Abstract
Autism spectrum disorder (ASD) is known as a group of neurodevelopmental conditions including stereotyped and repetitive behaviors, besides social and sensorimotor deficits. Anatomical and functional evidence indicates atypical maturation of the striatum. Astrocytes regulate the maturation and plasticity of synaptic circuits, and impaired calcium signaling is associated with repetitive behaviors and atypical social interaction. Spontaneous calcium transients (SCT) recorded in the striatal astrocytes of the rat were investigated in the preclinical model of ASD by prenatal exposure to valproic acid (VPA). Our results showed sensorimotor delay, augmented glial fibrillary acidic protein -a typical intermediate filament protein expressed by astrocytes- and diminished expression of GABAA-ρ3 through development, and increased frequency of SCT with a reduced latency that resulted in a diminished amplitude in the VPA model. The convulsant picrotoxin, a GABAA (γ-aminobutyric acid type A) receptor antagonist, reduced the frequency of SCT in both experimental groups but rescued this parameter to control levels in the preclinical ASD model. The amplitude and latency of SCT were decreased by picrotoxin in both experimental groups. Nipecotic acid, a GABA uptake inhibitor, reduced the mean amplitude only for the control group. Nevertheless, nipecotic acid increased the frequency but diminished the latency in both experimental groups. Thus, we conclude that striatal astrocytes exhibit SCT modulated by GABAA-mediated signaling, and prenatal exposure to VPA disturbs this tuning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is characterized by social and sensorimotor impairments, with repetitive patterns of interest and behaviors. On the other hand, valproic acid (VPA) is an antiepileptic medication also effective for bipolar disorder. However, administration of VPA during pregnancy increases the risk of ASD in offspring, and a single prenatal dose is used as a preclinical model [1, 2]. Motor stereotypies and repetitive behaviors observed in ASD are commonly associated to the dorsolateral striatum, while disordered aggregation of striosomal cells reported in the VPA model could potentially be related to social and sensorimotor impairments [3, 4]. The cellular organization of the striatum includes medium spiny neurons (MSNs; 95%) and interneurons (cholinergic and GABAergic). The expression of dopamine receptors D1 or D2 identifies MSNs of the direct or indirect pathways, respectively [5]. Communication between MSNs (D1 and D2) and astrocytes is circuit specific [6]. Interestingly, MSNs showed reduced tonic inhibition when GABA transporter 3 (GAT-3) expression was increased by calcium transient attenuation in astrocytes, affecting the striatal microcircuitry in vivo and leading to self-grooming behavior, associated with human obsessive-compulsive disorder [7]. Calcium release from intracellular stores requires activation of type 2 inositol 1,4,5-trisphosphate receptors (IP3R2) in astrocytes, and gene mutations of this receptor are linked to ASD. Accordingly, conditional knockout of the astrocytic IP3R2 in the prefrontal cortex resulted in reduced purinergic gliotransmission with abnormal social interaction and repetitive behavior [8]. Overall, these data strongly suggest that calcium signaling in astrocytes may be perturbed by ASD. Thus, we studied SCT and modulation by GABAA receptors is striatal astrocytes, and tested if this signaling is disturbed in mice prenatally exposed to VPA.
Materials and Methods
Animals
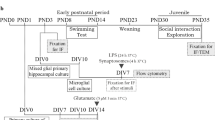
Mice were handled according to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and the Institutional Committee for the Care and Use of Laboratory Animals of Instituto de Neurobiología - UNAM. Briefly, pregnancy of Wistar rats or the transgenic mouse line GFAP-eGFP [9] were confirmed by a vaginal plug at embryonic day 0 (E0). The pregnant rodents were housed individually under controlled temperature and light/dark cycle (12/12 h); food and water were available ad libitum. Sterilized saline solution (0.9%) or VPA (Sigma-Aldrich, St. Louis, MO, U.S.) were administered in a single intraperitoneal injection on E12.5 for Wistar rats (500 mg/Kg) [10]. Only male pups were selected based on ASD incidence (4:1) [11,12,13]. At least three litters were used for each experimental group and animals (N) for histological and behavioral studies, while the number of slices (n) is referred for calcium imaging experiments.
Sensorimotor Testing
Sensorimotor impairments in pups (P8) were identified with a prognostic behavioral test that estimated the latency to reach the nest for control (CTL) and VPA groups (Nctl = 54, Nvpa = 68) as previously described [10]. The experiment ended once the head of the mouse touched the home bedding [1, 2, 10].
