Abstract
Researchers and clinicians have long used meaningful intransitive (i.e., not tool-related; MFI) gestures to assess apraxia—a complex and frequent motor-cognitive disorder. Nevertheless, the neurocognitive bases of these gestures remain incompletely understood. Models of apraxia have assumed that meaningful intransitive gestures depend on either long-term memory (i.e., semantic memory and action lexicons) stored in the left hemisphere, or social cognition and the right hemisphere. This meta-analysis of 42 studies reports the performance of 2659 patients with either left or right hemisphere damage in tests of meaningful intransitive gestures, as compared to other gestures (i.e., MFT or meaningful transitive and MLI or meaningless intransitive) and cognitive tests. The key findings are as follows: (1) deficits of meaningful intransitive gestures are more frequent and severe after left than right hemisphere lesions, but they have been reported in both groups; (2) we found a transitivity effect in patients with lesions of the left hemisphere (i.e., meaningful transitive gestures more difficult than meaningful intransitive gestures) but a “reverse” transitivity effect in patients with lesions of the right hemisphere (i.e., meaningful transitive gestures easier than meaningful intransitive gestures); (3) there is a strong association between meaningful intransitive and transitive (but not meaningless) gestures; (4) isolated deficits of meaningful intransitive gestures are more frequent in cases with right than left hemisphere lesions; (5) these deficits may occur in the absence of language and semantic memory impairments; (6) meaningful intransitive gesture performance seems to vary according to the emotional content of gestures (i.e., body-centered gestures and emotional valence-intensity). These findings are partially consistent with the social cognition hypothesis. Methodological recommendations are given for future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to produce and recognize gestures that convey meaning is an essential dimension of communication in everyday social interactions (for a review, see, for example, Yang et al., 2015). Imagine, for example, the gestures you would use to say goodbye, to let a colleague understand that a meeting is boring, to indicate your irritation to another driver, or to communicate in a noisy bar. You might also think of board games where players have to pantomime actions or concepts, or even of the gestures used to train a dog. Brain lesions can hamper this ability. Initially, Finkelnburg (1870) described the case of a stroke patient who was unable to produce gestures that conveyed conventional meaning, a clinical profile he termed “asymbolia.” Although this concept has since been abandoned (see Goldenberg et al., 2003), this observation emphasizes the communicative function of gestures and its potential alteration in the context of apraxia.
Apraxia is an acquired cognitive-motor disorder characterized by an inability to perform goal-directed or skilled movements that is not better explained by elementary motor or sensory deficits (e.g., weakness, deafferentation, tremor, abnormal posture, and ataxia), aphasia, inattention to commands, lack of motivation, or general cognitive decline (Foundas & Duncan, 2019; Rothi et al., 1991, 1997). Limb apraxia (hereafter referred to as “apraxia”) refers to disorders of skilled action most commonly affecting the upper limbs. The assessment of apraxia in neuropsychological evaluations is recommended because this syndrome can reduce functional recovery, prevent patients from returning to work, and increase caregiver burden (Bickerton et al., 2012; Chestnut & Haaland, 2008; Coslett, 2018; Donovan et al., 2008; Foundas, Macauley, et al., 1995a; Foundas & Duncan, 2019; Sunderland & Shinner, 2007). Apraxia is also a good predictor of communication deficits in patients with aphasia (e.g., Hogrefe et al., 2012). However, this syndrome has been less studied than other neuropsychological syndromes (e.g., see Lesourd et al., 2013). For example, a Title-Abstract Pubmed search (on 04-26-22) using the keywords “apraxia AND stroke” returned 494 results, compared to 3663 for aphasia, 3937 for memory, and 5960 for attention. There is therefore a need to better understand the prevalence, severity, and causes of apraxia in stroke patients.
Studies on apraxia have emphasized a left hemispheric dominance for gesture production (Liepmann, 1905, 1920). The estimated prevalence of apraxia varies from 40 to 70% in patients with left hemisphere damage (LHD) and from 8 to 30% in patients with right hemisphere damage (RHD; Foundas, 2013). In fact, the exact prevalence of apraxia depends on the task used for the diagnosis (Baumard et al., 2014; Baumard & Le Gall, 2021; Buchmann et al., 2020). Under some test conditions, apraxia may even be more common in RHD than in LHD patients (Goldenberg, 1996; Zwinkels et al., 2004). Recent studies have focused in particular on the contribution of the right hemisphere in producing meaningful intransitive gestures, a topic for which there is conflicting evidence (Bartolo & Ham, 2016; Foundas et al., 1995a, b; Foundas & Duncan, 2019; Hanna-Pladdy, 2001; Rapcsak et al., 1993) but no comprehensive review. As a result, the neurocognitive basis of these gestures remain unclear. The aim of this study was therefore to review the performance of patients with lesions confined to either the left or right hemisphere on tests of meaningful intransitive gestures.
Clinical and Experimental Tasks
Limb apraxia is a complex disorder that has been assessed using a wide variety of task batteries. However, there is a general consensus that the assessment of apraxia must take into consideration the gestures examined, the way gestures are presented (i.e., the input modality), and the required response modality (i.e., the output modality; Table 1; for reviews, see Dovern et al., 2012; Vanbellingen et al., 2010). Regarding the gestures used in apraxia, the taxonomy may vary across studies. In this paper, we refer to meaningful intransitive (MFI) gestures as communicative gestures that convey meaning, are recognizable by peers, and do not involve the reproduction of a tool-use action. Meaningful transitive (MFT) gestures, also called pantomime of tool use, convey meaning and are tool-related gestures. They consist in asking patients to show how they would use a given familiar tool, without touching or holding the tool in hand. Meaningless intransitive (MLI) gestures do not convey any recognizable meaning, and they are not tool-related (e.g., Achilles et al., 2016; Baumard et al., 2020, 2023; Goldenberg, 1996, 1999; Goldenberg & Hagmann, 1997). Unfortunately, most studies have used mixed sets of stimuli, making it difficult to test the effect of transitivity (comparison between meaningful intransitive and meaningful transitive gestures) and meaning (comparison between meaningful intransitive and meaningless gestures).
Meaningful intransitive gestures include a variety of gestures (Cochet & Vauclair, 2014; Ferri et al., 2014; Gallagher & Frith, 2004; Straube et al., 2011; Yang et al., 2015). They are generally assimilated to symbolic gestures, also called emblems or emblematic gestures. These gestures differ from the gestures that accompany speech (co-speech gestures) in that they are intentional, and they can be fully understood by peers without recourse to speech. Meaningful intransitive or symbolic gestures can be divided into subcategories. Iconic gestures illustrate concrete physical features of the world (e.g., making an arch with the hand to illustrate a “bridge”), whereas metaphoric gestures illustrate abstract concepts (e.g., making an arch with the hand to convey the idea of a “link” between two concepts; Straube et al., 2011). Some meaningful intransitive gestures do not convey concepts but rather express habits within a given culture (e.g., “waving goodbye”). All these gestures depend on conventional knowledge, shared by both the sender and the receiver. In addition, expressive gestures communicate an internal physical state (e.g., “hungry”) or an emotion (e.g., “angry”). Deictic gestures are pointing movements that draw to a referent in the environment, with the intention of showing interest in an object (expressive pointing), providing information to the receiver (informative pointing), or asking the receiver to grasp or act on the target (imperative pointing). Instrumental gestures are commands or requests (e.g., “shut up”). In the present review, we refer to meaningful intransitive gestures as a broad category of gestures that are understandable and performed independently of language—as in most apraxia studies.
Studies on apraxia have long emphasized the importance of controlling for input modality (Cubelli et al., 2000; Rothi et al., 1991, 1997). It is critical in patients with lesions of the left hemisphere, who often have aphasia, and in patients with lesions of the right hemisphere, who often have visuospatial impairments. Gesture production is generally assessed (1) by verbal command (e.g., “show me the sign of the cross” for meaningful intransitive gestures; “show me how you would use a hammer” for meaningful transitive gestures) and (2) by imitation. This can be concurrent imitation (i.e., the examiner continues to present the gesture until the patient imitates it, or holds the model while the patient imitates) or delayed imitation (i.e., the examiner makes a gesture and tells the patient to wait until the gesture is complete before starting to imitate it; Stamenova et al., 2010, 2012) (3) on visual input (e.g., showing the patient a scene that provides contextual cues to the expected corresponding gesture, for meaningful intransitive gestures; Mozaz et al., 2002; showing the picture of a tool, for meaningful transitive gestures).
Cognitive models of apraxia have made a distinction between the conception and the production of gestures, allowing for a distinction between action production and action reception (Rothi et al., 1991, 1997; Roy & Square, 1985). Meaningful intransitive and transitive gestures can be assessed with reception paradigms (e.g., Binder et al., 2017). In the naming condition, the patient is asked to name the action corresponding to the presented gesture. In the recognition condition, the patient has to discriminate correctly performed from incorrectly performed gestures. This condition tests whether the gesture is familiar to the patient with only minimal recourse to both speech production and explicit retrieval of semantic knowledge. In the comprehension condition, patients are asked to match a given gesture to a target picture (presented among distractors), which requires them to retrieve semantic knowledge about the meaning of the gesture.
The Cognitive Bases of Meaningful Intransitive Gestures
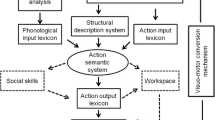
Previous studies have consistently found clinical dissociations between impaired meaningful and preserved meaningless gestures, and conversely (e.g., Bartolo et al., 2001; Goldenberg & Hagmann, 1997; Tessari et al., 2006). On this ground, cognitive models of apraxia have been conceived as dual-route models, thus including a lexical and a sub-lexical route (Cubelli et al., 2000; Rothi et al., 1991, 1997; Roy & Square, 1985; Stamenova et al., 2012). The non-lexical route is specific to meaningless gesture imitation thanks to visuomotor transformation mechanisms (see also Achilles et al., 2016, 2019), visuospatial skills (Dellasala et al., 2006; Goldenberg, 1996, 1999; Goldenberg et al., 2009) and to body representations (Buxbaum, 2001; Goldenberg, 1995, 2009; Goldenberg & Spatt, 2009). The lexical route is used to perform meaningful gestures (whether meaningful intransitive or transitive) on command (verbal or visual) or imitation. This route consists of two action lexicons, one of which allows the individual to recognize familiar gestures (input lexicon) and the other to perform them (output lexicon). Between the two lexicons, an action semantic system allows the retrieval of the conceptual information associated with the gesture.
Importantly, the model accounts for a meaning effect (i.e., the difference in performance between meaningful and meaningless gestures) but it does not predict a transitivity effect (i.e., the difference in performance between transitive and intransitive gestures). Nevertheless, there is some evidence that meaningful transitive gestures are more difficult than other gestures (Bartolo et al., 2003; Baumard et al., 2014; Buxbaum et al., 2007; Carmo & Rumiati, 2009; Dumont et al., 1999; Foundas et al., 1999; Haaland et al., 2000; Morlaas, 1928; Mozaz et al., 2002; Rapcsak et al., 1993; Roy et al., 1991). Indeed, meaningful transitive gestures are performed in the absence of feedback from the mechanical interactions of tools and objects (Hermsdörfer et al., 2006). As a consequence, they call for multiple cognitive processes (Bartolo et al., 2003; Bartolo & Ham, 2016; Buxbaum et al., 2005a, b; Goldenberg et al., 2003; Osiurak, 2014; Osiurak et al., 2021). The greater difficulty of meaningful transitive gestures, together with the lack of conceptual distinction between transitive and intransitive gestures, has long obscured the potentially specific nature of meaningful intransitive gestures. Most apraxia studies have therefore used mixed lists of transitive and intransitive gestures, making it difficult to document isolated impairments of meaningful intransitive gestures.
The Lexical-Semantic and Social Cognition Hypotheses
At least two hypotheses have been advanced to account for meaningful intransitive gesture production. According to the lexical-semantic hypothesis, the production and recognition of meaningful gestures (whether meaningful intransitive or transitive) rely on semantic memory or action lexicons that contain visuokinesthetic engrams of previously experienced gestures (Binkofski & Buxbaum, 2013; Buxbaum, 2001, 2017; Cubelli et al., 2000; Rothi et al., 1991, 1997; Roy & Square, 1985; Stamenova et al., 2012). Action lexicons and semantic memory are not specific to meaningful intransitive gestures; therefore, the disruption of these modules cannot predict isolated impairments in meaningful intransitive gesture tasks. A less strict version of this hypothesis suggests that meaningful transitive gestures, but not meaningful intransitive gestures, rely on action lexicons (Buxbaum et al., 2007; Buxbaum et al., 2005a, b). Thus, the lexical-semantic hypothesis predicts only two possible patterns of performance: either similar levels of performance between meaningful transitive and intransitive gestures or preserved meaningful intransitive gestures versus impaired meaningful transitive gestures. The opposite pattern is not envisaged. This hypothesis also predicts a lower performance of patients with left hemisphere lesions, compared to patients with right hemisphere lesions, as visuokinesthetic engrams are strongly dependent on the left parietal lobe (Binkofski & Buxbaum, 2013; Buxbaum, 2001, 2017; Buxbaum et al., 2007; Buxbaum et al., 2005a; Buxbaum, Kyle, et al., 2005b).
