., Chen, G.D., Tseng, T.C.: CuO nanowire synthesis catalyzed by a CoWP nanofilter. Acta Mater. 57, 1570–1576 (2009)
Wagner, C.: Beitrag zur theorie des anlaufvorgangs. Z. Phys. Chem. B 21, 25–41 (1933)
Xia, Y.N., Yang, P.D., Sun, Y.G.: One-dimensional nanostructures: synthesis characterization and applications. Adv. Mater. 15, 353–389 (2003)
Xu, J.F., Ji, W., Shen, Z.X.: Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 30, 413–415 (1999)
Xu, C.H., Woo, C.H., Shi, S.Q.: Formation of CuO nanowires on Cu foil. Chem. Phys. Lett. 399, 62–66 (2004)
Yu, T., Zhao, X., Shen, Z.X., Wu, Y.H., Sow, C.H.: Investigation of individual CuO nanorods by polarized micro-Raman scattering. J. Cryst. Growth 268, 590–595 (2004)
Zhang, K.L., Rossi, C., Tenailleau, C.: Synthesis of large-area and aligned copper oxide nanowires from copper thin film on silicon substrate. Nanotechnology 18, 275607 (2007)
Zheng, X.G., Xu, C.N., Tomokiyo, Y., Tanaka, E., Yamada, H., Soejima, Y.: Observation of charge stripes in cupric oxide. Phys. Rev. Lett. 85, 5170–5173 (2000)
Zhu, Y.W., Yu, T., Cheong, F.C., Xu, X.J., Lim, C.T., Tan, V.B.C.: Large-scale synthesis and field emission properties of vertically oriented CuO. Nanotechnology 16, 88–92 (2005)
Acknowledgments
Financial support from National Science Foundation of China (NSFC Nos: 11374091, 11344005, 11274100), Ministry of Education (211108), Hubei Provincial Department of Science and Technology (2011BAB032) and Department of Science and Technology of Wuhan city (201110821251) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, Y., Wen, Y. et al. Well-aligned CuO nanowires detached from Cu foil by a simple contact printing method. Opt Quant Electron 47, 2095–2102 (2015). https://doi.org/10.1007/s11082-014-0083-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11082-014-0083-0