Western Blot Analysis
Five independent sets of protein samples isolated from the striatum were collected in five different stages of development (E16 to P30): E16 (Nctl=8; Nvpa=8), P4 (Nctl=12; Nvpa=12), P8 (Nctl=16; Nvpa=16), P18 (Nctl=16; Nvpa=16), P30 (Nctl=14; Nvpa=16). Briefly, the tissue was homogenized in iced-cold glycine lysis buffer (in mM: 300 sucrose, 200 Glycine, 150 NaCl, 50 EDTA, 50 EGTA, pH 9.0) and protease inhibitor (Sigma-Aldrich, St. Louis, MO, U.S.), followed by isolation and quantification of the protein with a Bradford assay (Bio-Rad, Hercules, CA, U.S.) [14]. The protein (10 µg per lane) was resolved in a 10% polyacrylamide gel, transferred to PVDF membranes, blocked with 5% non-fat dry milk in Tris-buffered saline (TBS), 0.1% Tween 20 (TBS-T) for 3 h at room temperature. The membranes were incubated with the primary antibody goat polyclonal anti-GFAP 1:1,000 (Santa Cruz, Dallas, TX, U.S.), rabbit polyclonal anti- GABAA-ρ3 1:1,000 (Santa Cruz, Dallas, TX, U.S.) or rabbit anti-Actin 1:1,000 (Santa Cruz, Dallas, TX, U.S.) overnight at 4 °C. The membranes were rinsed with TBS-T (3 × 15 min each) and incubated (3 h) with the corresponding antibodies (rabbit anti-goat IgG-AP or goat anti-rabbit IgGAP; 1:2,000) (Santa Cruz, Dallas, TX, U.S.), washed again with TBS-T (15 min, 3 x) and detected through alkaline phosphatase activity with the BCIP/NBT AP-conjugate substrate reaction kit (Bio-Rad, Hercules, CA, U.S.). Western blot images were acquired with the image-based Gel Doc™ EZ Gel Documentation System (Bio-Rad, Hercules, CA, U.S.). Image Lab 3.0 software (BioRad, Hercules, CA, U.S.) was used for optical density estimation and normalized with the β-Actin bands.
Staining and Functional Identification Of Astrocytes
Sulforhodamine 101 (SR101), a red fluorescent xanthene derivative is widely used for astrocyte identification. SR101 uptake was observed in 92% of the GFAP-EGFP+ cells and approximately 1/3 of the SR101+ cells were GFAP-EGFP+ (data not shown). Astrocytes uptake SR101 through the thyroid hormone transporter OATP1C1 [15]. Thus, slices were incubated for 20 min with 1 µM of sulforhodamine 101 (SR101) added to the artificial cerebrospinal fluid (aCSF) at 37 °C. The slices were rinsed for 10 min [15, 16]. Striatal astrocytes were identified by SR101 staining in coronal slices, and 94% of the SR101 + cells uploaded the calcium indicator Fluo-4AM (378/404 cells, n = 12, N = 6). Astrocytes express purinergic receptors and functional response to ATP results in evoked calcium transients [17]. Thus, extracellular application of ATP (100 µM) on striatal slices evoked intracellular calcium transients in 88% of SR101 + cells (332/378 cells, n = 12, N = 6) (Suppl. Figure 1). We conclude that SR101 is a confident tool for identification of striatal astrocytes.