The social cognition hypothesis (Bartolo & Ham, 2016; see also Osiurak et al., 2021) posits that meaningful intransitive gesture production and recognition depend on a specific “social cognition” module that is independent of semantic memory and action lexicons. Social cognition refers to the psychological processes that enable individuals to interact with peers and to be part of a social group (Frith, 2008). It encompasses various mechanisms and phenomena, such as person recognition (e.g., face and body perception), personality perception (e.g., friendliness), membership perception (e.g., gender and age), social beliefs (e.g., stereotypes), social mechanisms (e.g., perspective-taking and intentionality detection), mind-reading (e.g., emotion and bodily sensations), social attitudes (e.g., like or dislike), and social interaction (e.g., imitation and communication; Goldman & de Vignemont, 2009; Quesque & Rossetti, 2020). In this view, communicating with gestures implies that the sender and receiver share a “common ground,” namely, “the knowledge, assumptions, and beliefs that people in interaction know (or assume) they share” (Holler & Stevens, 2007, p.5; see also Gerwing & Bavelas, 2005). This presumably relies not only on semantic memory (shared knowledge) but also on the theory of mind for the sender to infer the initial state of mind and knowledge of the receiver, to predict the expected state of mind and behavior to be induced by the gesture, and to select features of the gesture that will be recognizable to the observer. Emotion processing may also be necessary to experiment with and identify and express emotions with gestures (e.g., Blonder et al., 1995; Gallagher & Frith, 2004). Within the dual-route model, a social cognition mechanism has been included. This mechanism processes the social cognitive information necessary for meaningful intransitive gesture production (Bartolo & Ham, 2016). In the presence of social cognitive impairment, meaningful intransitive but not transitive gestures should be affected, as has been found in an individual with autism (Stieglitz Ham et al., 2010). Thus, in contrast to the lexical-semantic hypothesis, the social cognition hypothesis admits the existence of a pattern characterized by impaired meaningful intransitive gestures and preserved meaningful transitive gestures. This would be particularly true for patients with right hemisphere lesions, as the right hemisphere is considered the “social brain” (Happé et al., 1999).
Objectives of the Present Review
This study had three main goals: First, to compare the performance of patients with lesions confined to either the left or right hemisphere in the production and reception of meaningful intransitive gestures in order to test whether or not there is hemispheric asymmetry for these gestures; Second, to compare the performance of both patient groups on tests involving only meaningful intransitive gestures, only meaningful transitive or meaningless gestures, or a mixture of meaningful intransitive and other gestures. Our aim here was to investigate associations and dissociations between gesture types in stroke patients; Third, to study the relationship between meaningful intransitive gesture performance and other cognitive deficits, such as language and semantic memory, when available. We also studied how specific features of meaningful intransitive gestures (i.e., the body-centered nature of gestures and their emotional valence and intensity) influenced the performance of patients.
Methods
Monitoring and Selection of Studies
Three authors (JB, AL, VB) carried out several Title-Abstract “PubMed” researches, without date limitation, during the year 2020. PubMed was chosen because it accesses the MEDLINE database of references on biomedical and health topics. The following keywords were used: “symbolic gestures”, “meaningful gestures”, “intransitive gestures”, “social gestures”, “communicative gestures”, “deictic gestures”, “iconic gestures”, “depictive gestures”, “emblematic gestures”, “emblems”, “apraxia”, “limb apraxia”, “ideomotor apraxia”, “imitation”, “action understanding”, “gesture understanding”, “gesture comprehension”, “gesture observation”, “gesture recognition”, “gesture discrimination”, as well as “stroke”, “lesions”, “hemisphere”, “stroke”, “left brain damage”, “right brain damage”. This search returned 2505 results. Following the PRISMA guidelines, we removed duplicate records and clearly irrelevant studies and identified 399 potentially relevant studies (see Fig. 1). These studies were screened twice in 2021 and 2022 to select studies that met the following criteria:
-
1.
Only English-language clinical studies were included.
-
2.
Group studies, but not reviews or single case studies, were included.
-
3.
Studies using pure lists of meaningful intransitive gestures were included. Studies using mixed lists of meaningful intransitive and transitive gestures or meaningful intransitive and meaningless gestures were also included, but not studies mixing the three types of gestures.
-
4.
Studies were required to provide details on the input and response modalities (see the “Clinical and Experimental Tasks” section).
-
5.
Studies of patients with lesions limited to either the left (LHD group) or the right hemisphere (RHD group) were included. Subcortical lesions were included only if they were clearly circumscribed to one hemisphere. Studies involving only healthy controls, psychiatric patients, or patients with disconnection syndromes after lesions of the corpus callosum were not included.
-
6.
Studies with consecutive patients or studies providing data on apraxic and non-apraxic patients were included.
-
7.
Only studies that included a control sample consisting of either healthy individuals or non-neurological patients were included.
-
8.
Studies were required to provide quantitative data for both patients and controls, including either mean scores or the percentage of patients showing an impairment (or both). Studies reporting error rates were only included if they could be converted to a success rate to allow comparison between studies. Studies that included only Z-scores, qualitative data (e.g., spatial, temporal, and content errors), or kinematic data were not considered.
-
9.
Studies with data overlap (i.e., the same data used in two manuscripts, as explicitly mentioned in the manuscript) were not included to avoid overrepresenting some findings.
The final selection included 42 different studies between 1966 and 2017 (Fig. 2 and Tables 2 and 3). There were 22 “pure meaningful intransitive gesture” studies (left hemisphere lesions, n = 22; right hemisphere lesions, n = 8), of which 11 studies also reported pure meaningful transitive gesture scores (left hemisphere lesions, n = 11; right hemisphere lesions, n =6) and 9 also reported pure meaningless gesture scores (left hemisphere lesions, n = 9; right hemisphere lesions, n = 4). There were also 12 studies reporting mixed scores of “meaningful intransitive + transitive gestures” (left hemisphere lesions, n = 12; right hemisphere lesions, n = 5) and 12 studies reporting mixed scores of “meaningful intransitive + meaningless gestures” (left hemisphere lesions, n = 12; right hemisphere lesions, n = 5). Notably, four studies have used both pure meaningful intransitive tests and mixed “meaningful intransitive + meaningless” tests on the same clinical sample (Cubelli et al., 2000, 2006; Ferro et al., 1980; Mengotti et al., 2013). All these studies reported data from 2659 different patients, including 2115 patients with left hemisphere lesions and 544 patients with right hemisphere lesions (Table 2). The sample sizes for patients with left hemisphere lesions were as follows: pure meaningful intransitive gesture studies: n = 1209; pure meaningful transitive gesture studies: n = 469; pure meaningless studies: n = 391; mixed “meaningful intransitive + transitive” gesture studies: n = 559; mixed “meaningful intransitive + meaningless” gesture studies: n = 525. Sample sizes for patients with right hemisphere lesions were as follows: pure meaningful intransitive gesture studies: n = 267; pure meaningful transitive gesture studies: n = 187; pure meaningless gesture studies: n = 188; mixed “meaningful intransitive + transitive” gesture studies: n = 192; mixed meaningful intransitive + meaningless” gesture studies: n = 85). The 22 meaningful intransitive studies used 15 different tests that included 74 different meaningful intransitive gestures.
Number of studies on meaningful intransitive gestures. Notes. Overall, 42 studies met the selection criteria. Some studies have studied apraxia as a general cognitive domain and hence have used mixed lists of meaningful/meaningless or transitive/intransitive gestures while there has been growing interest in the specific nature of meaningful intransitive (MFI) gestures. Four studies have used both types of lists: Cubelli et al., 2000, 2006; Ferro et al., 1980; Mengotti et al., 2013
Data Analysis
We first extracted the data corresponding to tests of meaningful intransitive gestures. From these studies, we also extracted data from tests of meaningful transitive or meaningless gestures (when available), for comparison in the same patients. Many studies have used mixed lists of “meaningful intransitive + transitive” or “meaningful intransitive + meaningless” gestures. We extracted these data as well, allowing us to show a gradient of tasks ranging from “pure” meaningful intransitive gesture tests, to tests that mix meaningful intransitive and other gestures, to “pure” tests of meaningful transitive or meaningless gestures.
Particular Cases Met During Data Extraction
In some cases, we had to reconstruct or correct the data. Supplementary Table 1 shows particular cases encountered during data extraction. Where scores from apraxic and non-apraxic patients were available, we calculated the mean score of both groups to approach the effect of hemisphere damage on performance. When data were only available in histograms, we derived the raw data by calculating cross-products (i.e., score in pixels * maximum score/maximum score in pixels). This method expresses the raw data with only a small margin of error. Finally, where individual data were available, data from left-handers were removed before computing the mean score of the group for group comparisons (Bonivento et al., 2014; Frenkel-Toledo et al., 2016; Mengotti et al., 2013, 2015). As a result, the sample included almost exclusively right-handed participants (Table 2). Notably, in several studies, the handedness of the patients was not reported. These studies were nevertheless included for three reasons: (1) several of them had large samples (Bickerton et al., 2012; Dressing et al., 2018; Vanbellingen et al., 2010), which is rare and valuable in the field of apraxia; (2) large samples naturally control for laterality, as 90% of the population is right-handed (Papadatou-Pastou et al., 2020); (3) even among strong left-handers, only a minority of individuals shows right-hemisphere dominance for language (e.g., 22 to 27%; Knecht et al., 2000; Szaflarski et al., 2002), and there is no right-left difference for meaningful transitive gestures (Vingerhoets et al., 2012).
Measures Extracted
We first extracted demographic data (i.e., age, years of education, and percentage of females and right-handed patients) and clinical data (i.e., prevalence of aphasia and days post-onset) from each study to characterize the clinical samples. When available, we extracted the frequency of the deficit (i.e., the percentage of patients with a score below the cut-off score). We were also interested in the intensity of the deficit, i.e., the control-patient difference. Given the heterogeneity of assessment methods, we resorted to a qualitative meta-analysis method developed by our research group (Baumard et al., 2014; Lesourd et al., 2013). This method allows studies with different methodologies to be compared. It consists of two steps: (1) converting raw scores into percentages of the maximum score, for each study, condition, and group and (2) calculating a control-patient difference score, i.e., the difference between the clinical group and the matched control group (e.g., controls’ score = 90%, patients’ score = 50%, hence control-patient difference score = 40%). The greater the difference, the more impaired the patients. We controlled for sample sizes in two ways to avoid overrepresentation of small samples. In Table 4 (bottom rows), we calculated the mean of control-patient differences weighted by sample size. In Fig. 3, the colors correspond to the different studies and the size of the circles represents the sample size.
Control-patient difference, meaningful intransitive gestures. Notes. Figure based on the data available in Table 4. Only scores corresponding to production on verbal command or imitation are displayed given the lack of data regarding reception and other task modalities. Each color corresponds to a specific study. The larger the circle, the larger the sample size. LHD, left hemisphere damage; RHD, right hemisphere damage
Coding of the Emotional Valence of Gestures
Parameters other than meaning and transitivity may influence patients’ performance. In the light of the social cognition hypothesis, we investigated how some characteristics of gestures related to emotion processing might influence the performance of patient groups. First, gestures may or may be not body-centered (i.e., performed toward the body). Body-centered gestures may elicit different emotional and physiological responses compared to gestures performed away from the body (Bartolo et al., 2019). Second, gestures may express different emotional categories (e.g., happiness and fear) and may vary in terms of emotional valence (i.e., positive, neutral, and negative) and intensity. Previous studies have investigated the effect of this variable on reaching and grasping gestures (e.g., Esteves et al., 2016) but not on meaningful intransitive gestures. Third, the content of meaningful intransitive gestures may be important, as these gestures may be deictic, or may express an internal bodily state, an emotion, a command or request, or a concrete or an abstract concept (see the “Clinical and Experimental Tasks” section). This may have an impact on the neural networks involved in gesture production (Gallagher & Frith, 2004), and, thus, on the performance of patients with focal brain lesions.
Where possible, we performed a fine-grained analysis of the meaningful intransitive gestures used in the included studies. To decide whether gestures were body-centered or not, we asked three judges to rate video clips of 102 meaningful intransitive gestures (Supplementary Table 2). This allowed us to calculate the mean percentage of body-centered gestures used in each study (Supplementary Table 3). We also asked 28 healthy controls with no neurological or psychiatric history (mean age, 26.9; standard deviation, 8.1; 15 females; 26 right-handed; mean years of education, 15.2, standard deviation, 1.8) to rate the 102 gesture video clips for meaning (i.e., “Does the gesture have a meaning for you?”, yes or no), valence, and intensity (on a 7-point Likert scale; Supplementary Table 4). This allowed us to calculate the mean score for each of the studies included in this review (Supplementary Table 5). The results of these analyses are shown in Table 5.
Results
Methodological Considerations
In this section, we review differences between studies that may have influenced the findings and that may require experimental control in future studies.
Demographic and Clinical Data
Table 2 shows demographic data for each clinical group. The mean age was similar in both groups (patients with left hemisphere lesions, mean age = 61.2, sd = 5.7; patients with right hemisphere lesions, mean age = 63.9, sd = 3.8). The same was true for years of education (patients with left hemisphere lesions, mean = 10.2, sd = 2.9; patients with right hemisphere lesions, mean = 12.8, sd = 2.2) and for the percentage of right-handed participants (100% in both groups, considering the studies that provided this data). None of the studies made reference to race/ethnicity. Not surprisingly, 79.6% of cases with left hemisphere lesions (sd = 17.5) had aphasia. The number of days post-onset at the time of assessment was also comparable in both populations, although with high heterogeneity between studies (patients with left hemisphere lesions, mean = 305.7, sd = 473.2; range = 4–1825; patients with right hemisphere lesions, mean = 374.2, sd = 365.2; range = 75-860). Of note, two studies included patients more than 2 years after onset and still documented deficits in patients’ meaningful intransitive gestures (Haaland et al., 2000; Stamenova et al., 2010), suggesting limited spontaneous recovery.
Differences in Response Modality
As shown in Table 3, gesture production is clearly overrepresented in the literature, and there is only one study on the recognition and comprehension of meaningful intransitive gestures (Binder et al., 2017). Other studies have investigated these task modalities, but with mixed lists of meaningful intransitive and transitive gestures, making it difficult to draw conclusions about meaningful intransitive gestures. This is without mentioning differences in testing procedures, as we found tasks assessing gesture-tool matching (Gainotti & Lemmo, 1976), gesture-context matching (e.g., “military salute” goes with “officer”; Ferro et al., 1980, 1983), gesture-word matching (Bickerton et al., 2012), naming (Papeo & Rumiati, 2013), and judging of correctly or incorrectly performed gestures (Pazzaglia et al., 2008). The variety of assessment methods makes it difficult to draw firm conclusions about the patients’ ability to understand meaningful intransitive gestures.
Differences in Task Modality
We also found differences in task modality. Some older studies have tested gesture production on verbal command and then on imitation only if the patient failed on verbal command (Basso et al., 1981; De Renzi et al., 1966; Ferro et al., 1980). In contrast, more recent studies have tested verbal command and imitation independently from each other, allowing cognitive routes to be tested separately (Heath et al., 2001; Roy et al., 1991; Schnider et al., 1997; Stamenova et al., 2010; Vanbellingen et al., 2010). However, the type of imitation (concurrent or delayed) is rarely explicit, making it difficult to infer how working memory might have influenced the results.
Differences in Number of Items
Furthermore, the number of items varies widely across studies, ranging from 4 gestures (Weiss et al., 2016) to 40 gestures (Binder et al., 2017). A small number of items may either exaggerate or minimize the clinical deficit. Regarding mixed lists of gestures, the proportion of meaningful intransitive gestures was well controlled in studies that mixed meaningful intransitive and meaningless gestures, but not in studies that mixed meaningful intransitive and transitive gestures (Table 3).
Differences in Coding Systems
As shown in Table 3, there is significant heterogeneity between scoring systems, as we found four different types of coding systems. First, trial-based scores are scores that depend on the number of trials required to achieve normal performance (Binder et al., 2017; De Renzi, 1980; De Renzi et al., 1982; Mengotti et al., 2013) or the number of correct trials (Mengotti et al., 2015). Second, error-based scores correspond to error counts that are converted into scores (e.g., Haaland et al., 2000). Third, multidimensional coding systems encode for different dimensions of a given gesture. For instance, Heath et al. (2001; see also Stamenova et al., 2010) scored five dimensions on a 2-point scale: orientation of the hand (rotation of the palm relative to the arm), action (movement of the hand through space), hand posture (position or shape of the limb), plane of movement of the hand, and the location of the hand relative to the body. Fourthly, accuracy scores code for the quality of overall performance. These are the most widely used, but there is considerable heterogeneity between studies, which means that similar coding systems may actually correspond to very different errors. Some coding systems have coded the patient’s first response (Raade et al., 1991) while others have allowed for self-corrections (Ambrosoni et al., 2006; Bartolo et al., 2008; Schnider et al., 1997; Weiss et al., 2016). Binary pass-or-fail scores have been used (Cubelli et al., 2006; Dressing et al., 2018; Ortiz & Mantovani-Nagaoka, 2017), but also 3- to 7-point scales based on different error types such as abnormal amplitude, force, or speed; perseverations; oral performance; spatial errors (i.e., location or plane of movement); postural errors (e.g., wrong hand posture or no action); content errors such as visual errors and semantic parapraxias (e.g., a combination of two items or an action visually similar to the target) or movements unrelated to the task; hesitations, “robot-like” movements, omissions, substitutions, extra movements; and no response or unrecognizable gesture (Bonivento et al., 2014; De Renzi et al., 1966; Hanna-Pladdy, 2001; Roy et al., 1991; Schnider et al., 1997; Vanbellingen et al., 2010).
Are “Meaningful” Gestures Actually Meaningful?
In previous studies, gestures that were considered meaningless were in fact meaningful to naïve participants (Achilles et al., 2016). Our coding of gesture meaning (see the “Coding of the Emotional Valence of Gestures” section) confirms that the gestures used in previous studies were mostly meaningful, with very rare exception (Table 5).
Percentage of Patients Showing a Deficit
Eleven studies of patients with left hemisphere lesions (total n cases = 866) reported the number of patients showing a deficit in meaningful intransitive gestures (see details in Table 4 and Supplementary Tables 6 and 7). The mean percentage of patients performing below the cut-off score (across all studies and conditions) was 46.4% (standard deviation across studies: 12.4%). In comparison, the mean percentage of cases with a deficit was 53.5% (sd = 18.0%) for pure meaningful transitive gesture tests (N = 5 studies, total N cases = 272) and 35.9% (sd = 7.3%) for pure meaningless gesture tests (N = 3 studies, total N cases = 168). More specifically, 67 out of 118 cases (57%) failed the meaningful intransitive gesture test on verbal command, and 280 out of 847 cases (33%) on imitation. There were no data on visual input and reception.
Four studies of patients with right hemisphere lesions (total n cases = 188) reported the same data. The mean percentage of patients scoring below the cut-off score on pure meaningful intransitive gesture tests was 31.2% (standard deviation, 20.7%). In comparison, the mean percentage of cases with a deficit was 32.1% (sd = 1.1%) for pure meaningful transitive gesture tests (N = 2 studies, total N cases = 46) and 11.3% (N = 1 study, total N cases = 80) for meaningless gesture tests. More specifically, 48 out of 100 cases (48%) failed the meaningful intransitive gesture test on verbal command and 37 out of 188 cases (20%) failed the gesture test on imitation. There was no data on visual input or reception.
We performed chi-square tests with two factors (factor 1: presence or absence of a deficit; factor 2: group with left or right hemisphere damage) for meaningful intransitive gestures only, on verbal command (data available for n = 118 cases with left hemisphere lesions and 100 cases with right hemisphere lesions; cases with left hemisphere lesions with no deficit, n = 51 (43%), with a deficit, n = 67 (57%); cases with right hemisphere lesions with no deficit, n = 52 (52%), with a deficit, n = 48 (48%)) and on imitation (data available for 847 cases with left hemisphere lesions and 188 cases with right hemisphere lesions; cases with left hemisphere lesions with no deficit, n = 567 (67%), with a deficit, n = 280 (33%); cases with right hemisphere lesions with no deficit, n = 151 (80%), with a deficit, n = 37 (20%)). The test was significant for imitation (χ2 = 12.3, df = 1, p < 0.001) but not for verbal command (χ2 = 1.34, df = 1, p = 0.247). In conclusion, (1) previous studies have documented meaningful intransitive gesture impairments in both cases with left hemisphere lesions and cases with right hemisphere lesions, and in both verbal command and imitation; (2) the prevalence of meaningful intransitive gesture deficits is higher after left hemisphere lesions, especially when testing imitation. It is noteworthy, however, that in studies of patients with right hemisphere lesions, the prevalence of deficit in verbal command was higher for meaningful intransitive gestures (mean = 42.5%, sd = 30.4%) than for meaningful transitive gestures (mean = 26.0%, n = 1 study), whereas in studies of patients with left hemisphere lesions, the reverse pattern was found (meaningful intransitive mean = 54.5%, sd =12.7; meaningful transitive mean = 62.0%, n = 1 study). Overall, the results remained unchanged when strictly controlling for laterality (i.e., excluding studies that did not provide data on laterality from the analyses; Supplementary Fig. 1).
Control-Patient Difference Score
Table 4 and Fig. 4 show the mean control-patient difference (weighted by sample size) as a function of the clinical group, task, and modality. Control-patient differences were higher in patients with left than right hemisphere lesions in all tasks and modalities. Specifically, for meaningful intransitive gestures, 8 out of 9 studies of patients with left hemisphere lesions have documented significant control-patient differences in verbal command, and 6 out of 7 in imitation. In contrast, 2 out of 5 and 2 out of 4 studies of patients with right hemisphere lesions have documented significant control-patient differences in verbal command and imitation, respectively. However, patients with left or right hemisphere lesions have shown similar levels of performance on the meaningful intransitive gesture tests (especially after controlling for laterality, see Supplementary Fig. 2).
Mean control-patient difference. Notes. Figure based on the data available in Table 4. Standard deviations reflect the between-study discrepancy. Left panel: mean across all task modalities, including verbal command, imitation, production based on visual input, and reception. Data for visual input and reception are given in the text given the low number of studies. LHD, left hemisphere damage; RHD, right hemisphere damage; MFI, meaningful intransitive; MFT, meaningful transitive; MLI, meaningless intransitive
Gesture Production
With regard to gesture production in patients with left hemisphere lesions, the control-patient difference was smaller for meaningful intransitive gestures than for any other condition (see Fig. 4). A transitivity gradient was observed, with tests using meaningful intransitive gestures only being easier than tests mixing meaningful intransitive and transitive gestures, which in turn were easier than tests using meaningful transitive gestures only. Figure 4 shows that there was no clear meaning effect: tests with meaningless gestures seemed to be more difficult than tests with meaningful intransitive gestures, but this was strongly influenced by the verbal command modality, which is quite unusual for this type of gesture (i.e., it consists of giving complex instructions such as “put your right thumb on your right ear with the other fingers pointing upward”) and probably complex for aphasic patients with left hemisphere lesions. When it comes to imitation, and when laterality is strictly controlled for (Supplementary Fig. 2), there is actually little difference between meaningful intransitive and meaningless gestures for patients with left hemisphere lesions. In summary, we found a transitivity effect, but no clear meaning effect, in these patients.
Based on Fig. 4 again, gesture production performance followed a different pattern in patients with right hemisphere lesions. Pure meaningful intransitive gesture tests yielded the highest control-patient difference, while meaningful transitive gesture tests yielded the lowest one. This was true for verbal command and imitation and was confirmed after controlling for laterality (Supplementary Fig. 2). Again, meaningful intransitive and meaningless gestures yielded similar levels of performance. In conclusion, we found no meaning effect, but a “reverse” transitivity effect in patients with right hemisphere lesions, where the gesture production performance increased when patients were asked to pantomime the use of tools, but decreased when they were asked to make meaningful intransitive gestures.
Gesture Reception
Studies that have mixed meaningful intransitive and transitive gestures (see Table 4) have consistently found deficits in gesture reception in patients with left hemisphere lesions, with 30 to 58% of cases showing a deficit and a mean control-patient difference similar to that observed for gesture production. In contrast, gesture reception deficits were rarer in patients with right hemisphere lesions (7–12% of cases), and control-patient differences were smaller. Nevertheless, different tasks were used (see the “Methodological Considerations” section) and there may be a confounding transitivity effect due to the presence of meaningful transitive gestures in the tests. Only one study (of patients with left hemisphere lesions) has investigated and documented gesture recognition and gesture comprehension deficits with meaningful intransitive gestures (see definitions in the “Methodological Considerations” section; Binder et al., 2017). Notably, in a study not included in this review, patients with right hemisphere lesions performed worse than patients with left hemisphere lesions in a comprehension task (i.e., describing the meaning of gestures and indicating the context in which they appear; Halsband et al., 2001). In conclusion, based on the available data, both patient groups seem to have meaningful intransitive gesture reception impairments, although with a high degree of uncertainty due to the low number of studies.
Relationship Between Types of Gestures
We found 11 pure meaningful intransitive studies that also provided gesture scores obtained with pure meaningful transitive gesture tests. Similarly, we found 9 pure meaningful intransitive studies that also provided gesture scores from pure meaningless tests. This made it possible to test the relationship between meaningful intransitive gesture production scores on the one hand and meaningful transitive and meaningless gesture production scores on the other hand—in the same task modality. As shown in Fig. 5, there was a strong association between meaningful intransitive and transitive gesture scores, but a weak association between meaningful intransitive and meaningless gesture scores.
Association between scores obtained with meaningful intransitive, meaningful transitive, and meaningless gestures. Notes. Figure based on the data available in Table 4. Only scores corresponding to production on verbal command or imitation are displayed given the lack of data regarding reception and other task modalities. Each color corresponds to a specific study. The larger the circle, the larger the sample size. Full circles and lines: patients with left hemisphere lesions. Dotted circles and lines: patients with right hemisphere lesions. MFI, meaningful intransitive; MFT, meaningful transitive; MLI, meaningless intransitive
Dissociations Between Types of Gestures
We examined individual cases in all the studies that had used both pure meaningful intransitive and either pure meaningful transitive or pure meaningless gesture lists. Only five studies have provided individual scores (see Table 6) for a total subsample of n = 259 cases with left hemisphere lesions and n = 43 cases with right hemisphere lesions.
As shown in Table 6, double dissociations (i.e., normal performance with gesture type 1 but impaired performance with gesture type 2 in some cases, and the opposite pattern in some other cases) were documented between meaningful intransitive and transitive gesture production tasks, in both patient groups. In verbal command, selective meaningful intransitive gesture impairments were documented in 4 out of 35 cases with right hemisphere lesions (11.4%) but were not observed in the patients with left hemisphere lesions (Stamenova et al., 2010). In imitation, selective impairments of meaningful intransitive gestures were documented in 6 out of 43 RHD cases (13.9%) and 11 out of 225 LHD cases (4.9%). The difference was significant (chi-square test with “meaningful intransitive impaired but meaningful transitive normal” versus “any other profile,” and left versus right hemisphere damage; χ2 = 4.49, df = 1, p = 0.034).
Finally, only two imitation studies (of patients with left hemisphere lesions) provided individual meaningful intransitive and meaningless gesture scores (see Table 6). Again, double dissociations were found. For example, in Mengotti et al.’s study, three cases showed impaired performance with meaningful intransitive gestures but normal performance with meaningless gestures (DR: meaningful 25/36, cut-off 30, meaningless 29/36, cut-off 29; BV: meaningful 28/36, cut-off 33.7, meaningless 31/36, cut-off 29.6; SS: meaningful 33/36, cut-off 33.7, meaningless 32/36, cut-off 29.6), while three cases showed the opposite pattern (FN: meaningful 33/36, meaningless 27/36; PI: meaningful 33/36, meaningless 25/36; SR: meaningful 30/36, meaningless 24/36; cut-off meaningful 30, meaningless 29 for the three cases). Selective impairments of meaningful intransitive gestures were documented in 5 out of 52 LHD cases (9.6%).
It should be noted, however, that in the only study that provided individual scores for the three types of gestures separately deficits in meaningful intransitive gestures were always associated with deficits of either meaningful transitive or meaningless gestures (or both; Vanbellingen et al., 2015).
Links to Aphasia
Nine pure meaningful intransitive gesture studies of patients with left hemisphere lesions have provided language scores. We extracted comprehension scores (as this was the most commonly reported testing condition) and, were these not available, global aphasia scores. We found an association between the results of the language tests and meaningful intransitive gesture production in patients with left hemisphere lesions (Supplementary Fig. 3), both for verbal command and imitation. Furthermore, the prevalence of meaningful intransitive gesture production deficits varies according to the presence or absence of aphasia. In the study by Basso et al. (1981), 3 out of 32 (9%) non-aphasic patients with left hemisphere lesions failed the gesture test compared with 57 out of 141 (40%) aphasic patients. De Renzi (1980) documented meaningful intransitive gesture production deficits in 1 out of 40 (3%) non-aphasic patients with lesions of the left hemisphere, but 41 out of 60 (68%) aphasic patients. Cubelli et al. (2000) and Ferro et al. (1980) found similar values (53% and 31% of aphasic patients, respectively). Two studies have reported meaningful intransitive gesture production scores for aphasic vs. non-aphasic patients. In the study by Cubelli et al. (2000), aphasic patients (n = 17) had a lower mean performance than non-aphasic patients with left hemisphere lesions (n = 2; 44% and 65% of the maximum score, respectively). In the study of Mengotti et al. (2013), aphasic patients had a lower score than non-aphasic patients (66% and 71%, respectively), and both subgroups had a lower score than the control group (94%). In conclusion, the presence of aphasia is a good predictor of meaningful intransitive gesture production deficits in patients with lesions of the left hemisphere.
However, both disorders can occur independently. Misunderstanding of gesture labels cannot explain the failure to imitate gestures. In addition, some patients may have gesture production deficits without aphasia. For example, in Cubelli et al.’s (2000) study, one patient with left hemisphere lesions (case 16) scored in the normal range on all language tests while showing a marked impairment in the production of meaningful intransitive gestures on verbal command (16/40, cut-off score 24). Case 9, on the other hand, had severe aphasia but no gesture impairment. These findings suggest that language and meaningful intransitive gesture production are independent from each other, in line with a previous study (Papagno et al., 1993). Thus, aphasia alone cannot account for meaningful intransitive gesture production deficits.
Links to Semantic Memory
There is a lack of data on the relationship between meaningful intransitive gesture production and semantic memory. Based on the only study that has provided quantitative scores for both meaningful intransitive gesture production and semantic cognition (Mengotti et al., 2013), there is no clear association between these dimensions (Supplementary Fig. 4) —possibly due to ceiling effects. Most apraxia studies that have provided semantic scores have used mixed lists of meaningful intransitive and transitive gestures (Papeo & Rumiati, 2013; van Nispen et al., 2016) or meaningful intransitive and meaningless gestures (Negri et al., 2007). These studies found either no correlation or a moderate correlation (r = 0.33) between the two dimensions (Supplementary Fig. 4). In the Van Nispen et al. study, 7 out of 38 cases with left hemisphere lesions (18%) had semantic memory deficits, and all of them failed to communicate with gestures.
Nevertheless, some patients may show normal gesture performance despite impaired semantic knowledge, and conversely. First, with regard to mixed lists studies, in the study by Papeo and Rumiati (2013), one case with left hemisphere lesions (C7) scored in the normal range in the semantic test but failed the gesture test, while several other cases showed the opposite pattern. In the study by Van Nispen et al. (2016), several cases with left hemisphere lesions failed the gesture test despite having normal semantic memory. In the study by Negri et al. (2007), none of the patients had severe semantic deficits, and yet 6 out of 22 patients with left hemisphere lesions (27%) and 1 out of 14 patients with right hemisphere lesions (7%) failed the gesture test. In the study by Mengotti et al., two patients with selective meaningful intransitive gesture imitation impairments (without meaningless gesture imitation impairments), DR and SS, scored in the normal range on tests assessing semantic cognition.Footnote 1 Thus, in these two cases, neither general imitation impairments (as meaningless gesture imitation was normal) nor semantic deficits (as semantic cognition was normal) could account for the selective meaningful intransitive gesture imitation deficit. Another case, GU, performed below the cut-off score on the semantic test (32/52, cut-off score 40 based on Gamboz et al., 2009) but above the cut-off score on both the meaningful intransitive and meaningless gesture tests (35/36 and 34/36, cut-off score 32 and 31, respectively). This means that impaired production of meaningful intransitive gestures can be observed even in the context of normal semantic cognition. Thus, semantic memory deficits are not sufficient to explain meaningful intransitive gesture production impairments.
Effect of Characteristics of Meaningful Intransitive Gestures
Eleven meaningful intransitive gesture studies provided both the full list of gestures and the control-patient difference (Tables 3 and 4), allowing us to test associations between gesture characteristics (i.e., body-centered or not; emotional valence and intensity) and the control-patient difference score. Figure 6 shows the association between the percentage of body-centered gestures used in the meaningful intransitive gesture tests and the control-patient difference score. There was an association in the group of patients with left hemisphere lesions (mainly on verbal command) as well as in the group of patients with right hemisphere lesions (only on imitation). This dimension therefore predicts a portion of the gesture performance: the higher the number of meaningful intransitive body-centered gestures in the task, the greater the impairment.
Figure 6 also shows that the valence and intensity of gestures influence the performance. Studies that have used gestures with negative emotional valence, or gestures with high emotional intensity, seem to yield higher control-patient difference, at least on verbal command (see Supplementary Fig. 5). The influence of emotional valence and intensity is less clear on imitation, probably because when a model is presented, individuals focus on the shape of the gesture rather than on its meaning.
Effect of gesture characteristics on meaningful intransitive gesture performance. Notes. The figure displays the control-patient difference score (y-axis) as a function of either the percentage of body-centered gestures used in the task (upper panels) or the mean valence intensity of the gestures used in the task (lower panels). Full line circles and regression lines correspond to patients with left hemisphere lesions. Dotted circles and regression lines correspond to patients with right hemisphere lesions. Each color corresponds to a specific study. The larger the circle, the larger the sample size. MFI, meaningful intransitive
General Discussion
Summary of the Findings
The first aim of this review was to compare the performance of patients with left versus right hemisphere lesions on meaningful intransitive gesture production and recognition in order to verify the hemispheric dominance for meaningful intransitive gestures. Our results showed that 46% of cases with left hemisphere lesions and 31% of cases with right hemisphere lesions had a deficit in meaningful intransitive gestures. The control-patient difference score was also higher in patients with left than right hemisphere lesions, although RHD studies have documented significant control-patient differences. The second aim of this review was to compare meaningful intransitive gesture performance with meaningful transitive and meaningless gesture performance. We documented a transitivity effect in patients with left hemisphere lesions (i.e., meaningful transitive gestures are more difficult than meaningful intransitive gestures) and a “reverse” transitivity effect in patients with right hemisphere lesions (i.e., meaningful transitive gestures are easier than meaningful intransitive gestures) while meaning (i.e., meaningful intransitive versus meaningless gestures) had only little effect on the performance. We also found associations, in both clinical groups, between meaningful intransitive and transitive gestures, but not between meaningful intransitive and meaningless gestures. Finally, we reported selective impairments of meaningful intransitive gestures, suggesting that the latter require specific cognitive mechanisms. Finally, the third aim of this study was to describe associations between meaningful intransitive gesture impairments, and other cognitive impairments. We found associations with measures of language and semantic memory, but also selective impairments of meaningful intransitive gestures without language or semantic memory impairments.
According to cognitive models of apraxia, the production of meaningful gestures requires access to semantic memory or action lexicons, whereas the production of meaningless gestures depends on direct visuomotor connections (Achilles et al., 2016; Raade et al., 1991) or body representations (Buxbaum, 2001; Cubelli et al., 2000; Goldenberg, 1995). The finding of an association between meaningful intransitive and transitive gestures, but not between meaningful intransitive and meaningless gestures, is consistent with this view. However, the finding of a transitivity effect and selective impairments for meaningful intransitive gestures suggests that the latter require specific cognitive mechanisms. This finding contradicts the lexical-semantic hypothesis, which predicted either no difference of performance between meaningful intransitive and transitive gestures, or selective deficits of meaningful transitive gestures. The results are more consistent with the social cognition hypothesis, which predicted selective deficits in meaningful intransitive gestures. The following sections discuss the hemispheric dominance and the cognitive processes that may be involved in meaningful intransitive gestures.
Is There Hemispheric Dominance for Meaningful Intransitive Gestures?
Over the last two decades, it has become clear that the left parietal lobe plays an important role in the comprehension and production of tool-related actions (Binkofski & Buxbaum, 2013; Buxbaum, 2001, 2017; Buxbaum & Kalénine, 2010; Osiurak et al., 2021; Reynaud et al., 2016, 2019). In contrast, it has been suggested that meaningful intransitive gestures rely on a bilateral network (Buxbaum et al., 2007). This may be due to their social nature and the involvement of the right hemisphere in social skills (Bartolo & Ham, 2016; Happé et al., 1999). Our results are consistent with this hypothesis: although meaningful intransitive gestures are impaired after lesions of both the left and right hemispheres, selective impairments of meaningful intransitive gesture production are more frequent after right than left hemisphere lesions (see the “Dissociations Between Types of Gestures” section). It is concluded that both hemispheres are involved in meaningful intransitive gesture processing, with a possible specific role of the right hemisphere in the processing of the social content of gestures. A similar “contextual hypothesis” has been formulated, according to which the left hemisphere is involved in the programming of an abstract, decontextualized gestures, whereas the right hemisphere is involved in the programming of concrete gestures performed in an ecological context (Rapcsak et al., 1993; see also "abstract attitude", Goldstein, 1948; "automatic-voluntary dissociation", Jackson, 1866). In this view, meaningful intransitive gestures may be represented bilaterally in the brain because of their ecological, context-related nature—in contrast to meaningful transitive and meaningless gestures which are complex, creative tasks (see Osiurak et al., 2021). However, in the current state of the literature, the role of each hemisphere remains unclear. After all, the emotional valence and intensity also seemed to influence the performance of patients with left hemisphere lesions as well, and different subprocesses of social cognition are distributed across bilateral brain regions (Adolphs, 1999; Van Overwalle, 2009). Future studies should investigate the specific contribution of each hemisphere to these gestures.
Methodological issues may also explain the bilateral representation of meaningful intransitive gestures. Since the assessment of apraxia generally consists of asking patients to perform gestures out of context, this may explain the contribution of the left hemisphere but it does not mean that in an ecological setting, some gestures are not processed in the right hemisphere. Indeed, some studies have highlighted the difficulties of patients with right hemisphere lesions in tasks requiring spontaneous gestures (e.g., Blonder et al., 1995; Jason, 1985). These studies have suggested that these patients do not have a reduced ability to generate gestures, but rather difficulties in generating gestures that meet the requirements of the task. In this view, the problem for them may not be generating gestures, but using contextual cues or increasing emotional arousal to generate the right gestures in the right situation. To test this assumption, future studies should not only test meaningful intransitive gesture production in verbal command and imitation but also in ecological settings (see also Sekine & Rose, 2013).
Furthermore, while apraxia studies have focused on distinctions between broad gesture categories (meaningful intransitive, meaningful transitive, and meaningless), the performance may vary as a function of the “socioemotional complexity” of meaningful intransitive gestures. We found that the number of body-centered gestures, as well as the emotional valence and intensity of the gestures, may influence the results. Thus, subtle differences between meaningful intransitive gestures can lead to different results. For instance, giving simple commands or expressing feelings or inner states produce different brain activations, as suggested by an fMRI study (Gallagher & Frith, 2004). Similarly, some meaningful intransitive gestures are likely to be triggered by contextual cues in a highly automatic manner (e.g., waving goodbye), whereas other meaningful intransitive gestures may depend on conscious cognitive control (e.g., miming a spider). As most studies have used mixed lists of emotional, instrumental, deictic, and conceptual gestures, this may explain differences in findings when comparing patient groups. Future studies on meaningful intransitive gestures should control for these dimensions.
The coding systems used can also strongly influence the results (see the “Methodological Considerations” section). Most coding systems have focused on the kinematic components of movements (e.g., spatial, temporal, and postural errors) rather than on the communicative nature of gestures (e.g., the recognizability of gestures and independent of execution errors). Thus, they may have exaggerated the prevalence and severity of meaningful intransitive gesture impairments in patients with left hemisphere lesions and failed to capture deficits in patients with right hemisphere lesions. For the same reason, tasks assessing gesture production (focusing on spatiotemporal parameters) and gesture reception (focusing on gesture recognizability and knowledge) are not fully comparable. For this reason, differences in performance do not necessarily correspond to differences in cognitive architectures (e.g., production vs. reception action lexicon) but probably reflect differences in task design and encoding systems. By controlling for meaningful intransitive gesture types and using both production and reception tasks with similar encoding systems, future studies may reveal specific patterns of hemispheric dominance.
Do Meaningful Intransitive Gestures Depend on Language and Semantic Memory?
There is a link between measures of meaningful intransitive gestures and language, probably because of the similarity of the two dimensions. Indeed, both are complex motor acts of a communicative nature, in which a sender conveys symbolic content to a receiver (Xu et al., 2009). Thus, they probably share a common ground, which has been proposed to be the need to access semantic memory (to retrieve knowledge about words and gestures) or lexicons (containing words for language, spatial coordinates, and kinematics of the movement for gestures; e.g., Cubelli et al., 2000; Stamenova et al., 2012). Some theories of the origin of language suggest that communication was first gestural before evolving into the modern language (Armstrong, 2008; Corballis, 2010; Gentilucci & Corballis, 2006). In all likelihood, gestures and language also share common neural networks (Devlin & Watkins, 2007; Willems et al., 2007). It has been proposed that semantic memory is strongly dependent on the temporal lobes, whereas action lexicons are dependent on the left parietal lobe (Binkofski & Buxbaum, 2013; Buxbaum, 2001, 2017; Buxbaum & Kalénine, 2010). The anatomical proximity of these structures to brain regions underlying language may explain the frequent co-occurrence of aphasia and meaningful intransitive gesture impairments, in line with what Liepmann originally emphasized for apraxia in general. However, after controlling for lesion volume and stroke-test interval, the aphasia severity actually predicts only a marginal amount of variance of meaningful intransitive gesture production (Hanna-Pladdy, 2001; see also Goodglass & Kaplan, 1963). Indeed, meaningful intransitive gesture production impairments without language impairments have been documented, implying that meaningful intransitive gesture performance depends on other cognitive mechanisms.
Semantic memory plays a role in the production of meaningful transitive gestures (e.g., Baumard et al., 2016, 2019; Bozeat et al., 2002; Hodges, 2000), and it is likely to play a role in the production of meaningful intransitive gestures—especially given the association between meaningful intransitive and transitive gestures. Indeed, the production or recognition of a gesture requires that the sender and receiver share knowledge about the content of the gesture. However, while some studies have suggested an association between gesture production and semantic scores (Van Nispen et al., 2016), this association is not clear when only meaningful intransitive gestures are considered (Mengotti et al., 2013). This may be due to a ceiling effect of semantic measures, as well as to the use of semantic measures that are not specific to gestures (Mengotti et al., 2013; Negri et al., 2007; Papeo & Rumiati, 2013). Based on the available data, the involvement of semantic memory in the production and reception of meaningful intransitive gestures remains an open issue. More importantly, given the observation of selective impairments of meaningful intransitive gesture production in the context of normal semantic memory (and conversely), semantic deficits are neither necessary nor sufficient to explain meaningful intransitive gesture impairments.
Do Meaningful Intransitive Gestures Depend on Social Cognition?
The results are consistent with the social cognition hypothesis, which predicted selective impairments of meaningful intransitive gestures. We also found that the emotional valence and intensity of gestures appeared to influence the performance. These findings are consistent with the possible role of socioemotional processes in meaningful intransitive gesture processing (Bartolo & Ham, 2016; Gallagher & Frith, 2004). Social cognition may be required to perform meaningful intransitive gestures, because of their social nature (Bartolo & Cubelli, 2014; Cubelli et al., 2000), or because the production of such gestures involves the selection of gesture features that the observer may be able to recognize (for a similar view on meaningful transitive gestures, see Goldenberg et al., 2003; Osiurak et al., 2021).
The hypothesis of a link between social skills and gesture performance is appealing, especially in the light of the development of apraxia studies in some pathologies with communicative disorders such as autism spectrum disorders (e.g., Mostofsky et al., 2006; Stieglitz Ham et al., 2010, 2011) or schizophrenia (e.g., Walther et al., 2013, 2020). However, it raises three issues. The first one is the lack of data in the international literature. If social cognition deficits explain meaningful intransitive gesture impairments, then the latter should be found in all the patients with social skills deficits, an assumption that remains to be demonstrated, as patients with social skills deficits are not systematically tested for apraxia.
The second issue is that social cognition has been proposed to play a role in the production of meaningful transitive gestures as well (e.g., Osiurak et al., 2021). If social cognition plays a role in both meaningful intransitive and transitive gestures, then how can the difference in performance between these gestures be explained? One possible explanation is that different social skills underlie meaningful intransitive and transitive gesture production. Both gestures presumably require theory of mind and perspective-taking in order to select features of the gesture that will be recognizable to the observer. Because pantomime is a novel gesture, meaningful transitive gestures specifically require working memory, to hold semantic and procedural information about the tool to be mimed in order to create the gesture (Bartolo et al., 2003; Goodglass & Kaplan, 1963; Hanna-Pladdy, 2001). In contrast, knowledge of social situations and rules (i.e., knowing which gesture to perform in which social context), as well as retrieval of the emotional content of gestures, may be important for performing meaningful intransitive gestures. However, it is also plausible that meaningful intransitive gestures require basic communication but not high-level social cognition processes. After all, intuitively, waving goodbye does not necessarily require perspective-taking, emotion processing, or theory of mind. Future studies should investigate the associations between specific social skills and different types of meaningful intransitive gestures.
This raises a third issue: social cognition, and its subcomponents, remain a topic of debate. Some tests widely used to detect deficits of social cognition, especially theory of mind, show poor psychometric properties and specificity as they may measure other cognitive or socio-cognitive constructs (Higgins et al., 2023; Kittel et al., 2022; Pavlova & Sokolov, 2022; Quesque & Rossetti, 2020). There is indeed uncertainty about what tests of social cognition actually measure. For instance, Olderbak and Wilhelm (2020) emphasized both jingle fallacies, when tests designed to assess the same construct actually assess different constructs (e.g., “emotion perception” may be assessed with self-report questionnaires, facial emotion processing or prosody processing, with actually poor between-task correlations) and jangle fallacies, when tests designed to assess different constructs actually assess the same construct (e.g., tests designed to assess “empathy,” “theory of mind,” or “emotion perception” may use a very similar design consisting in presenting emotional faces, resulting in strong between-task correlations). This will be a major challenge in the study of the relationships between meaningful intransitive gestures and social cognition because the findings and conclusions of future studies may depend on methodological choices, that is, on the nature of the gestures used on the one hand and the nature of the social cognition tests used on the other hand.
Conclusion and Recommendations
The ability to make and recognize meaningful intransitive gestures is an important component of non-verbal communication, with great ecological significance. It is therefore important to test meaningful intransitive gesture production and reception in neuropsychological assessments. This article is a groundbreaking contribution to the field of apraxia in that it is the first to provide a comprehensive review of the literature on the subject of intransitive gestures. The results showed that impairments in meaningful intransitive gestures were documented in patients with left but also right hemisphere lesions, suggesting that the right hemisphere contributes to meaningful intransitive gesture processing. Different levels of performance between meaningful intransitive, meaningful transitive, and meaningless gestures, as well as between meaningful intransitive gestures on the one hand and language and semantic memory on the other hand, suggest that meaningful intransitive gestures require specific cognitive processes. These findings are not consistent with cognitive models of apraxia but rather invite us to explore the links between meaningful intransitive gesture processing and socioemotional processing. Finally, this review allows us to make a series of recommendations for future neuropsychological studies:
-
Investigate meaningful intransitive gestures in patients not only with left but also with right hemisphere lesions.
-
Test meaningful intransitive gestures together with meaningful transitive and meaningless gestures in order to detect selective impairments of meaningful intransitive gestures.
-
Test meaningful intransitive gesture production but also recognition and comprehension to distinguish between conceptual and production disorders.
-
Test different subtypes of meaningful intransitive gestures (e.g., instrumental, emotional, deictic, and conceptual gestures).
-
Control for the content, meaning, and emotional valence of meaningful intransitive gestures.
-
Test meaningful intransitive gestures using classical methods (i.e., on verbal command and imitation) but also in more ecological settings (e.g., during actual social interactions or based on contextual cues).
-
Test the relationship between meaningful intransitive gesture performance and cognitive dimensions such as language, semantic memory, working memory, visual-spatial skills, and social cognition.
Availability of Data and Materials
This is a meta-analysis of already published studies. The data are available in tables/figures in the manuscript and in the supplement. The dataset is available on reasonable request to the corresponding author.
References
Achilles, E. I. S., Ballweg, C. S., Niessen, E., Kusch, M., Ant, J. M., Fink, G. R., & Weiss, P. H. (2019). Neural correlates of differential finger gesture imitation deficits in left hemisphere stroke. NeuroImage: Clinical, 23, 101915. https://doi.org/10.1016/j.nicl.2019.101915
Achilles, E. I. S., Fink, G. R., Fischer, M. H., Dovern, A., Held, A., Timpert, D. C., Schroeter, C., Schuetz, K., Kloetzsch, C., & Weiss, P. H. (2016). Effect of meaning on apraxic finger imitation deficits. Neuropsychologia, 82, 74–83. https://doi.org/10.1016/j.neuropsychologia.2015.12.022
Adolphs, R. (1999). Social cognition and the human brain. Trends in Cognitive Sciences, 3(12), 469–479. https://doi.org/10.1016/S1364-6613(99)01399-6
Ambrosoni, E., Sala, S., Motto, C., Oddo, S., & Spinnler, H. (2006). Gesture imitation with lower limbs following left hemisphere stroke. Archives of Clinical Neuropsychology, 21(4), Article 4. https://doi.org/10.1016/j.acn.2006.05.001
Armstrong, D. F. (2008). The gestural theory of language origins. Sign Language Studies, 8(3), 289–314.
Barbieri, C., & De Renzi, E. (1988). The executive and ideational components of apraxia. Cortex, 24(4), Article 4. https://doi.org/10.1016/S0010-9452(88)80047-9
Bartolo, A., Cubelli, R., Sala, S. D., Drei, S., & Marchetti, C. (2001). Double dissociation between meaningful and meaningless gesture reproduction in apraxia. Cortex, 37(5), Article 5. https://doi.org/10.1016/S0010-9452(08)70617-8
Bartolo, A., Cubelli, R., Della Sala, S., & Drei, S. (2003). Pantomimes are special gestures which rely on working memory. Brain and Cognition, 53(3), Article 3. https://doi.org/10.1016/S0278-2626(03)00209-4
Bartolo, A., & Ham, H. S. (2016). A cognitive overview of limb apraxia. Current Neurology and Neuroscience Reports, 16(8), Article 8. https://doi.org/10.1007/s11910-016-0675-0
Bartolo, A., Claisse, C., Gallo, F., Ott, L., Sampaio, A., & Nandrino, J.-L. (2019). Gestures convey different physiological responses when performed toward and away from the body. Scientific Reports, 9(1), 12862. https://doi.org/10.1038/s41598-019-49318-3
Bartolo, A., & Cubelli, R. (2014). The cognitive models of limb apraxia and the specific properties of meaningful gestures. Cortex, 57, 297–298. https://doi.org/10.1016/j.cortex.2014.01.007
Basso, A., Capitani, E., Luzzatti, C., & Spinnler, H. (1981). Intelligence and left hemisphere disease the role of aphasia, apraxia and size of lesion : The role of aphasia, apraxia and size of lesion. Brain, 104(4), 721–734. https://doi.org/10.1093/brain/104.4.721
Basso, A., Capitani, F., Sala, S. D., Laiacona, M., & Spinnler, H. (1987). Ideomotor apraxia : A study of initial severity. Acta Neurologica Scandinavica, 76(2), 142–146. https://doi.org/10.1111/j.1600-0404.1987.tb03557.x
Baumard, J., Osiurak, F., Lesourd, M., & Le Gall, D. (2014). Tool use disorders after left brain damage. Frontiers in Psychology, 5. https://doi.org/10.3389/fpsyg.2014.00473
Baumard, J., Lesourd, M., Remigereau, C., Laurent, L., Jarry, C., Etcharry-Bouyx, F., Chauviré, V., Osiurak, F., & Le Gall, D. (2023). Meaningless imitation in neurodegenerative diseases : Effects of body part, bimanual imitation, asymmetry, and body midline crossing. Cognitive Neuropsychology, 1‑22. https://doi.org/10.1080/02643294.2022.2164487
Baumard, J., & Le Gall, D. (2021). The challenge of apraxia : Toward an operational definition? Cortex, 141, 66–80. https://doi.org/10.1016/j.cortex.2021.04.001
Baumard, J., Lesourd, M., Jarry, C., Merck, C., Etcharry-Bouyx, F., Chauviré, V., Belliard, S., Moreaud, O., Croisile, B., Osiurak, F., & Le Gall, D. (2016). Tool use disorders in neurodegenerative diseases : Roles of semantic memory and technical reasoning. Cortex, 82, 119–132. https://doi.org/10.1016/j.cortex.2016.06.007
Baumard, J., Lesourd, M., Remigereau, C., Lucas, C., Jarry, C., Osiurak, F., & Le Gall, D. (2020). Imitation of meaningless gestures in normal aging. Aging, Neuropsychology, and Cognition, 27(5), 729–747. https://doi.org/10.1080/13825585.2019.1674773
Baumard, J., Lesourd, M., Remigereau, C., Merck, C., Jarry, C., Etcharry-Bouyx, F., Chauviré, V., Belliard, S., Moreaud, O., Osiurak, F., & Le Gall, D. (2019). The – weak – role of memory in tool use : Evidence from neurodegenerative diseases. Neuropsychologia, 129, 117–132. https://doi.org/10.1016/j.neuropsychologia.2019.03.008
Bickerton, W.-L., Riddoch, M. J., Samson, D., Balani, A. B., Mistry, B., & Humphreys, G. W. (2012). Systematic assessment of apraxia and functional predictions from the Birmingham Cognitive Screen. Journal of Neurology, Neurosurgery & Psychiatry, 83(5), Article 5. https://doi.org/10.1136/jnnp-2011-300968
Binder, E., Dovern, A., Hesse, M. D., Ebke, M., Karbe, H., Saliger, J., Fink, G. R., & Weiss, P. H. (2017). Lesion evidence for a human mirror neuron system. Cortex, 90, 125–137. https://doi.org/10.1016/j.cortex.2017.02.008
Binkofski, F., & Buxbaum, L. J. (2013). Two action systems in the human brain. Brain and Language, 127(2), Article 2. https://doi.org/10.1016/j.bandl.2012.07.007
Blonder, L. X., Burns, A. F., Bowers, D., Moore, R. W., & Heilman, K. M. (1995). Spontaneous gestures following right hemisphere infarct. Neuropsychologia, 33(2), 203–213. https://doi.org/10.1016/0028-3932(94)00099-B
Bonivento, C., Rothstein, P., Humphreys, G., & Chechlacz, M. (2014). Neural correlates of transitive and intransitive action imitation : An investigation using voxel-based morphometry. NeuroImage: Clinical, 6, 488‑497. https://doi.org/10.1016/j.nicl.2014.09.010
Bozeat, S., Lambon Ralph, M. A. L., Patterson, K., & Hodges, J. R. (2002). When objects lose their meaning : What happens to their use? Cognitive, Affective, & Behavioral Neuroscience, 2(3), Article 3. https://doi.org/10.3758/CABN.2.3.236
Buchmann, I., Dangel, M., Finkel, L., Jung, R., Makhkamova, I., Binder, A., Dettmers, C., Herrmann, L., Liepert, J., Möller, J. C., Richter, G., Vogler, T., Wolf, C., & Randerath, J. (2020). Limb apraxia profiles in different clinical samples. The Clinical Neuropsychologist, 34(1), Article 1. https://doi.org/10.1080/13854046.2019.1585575
Buxbaum, L. J., Johnson-Frey, S. H., & Bartlett-Williams, M. (2005a). Deficient internal models for planning hand–object interactions in apraxia. Neuropsychologia, 43(6), Article 6. https://doi.org/10.1016/j.neuropsychologia.2004.09.006
Buxbaum, L. J., Kyle, K. M., & Menon, R. (2005b). On beyond mirror neurons : Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research, 25(1), Article 1. https://doi.org/10.1016/j.cogbrainres.2005.05.014
Buxbaum, L. J., Kyle, K., Grossman, M., & Coslett, B. (2007). Left inferior parietal representations for skilled hand-object interactions : Evidence from stroke and corticobasal degeneration. Cortex, 43(3), Article 3. https://doi.org/10.1016/S0010-9452(08)70466-0
Buxbaum, L. J., & Kalénine, S. (2010). Action knowledge, visuomotor activation, and embodiment in the two action systems. Annals of the New York Academy of Sciences, 1191(1), Article 1. https://doi.org/10.1111/j.1749-6632.2010.05447.x
Buxbaum, L. J. (2001). Ideomotor apraxia : A call to action. Neurocase, 7, 445–458.
Buxbaum, L. J. (2017). Learning, remembering, and predicting how to use tools : Distributed neurocognitive mechanisms: Comment on Osiurak and Badets (2016). Psychological Review, 124(3), 346–360. https://doi.org/10.1037/rev0000051
Carmo, J. C., & Rumiati, R. I. (2009). Imitation of transitive and intransitive actions in healthy individuals. Brain and Cognition, 69(3), 460–464. https://doi.org/10.1016/j.bandc.2008.09.007
Chestnut, C., & Haaland, K. Y. (2008). Functional significance of ipsilesional motor deficits after unilateral stroke. Archives of Physical Medicine and Rehabilitation, 89(1), Article 1. https://doi.org/10.1016/j.apmr.2007.08.125
Cochet, H., & Vauclair, J. (2014). Deictic gestures and symbolic gestures produced by adults in an experimental context : Hand shapes and hand preferences. Laterality: Asymmetries of Body, Brain and Cognition, 19(3), Article 3. https://doi.org/10.1080/1357650X.2013.804079
Corballis, M. C. (2010). Mirror neurons and the evolution of language. Brain and Language, 112(1), 25–35. https://doi.org/10.1016/j.bandl.2009.02.002
Coslett, H. B. (2018). Apraxia, neglect, and agnosia. Continuum: Lifelong Learning in Neurology, 24(3), 768‑782. https://doi.org/10.1212/CON.0000000000000606
Cubelli, R., Marchetti, C., Boscolo, G., & Della Sala, S. (2000). Cognition in action : Testing a model of limb apraxia. Brain and Cognition, 44(2), Article 2. https://doi.org/10.1006/brcg.2000.1226
Cubelli, R., Bartolo, A., Nichelli, P., & Della Sala, S. (2006). List effect in apraxia assessment. Neuroscience Letters, 407(2), Article 2. https://doi.org/10.1016/j.neulet.2006.08.019
De Renzi, E. (1980). Imitating gestures : A quantitative approach to ideomotor apraxia. Archives of Neurology, 37(1), Article 1. https://doi.org/10.1001/archneur.1980.00500500036003
De Renzi, E., Faglioni, P., & Sorgato, P. (1982). Modality-specific and supramodal mechanisms of apraxia. Brain, 105(2), 301–312. https://doi.org/10.1093/brain/105.2.301
De Renzi, E., Faglioni, P., Scarpa, M., & Crisi, G. (1986). Limb apraxia in patients with damage confined to the left basal ganglia and thalamus. Journal of Neurology, Neurosurgery & Psychiatry, 49(9), 1030–1038. https://doi.org/10.1136/jnnp.49.9.1030
De Renzi, E., Pieczuro, A., & Vignolo, L. A. (1966). Oral apraxia and aphasia. Cortex, 2(1), Article 1. https://doi.org/10.1016/S0010-9452(66)80028-X
Dellasala, S., Faglioni, P., Motto, C., & Spinnler, H. (2006). Hemisphere asymmetry for imitation of hand and finger movements, Goldenberg’s hypothesis reworked☆. Neuropsychologia, 44(8), Article 8. https://doi.org/10.1016/j.neuropsychologia.2005.11.011
Devlin, J. T., & Watkins, K. E. (2007). Stimulating language : Insights from TMS. Brain, 130(3), 610–622. https://doi.org/10.1093/brain/awl331
Donovan, N. J., Kendall, D. L., Heaton, S. C., Kwon, S., Velozo, C. A., & Duncan, P. W. (2008). Conceptualizing functional cognition in stroke. Neurorehabilitation and Neural Repair, 22(2), Article 2. https://doi.org/10.1177/1545968307306239
Dovern, A., Fink, G. R., & Weiss, P. H. (2012). Diagnosis and treatment of upper limb apraxia. Journal of Neurology, 259(7), 1269–1283. https://doi.org/10.1007/s00415-011-6336-y
Dressing, A., Nitschke, K., Kümmerer, D., Bormann, T., Beume, L., Schmidt, C. S. M., Ludwig, V. M., Mader, I., Willmes, K., Rijntjes, M., Kaller, C. P., Weiller, C., & Martin, M. (2018). Distinct contributions of dorsal and ventral streams to imitation of tool-use and communicative gestures. Cerebral Cortex, 28(2), Article 2. https://doi.org/10.1093/cercor/bhw383
Dumont, C., Ska, B., & Schiavetto, A. (1999). Selective impairment of transitive gestures : An unusual case of apraxia. Neurocase, 5(5), 447–458. https://doi.org/10.1080/13554799908402739
Esteves, P. O., Oliveira, L. A. S., Nogueira-Campos, A. A., Saunier, G., Pozzo, T., Oliveira, J. M., Rodrigues, E. C., Volchan, E., & Vargas, C. D. (2016). Motor planning of goal-directed action is tuned by the emotional valence of the stimulus : A kinematic study. Scientific Reports, 6(1), Article 1. https://doi.org/10.1038/srep28780
Ferri, F., Busiello, M., Campione, G. C., De Stefani, E., Innocenti, A., Romani, G. L., Costantini, M., & Gentilucci, M. (2014). The eye contact effect in request and emblematic hand gestures. European Journal of Neuroscience, 39(5), 841–851. https://doi.org/10.1111/ejn.12428
Ferro, J. M., Martins, I. P., Mariano, G., & Caldas, A. C. (1983). CT scan correlates of gesture recognition. Journal of Neurology, Neurosurgery & Psychiatry, 46(10), 943–952. https://doi.org/10.1136/jnnp.46.10.943
Ferro, J. M., Santos, M. E., Castro-caldas, A., & Mariano, M. G. (1980). Gesture recognition in aphasia. Journal of Clinical Neuropsychology, 2(4), 277–292. https://doi.org/10.1080/01688638008403800
Finkelnburg. (1870). Vortrag über aphasie. Berliner Klinische Wochenschrift, 7, 449–450.
Foundas, A. L., Macauley, B. L., Raymer, A. M., Maher, L. M., Heilman, K. M., & Rothi, L. J. G. (1995a). Ecological implications of limb apraxia : Evidence from mealtime behavior. Journal of the International Neuropsychological Society, 1(1), Article 1. https://doi.org/10.1017/S1355617700000114
Foundas, A. L. (2013). Apraxia. In Handbook of clinical neurology (Vol. 110, p. 335‑345). Elsevier. https://doi.org/10.1016/B978-0-444-52901-5.00028-9
Foundas, A. L., & Duncan, E. S. (2019). Limb apraxia : A disorder of learned skilled movement. Current Neurology and Neuroscience Reports, 19(10), 82. https://doi.org/10.1007/s11910-019-0989-9
Foundas, A. L., Henchey, R., Gilmore, R. L., Fennell, E. B., & Heilman, K. M. (1995b). Apraxia during Wada testing. Neurology, 45(7), 1379–1383. https://doi.org/10.1212/WNL.45.7.1379
Foundas, A. L., Macauley, B. L., Raymer, A. M., Maher, L. M., Rothi, L. J., & Heilman, K. M. (1999). Ideomotor apraxia in Alzheimer disease and left hemisphere stroke : Limb transitive and intransitive movements. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 12(3), 161–166.
Frenkel-Toledo, S., Liebermann, D. G., Bentin, S., & Soroker, N. (2016). Dysfunction of the human mirror neuron system in ideomotor apraxia : Evidence from Mu suppression. Journal of Cognitive Neuroscience, 28(6), 775–791. https://doi.org/10.1162/jocn_a_00936
Frith, C. D. (2008). Social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1499), 2033–2039. https://doi.org/10.1098/rstb.2008.0005
Gainotti, G., & Lemmo, M. A. (1976). Comprehension of symbolic gestures in aphasia. Brain and Language, 3(3), Article 3. https://doi.org/10.1016/0093-934X(76)90039-0
Gallagher, H. L., & Frith, C. D. (2004). Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia, 42(13), 1725–1736. https://doi.org/10.1016/j.neuropsychologia.2004.05.006
Gamboz, N., Coluccia, E., Iavarone, A., & Brandimonte, M. A. (2009). Normative data for the pyramids and palm trees test in the elderly Italian population. Neurological Sciences, 30(6), 453–458. https://doi.org/10.1007/s10072-009-0130-y
Gentilucci, M., & Corballis, M. (2006). From manual gesture to speech : A gradual transition. Neuroscience & Biobehavioral Reviews, 30(7), 949–960. https://doi.org/10.1016/j.neubiorev.2006.02.004
Gerwing, J., & Bavelas, J. (2005). Linguistic influences on gesture’s form. Gesture, 4(2), 157–195. https://doi.org/10.1075/gest.4.2.04ger
Goldenberg, G. (1995). Imitating gestures and manipulating a mannikin—The representation of the human body in ideomotor apraxia. Neuropsychologia, 33(1), Article 1. https://doi.org/10.1016/0028-3932(94)00104-W
Goldenberg, G., & Hagmann, S. (1997). The meaning of meaningless gestures : A study of visuo-imitative apraxia. Neuropsychologia, 35(3), Article 3. https://doi.org/10.1016/S0028-3932(96)00085-1
Goldenberg, G., Hartmann, K., & Schlott, I. (2003). Defective pantomime of object use in left brain damage : Apraxia or asymbolia? Neuropsychologia, 41(12), Article 12. https://doi.org/10.1016/S0028-3932(03)00120-9
Goldenberg, G., & Spatt, J. (2009). The neural basis of tool use. Brain, 132(6), Article 6. https://doi.org/10.1093/brain/awp080
Goldenberg, G. (2009). Apraxia and the parietal lobes. Neuropsychologia, 47(6), Article 6. https://doi.org/10.1016/j.neuropsychologia.2008.07.014
Goldenberg, G. (1996). Defective imitation of gestures in patients with damage in the left or right hemispheres. Journal of Neurology, Neurosurgery & Psychiatry, 61(2), 176–180. https://doi.org/10.1136/jnnp.61.2.176
Goldenberg, G. (1999). Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia, 37, 559–566.
Goldenberg, G., Münsinger, U., & Karnath, H.-O. (2009). Severity of neglect predicts accuracy of imitation in patients with right hemisphere lesions. Neuropsychologia, 47(13), 2948–2952. https://doi.org/10.1016/j.neuropsychologia.2009.06.024
Goldman, A., & de Vignemont, F. (2009). Is social cognition embodied? Trends in Cognitive Sciences, 13(4), 154–159. https://doi.org/10.1016/j.tics.2009.01.007
Goldstein, K. (1948). Language and language disturbances: Aphasic symptom complexes and their significance for medicine and theory of language. Grune & Stratton.
Goodglass, H., & Kaplan, E. (1963). Disturbance of gesture and pantomime in aphasia. Brain, 86(4), 703–720. https://doi.org/10.1093/brain/86.4.703
Haaland, K. Y., Harrington, D. L., & Knight, R. T. (2000). Neural representations of skilled movement. Brain, 123(11), Article 11. https://doi.org/10.1093/brain/123.11.2306
Halsband, U., Schmitt, J., Weyers, M., Binkofski, F., Grützner, G., & Freund, H.-J. (2001). Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions : A perspective on apraxia. Neuropsychologia, 39(2), 200–216. https://doi.org/10.1016/S0028-3932(00)00088-9
Hanna-Pladdy, B. (2001). Cortical and subcortical contributions to ideomotor apraxia : Analysis of task demands and error types. Brain, 124(12), 2513–2527. https://doi.org/10.1093/brain/124.12.2513
Hanna-Pladdy, B., Heilman, K. M., & Foundas, A. L. (2003). Ecological implications of ideomotor apraxia : Evidence from physical activities of daily living. Neurology, 60(3), Article 3. https://doi.org/10.1212/WNL.60.3.487
Happé, F., Brownell, H., & Winner, E. (1999). Acquired ‘theory of mind’ impairments following stroke. Cognition, 70(3), 211–240. https://doi.org/10.1016/S0010-0277(99)00005-0
Heath, M., Roy, E. A., Black, S. E., & Westwood, D. A. (2001). Intransitive limb gestures and apraxia following unilateral stroke*. Journal of Clinical and Experimental Neuropsychology, 23(5), Article 5. https://doi.org/10.1076/jcen.23.5.628.1240
Hermsdörfer, J., Hentze, S., & Goldenberg, G. (2006). Spatial and kinematic features of apraxic movement depend on the mode of execution. Neuropsychologia, 44(10), 1642–1652. https://doi.org/10.1016/j.neuropsychologia.2006.03.023
Higgins, W. C., Ross, R. M., Langdon, R., & Polito, V. (2023). The “reading the mind in the eyes” test shows poor psychometric properties in a large, demographically representative U.S. sample. Assessment, 30(6), 1777–1789. https://doi.org/10.1177/10731911221124342
Hodges, J. R. (2000). The role of conceptual knowledge in object use evidence from semantic dementia. Brain, 123(9), Article 9. https://doi.org/10.1093/brain/123.9.1913
Hogrefe, K., Ziegler, W., Weidinger, N., & Goldenberg, G. (2012). Non-verbal communication in severe aphasia : Influence of aphasia, apraxia, or semantic processing? Cortex, 48(8), Article 8. https://doi.org/10.1016/j.cortex.2011.02.022
Holler, J., & Stevens, R. (2007). The effect of common ground on how speakers use gesture and speech to represent size information. Journal of Language and Social Psychology, 26(1), 4–27. https://doi.org/10.1177/0261927X06296428
Jackson, H. (1866). Notes on the physiology and pathology of language. Brain, 38, 48–58.
Jason, G. W. (1985). Gesture fluency after focal cortical lesions. Neuropsychologia, 23(4), Article 4. https://doi.org/10.1016/0028-3932(85)90002-8
Kaya, K., Unsal-Delialioglu, S., Kurt, M., Altinok, N., & Ozel, S. (2006). Evaluation of ideomotor apraxia in patients with stroke : A study of reliability and validity. Journal of Rehabilitation Medicine, 38(2), Article 2. https://doi.org/10.1080/16501970500312255
Kittel, A. F. D., Olderbak, S., & Wilhelm, O. (2022). Sty in the mind’s eye : A meta-analytic investigation of the nomological network and internal consistency of the “reading the mind in the eyes” test. Assessment, 29(5), 872–895. https://doi.org/10.1177/1073191121996469
Knecht, S., Dräger, B., Deppe, M., Bobe, L., Lohmann, H., Flöel, A., Ringelstein, E.-B., & Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123(12), 2512–2518. https://doi.org/10.1093/brain/123.12.2512
Lesourd, M., Le Gall, D., Baumard, J., Croisile, B., Jarry, C., & Osiurak, F. (2013). Apraxia and Alzheimer’s disease : Review and perspectives. Neuropsychology Review, 23(3), Article 3. https://doi.org/10.1007/s11065-013-9235-4
Liepmann, H. (1905). Die linke hemisphere und das handeln. Münchener Medizinische Wochenschrift, 49, 2322–2326.
Liepmann, H. (1920). Apraxie. Ergebnisse der gesamten Medizin, 1, 516–540.
Mengotti, P., Corradi-Dell’Acqua, C., Negri, G. A. L., Ukmar, M., Pesavento, V., & Rumiati, R. I. (2013). Selective imitation impairments differentially interact with language processing. Brain, 136(8), Article 8. https://doi.org/10.1093/brain/awt194
Mengotti, P., Ripamonti, E., Pesavento, V., & Rumiati, R. I. (2015). Anatomical and spatial matching in imitation : Evidence from left and right brain-damaged patients. Neuropsychologia, 79, 256–271. https://doi.org/10.1016/j.neuropsychologia.2015.06.038
Morlaas, J. (1928). Contribution à l’étude de l’apraxie. Legrand.
Mostofsky, S. H., Dubey, P., Jerath, V. K., Jansiewicz, E. M., Goldberg, M. C., & Denckla, M. B. (2006). Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society, 12(03). https://doi.org/10.1017/S1355617706060437
Mozaz, M., Rothi, L. J. G., Anderson, J. M., Crucian, G. P., & Heilman, K. M. (2002). Postural knowledge of transitive pantomimes and intransitive gestures. Journal of the International Neuropsychological Society, 8(7), Article 7. https://doi.org/10.1017/S1355617702870114
Negri, G. A. L., Rumiati, R. I., Zadini, A., Ukmar, M., Mahon, B. Z., & Caramazza, A. (2007). What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology, 24(8), Article 8. https://doi.org/10.1080/02643290701707412
Olderbak, S., & Wilhelm, O. (2020). Overarching principles for the organization of socioemotional constructs. Current Directions in Psychological Science, 29(1), 63–70. https://doi.org/10.1177/0963721419884317
Ortiz, K. Z., & Mantovani-Nagaoka, J. (2017). Limb apraxia in aphasic patients. Arquivos de Neuro-Psiquiatria, 75(11), Article 11. https://doi.org/10.1590/0004-282x20170150
Osiurak, F. (2014). What neuropsychology tells us about human tool use? The four constraints theory (4CT): Mechanics, space, time, and effort. Neuropsychology Review, 24(2), 88–115. https://doi.org/10.1007/s11065-014-9260-y
Osiurak, F., Reynaud, E., Baumard, J., Rossetti, Y., Bartolo, A., & Lesourd, M. (2021). Pantomime of tool use : Looking beyond apraxia. Brain Communications, fcab263. https://doi.org/10.1093/braincomms/fcab263
Papadatou-Pastou, M., Ntolka, E., Schmitz, J., Martin, M., Munafò, M. R., Ocklenburg, S., & Paracchini, S. (2020). Human handedness : A meta-analysis. Psychological Bulletin, 146(6), 481–524. https://doi.org/10.1037/bul0000229
Papagno, C., Della Sala, S., & Basso, A. (1993). Ideomotor apraxia without aphasia and aphasia without apraxia : The anatomical support for a double dissociation. Journal of Neurology, Neurosurgery & Psychiatry, 56(3), 286–289. https://doi.org/10.1136/jnnp.56.3.286
Papeo, L., & Rumiati, R. I. (2013). Lexical and gestural symbols in left-damaged patients. Cortex, 49(6), Article 6. https://doi.org/10.1016/j.cortex.2012.09.003
Pavlova, M. A., & Sokolov, A. A. (2022). Reading language of the eyes. Neuroscience & Biobehavioral Reviews, 140, 104755. https://doi.org/10.1016/j.neubiorev.2022.104755
Pazzaglia, M., Smania, N., Corato, E., & Aglioti, S. M. (2008). Neural underpinnings of gesture discrimination in patients with limb apraxia. Journal of Neuroscience, 28(12), Article 12. https://doi.org/10.1523/JNEUROSCI.5748-07.2008
Quental, N. B. M., Brucki, S. M. D., & Bueno, O. F. A. (2013). Visuospatial function in early Alzheimer’s disease—the use of the visual object and space perception (VOSP) battery. PLoS ONE, 8(7), e68398. https://doi.org/10.1371/journal.pone.0068398
Quesque, F., & Rossetti, Y. (2020). What do theory-of-mind tasks actually measure? Theory and practice. Perspectives on Psychological Science, 15(2), 384–396. https://doi.org/10.1177/1745691619896607
Raade, A. S., Gonzalez Rothi, L. J., & Heilman, K. M. (1991). The relationship between buccofacial and limb apraxia. Brain and Cognition, 16(2), Article 2. https://doi.org/10.1016/0278-2626(91)90002-P
Rapcsak, S. Z., Ochipa, C., Beeson, P. M., & Rubens, A. B. (1993). Praxis and the right hemisphere. Brain and Cognition, 23(2), 181–202. https://doi.org/10.1006/brcg.1993.1054
Reynaud, E., Lesourd, M., Navarro, J., & Osiurak, F. (2016). On the neurocognitive origins of human tool use : A critical review of neuroimaging data. Neuroscience & Biobehavioral Reviews, 64, 421–437. https://doi.org/10.1016/j.neubiorev.2016.03.009
Reynaud, E., Navarro, J., Lesourd, M., & Osiurak, F. (2019). To watch is to work : A review of neuroimaging data on tool use observation network. Neuropsychology Review, 29(4), 484–497. https://doi.org/10.1007/s11065-019-09418-3
Rothi, L. J., Ochipa, C., & Heilman, K. M. (1991). A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology, 8(6), Article 6. https://doi.org/10.1080/02643299108253382
Rothi, L. J. G., Ochipa, C., & Heilman, K. M. (1997). A cognitive neuropsychological model of limb praxis and apraxia. In L. J. G. Rothi & K. M. Heilman (Eds.), Apraxia: The neuropsychology of action (pp. 29–49). Psychology Press/Erlbaum (UK) Taylor & Francis. (Reprinted in modified form from Cognitive Neuropsychology, 8, 1991, pp. 443–458).
Roy, E. A., & Square, P. A. (1985). Common considerations in the study of limb, verbal and oral apraxia. In Advances in psychology (Vol. 23, p. 111‑161). Elsevier. https://doi.org/10.1016/S0166-4115(08)61139-5
Roy, E. A., Square-storer, P., Hogg, S., & Adams, S. (1991). Analysis of task demands in apraxia. International Journal of Neuroscience, 56(1‑4), Article 1‑4. https://doi.org/10.3109/00207459108985414
Schnider, A., Hanlon, R. E., Alexander, D. N., & Benson, D. F. (1997). Ideomotor apraxia : behavioral dimensions and neuroanatomical basis. Brain and Language, 58(1), Article 1. https://doi.org/10.1006/brln.1997.1770
Schwoebel, J., Buxbaum, L. J., & Branch Coslett, H. (2004). Representations of the human body in the production and imitation of complex movements. Cognitive Neuropsychology, 21(2–4), Article 2–4. https://doi.org/10.1080/02643290342000348
Sekine, K., & Rose, M. L. (2013). The relationship of aphasia type and gesture production in people with aphasia. American Journal of Speech-Language Pathology, 22(4), Article 4. https://doi.org/10.1044/1058-0360(2013/12-0030
Stamenova, V., Roy, E. A., & Black, S. E. (2010). Associations and dissociations of transitive and intransitive gestures in left and right hemisphere stroke patients. Brain and Cognition, 72(3), Article 3. https://doi.org/10.1016/j.bandc.2010.01.004
Stamenova, V., Black, S. E., & Roy, E. A. (2012). An update on the conceptual–production systems model of apraxia : Evidence from stroke. Brain and Cognition, 80(1), Article 1. https://doi.org/10.1016/j.bandc.2012.03.009
Stieglitz Ham, H., Bartolo, A., Corley, M., Rajendran, G., Szabo, A., & Swanson, S. (2011). Exploring the relationship between gestural recognition and imitation : Evidence of dyspraxia in autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(1), 1–12. https://doi.org/10.1007/s10803-010-1011-1
Stieglitz Ham, H., Bartolo, A., Corley, M., Swanson, S., & Rajendran, G. (2010). Case report : Selective deficit in the production of intransitive gestures in an individual with autism. Cortex, 46(3), 407–409. https://doi.org/10.1016/j.cortex.2009.06.005
Straube, B., Green, A., Bromberger, B., & Kircher, T. (2011). The differentiation of iconic and metaphoric gestures : Common and unique integration processes. Human Brain Mapping, 32(4), 520–533. https://doi.org/10.1002/hbm.21041
Sunderland, A., & Shinner, C. (2007). Ideomotor apraxia and functional ability. Cortex, 43(3), Article 3. https://doi.org/10.1016/S0010-9452(08)70461-1
Sunderland, A., Bowers, M. P., Sluman, S.-M., Wilcock, D. J., & Ardron, M. E. (1999). Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke, 30(5), 949–955. https://doi.org/10.1161/01.STR.30.5.949
Szaflarski, J. P., Binder, J. R., Possing, E. T., McKiernan, K. A., Ward, B. D., & Hammeke, T. A. (2002). Language lateralization in left-handed and ambidextrous people : fMRI data. Neurology, 59(2), 238–244. https://doi.org/10.1212/wnl.59.2.238
Tessari, A., Canessa, N., Ukmar, M., & Rumiati, R. I. (2006). Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain, 130(4), Article 4. https://doi.org/10.1093/brain/awm003
Unsal-Delialioglu, S., Kurt, M., Kaya, K., Culha, C., & Ozel, S. (2008). Effects of ideomotor apraxia on functional outcomes in patients with right hemiplegia. International Journal of Rehabilitation Research, 31(2), Article 2. https://doi.org/10.1097/MRR.0b013e3282fc0fb9
van Nispen, K., van de Sandt-Koenderman, M., Mol, L., & Krahmer, E. (2016). Pantomime production by people with aphasia : what are influencing factors? Journal of Speech, Language, and Hearing Research, 59(4), Article 4. https://doi.org/10.1044/2015_JSLHR-L-15-0166
Van Overwalle, F. (2009). Social cognition and the brain : A meta-analysis. Human Brain Mapping, 30(3), 829–858. https://doi.org/10.1002/hbm.20547
Vanbellingen, T., Kersten, B., Van Hemelrijk, B., Van de Winckel, A., Bertschi, M., Müri, R., De Weerdt, W., & Bohlhalter, S. (2010). Comprehensive assessment of gesture production : A new test of upper limb apraxia (TULIA): A new test of upper limb apraxia. European Journal of Neurology, 17(1), Article 1. https://doi.org/10.1111/j.1468-1331.2009.02741.x
Vanbellingen, T., Schumacher, R., Eggenberger, N., Hopfner, S., Cazzoli, D., Preisig, B. C., Bertschi, M., Nyffeler, T., Gutbrod, K., Bassetti, C. L., Bohlhalter, S., & Müri, R. M. (2015). Different visual exploration of tool-related gestures in left hemisphere brain damaged patients is associated with poor gestural imitation. Neuropsychologia, 71, 158–164. https://doi.org/10.1016/j.neuropsychologia.2015.04.001
Vingerhoets, G., Acke, F., Alderweireldt, A.-S., Nys, J., Vandemaele, P., & Achten, E. (2012). Cerebral lateralization of praxis in right- and left-handedness : Same pattern, different strength. Human Brain Mapping, 33(4). https://doi.org/10.1002/hbm.21247
Walther, S., Mittal, V. A., Stegmayer, K., & Bohlhalter, S. (2020). Gesture deficits and apraxia in schizophrenia. Cortex, 133, 65–75. https://doi.org/10.1016/j.cortex.2020.09.017
Walther, S., Vanbellingen, T., Müri, R., Strik, W., & Bohlhalter, S. (2013). Impaired gesture performance in schizophrenia : Particular vulnerability of meaningless pantomimes. Neuropsychologia, 51(13), 2674–2678. https://doi.org/10.1016/j.neuropsychologia.2013.08.017
Weiss, P. H., Ubben, S. D., Kaesberg, S., Kalbe, E., Kessler, J., Liebig, T., & Fink, G. R. (2016). Where language meets meaningful action : A combined behavior and lesion analysis of aphasia and apraxia. Brain Structure and Function, 221(1), Article 1. https://doi.org/10.1007/s00429-014-0925-3
Willems, R. M., Özyürek, A., & Hagoort, P. (2007). When language meets action : The neural integration of gesture and speech. Cerebral Cortex, 17(10), 2322–2333. https://doi.org/10.1093/cercor/bhl141
Xu, J., Gannon, P. J., Emmorey, K., Smith, J. F., & Braun, A. R. (2009). Symbolic gestures and spoken language are processed by a common neural system. Proceedings of the National Academy of Sciences, 106(49), Article 49. https://doi.org/10.1073/pnas.0909197106
Yang, J., Andric, M., & Mathew, M. M. (2015). The neural basis of hand gesture comprehension : A meta-analysis of functional magnetic resonance imaging studies. Neuroscience & Biobehavioral Reviews, 57, 88–104. https://doi.org/10.1016/j.neubiorev.2015.08.006
Zwinkels, A., Geusgens, C., van de Sande, P., & van Heugten, C. (2004). Assessment of apraxia : Inter-rater reliability of a new apraxia test, association between apraxia and other cognitive deficits and prevalence of apraxia in a rehabilitation setting. Clinical Rehabilitation, 18(7), 819–827. https://doi.org/10.1191/0269215504cr816oa
Funding
This work was supported by grants from Region Normandie (“PEREMO” Project; JB and VB), from the University of Rouen Normandie (UNIROUEN, ED 556 HSRT, RIN Doctorant 2020 100%; JB), from the French National Research Agency (ANR; Project TECHNITION: ANR-21-CE28-0023-01; FO and ML) and the Région Auvergne-Rhône-Alpes (NUMERICOG-2017-900-EA 3082 EMC-R-2075; FO).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the work, proofread the manuscript, and approved the version to be published. The specific contributions were as follows: Josselin Baumard: conception and design, data collection and analysis, interpretation, drafting, and submission process. Alice Laniepce: data collection and analysis. Mathieu Lesourd: conception and design. Léna Guézouli: conception and design. Virginie Beaucousin: data collection and analysis. Maureen Gehin: conception and design, data collection, and analysis. François Osiurak: conception and design. Angela Bartolo: conception and design.
Corresponding author
Ethics declarations
Ethics Approval
This is a meta-analysis of already published studies. We have followed the PRISMA guidelines. The data given in the supplement were from healthy human participants who all gave informed consent to participate in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baumard, J., Laniepce, A., Lesourd, M. et al. The Neurocognitive Bases of Meaningful Intransitive Gestures: A Systematic Review and Meta-analysis of Neuropsychological Studies. Neuropsychol Rev (2024). https://doi.org/10.1007/s11065-024-09634-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11065-024-09634-6