Brain Slices and Calcium Imaging
The protocol was described before [18,19,20]. Briefly, coronal slices (250 μm) containing the striatum were obtained from Wistar rats (P8-P10) with a vibratome (VT1000s, Leica) and transferred to ice-cold oxygenated aCSF (in mM: 134 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, 1.25 K2HPO4, pH = 7.4). The slices were recovered (30 min at least), in oxygenated aCSF and incubated further (30–40 min, at 37 °C) with Fluo-4 AM (10 µM, AAT Bioquest, Sunnyvale, CA, U.S.). The slices were rinsed for 30 min with aCSF, transferred to the recording chamber and perfused with oxygenated aCSF (2 ml/min) at room temperature (20–22 °C). Calcium imaging experiments were performed under a cooled camera (SensiCam; PCO.Edge 4.2, Kelheim, Germany) coupled to an Olympus upright microscope (BX51WI, Miami, FL, U.S.) and X-Cite X-LED1 module (Lumen Dynamics Fremont, CA, U.S.) [18, 19]. Additionally, Picrotoxin (PTX; 50 µM) or Nipecotic acid (NIP; 100 µM) were added to the aCSF preincubated (∼2 min) and perfused during calcium imaging recordings. Astrocytes located in the dorsal striatum were selected for imaging. Briefly, individual cells were selected as regions of interests. The protocol for image acquisition was for 300s at 1 Hz with a X-Cite XLED1 module (Excelitas Technologies). ImageJ/FIJI and OriginPro 8 software were used for image processing and data analysis [20,21,22]. SCT were only considered for analysis when they were greater than twice the standard deviation of basal noise. SCT are reported as relative changes in fluorescence (ΔF/F). SCT kinetics were analyzed in ClampFit10.4 (Molecular Devices) and OriginPro 8 (Origin Labs. Northampton, MA.U.S.).
Statistical Analysis
Graphs and statistical analysis were performed with OriginPro 8 and GraphPad Prism software. The analysis included the Shapiro-Wilk test for normal distribution. Data are mean ± S.E.M. Parametric tests were applied to data sets with normal distribution, otherwise non-parametric tests were used. Student’s two-tailed t-tests (paired and unpaired) and two-tailed Mann–Whitney tests, with significances at P < 0.05, were applied for most statistical analyses. One-way or two-way ANOVA tests were used for data sets with more than two conditions, followed by Bonferroni post-hoc tests.
Results
Sensorimotor Delay and Increased Expression of Striatal GFAP
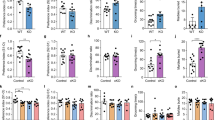
The sensorimotor performance of mice was tested for both experimental groups (CTL and VPA). The latency to reach the nest showed a significant increase in the VPA group (73.2 ± 8.2 s, Nvpa = 68, p = 0.0002) when compared to CTL (35.9 ± 3.1 s, Nctl = 54) (Fig. 1A).
Western blot studies showed that GFAP expression was significantly augmented by VPA through development (from E16 to P30). The peak of GFAP expression was observed on P4 (+ 30%) (Fig. 1B). We conclude that prenatal exposure to VPA consistently increases GFAP expression through the postnatal development of the striatum.
Sensorimotor deficits and increased GFAP expression in the VPA model. (A) The latency to reach the nest was significantly augmented by VPA (Nvpa = 68 from) when compared to CTL (Nctl = 54) at P8. (B) Western blot experiments showed that prenatal exposure to VPA resulted in augmented expression of GFAP through development (from E16 to P30). Data were collected from at least three different litters for each developmental stage, in each group (CTL and VPA). Statistical tests used were Shapiro-Wilk and Mann-Whitney (A) and two-way ANOVA followed by a Bonferroni test (B). Data are expressed as mean ± S.E.M., *p < 0.05, **p < 0.01, ***p < 0.001
Prenatal Exposure to VPA Increases the Frequency of Spontaneous Calcium Transients
SCT were recorded in striatal astrocytes from rat brain slices (P8-P10). The heat map revealed an increased frequency of SCT in the VPA group (0.0094+/- 0.0005 Hz; from 1067 cells, nvpa=34, Nvpa=12) when compared to CTL (0.0045+/- 0.0008 Hz; from 1212 cells (nctl=35; Nctl=12). In contrast, latency of the SCT was reduced by VPA (23 +/- 2 s; from 1067 cells) when compared to CTL (34 +/- 3 s; from 1212 cells) (Fig. 2A, B). Overall, SCT amplitudes were reduced by VPA (-39%; 27 ± 5 a.u., p = 0.01 from 172 cells, nvpa=6, Nvpa=6) when compared to CTL (45 ± 5 a.u. from 194 cells, nctl=6, Nctl=6).
Spontaneous calcium transients (SCT) are modulated by GABAA receptors, and the frequency is increased by VPA. (A) Heatmaps showing the temporal course (300 s) of SCT recorded in striatal cells from CTL (left) and VPA (right) experimental groups (B) Summary of the mean frequency and latency recorded in CTL (blue bars) and VPA (magenta bars) groups. The effect of picrotoxin (PTX, 50 µM; a non-competitive antagonist of GABAA receptors) and nipecotic acid (NIP, 100 µM; a GABA uptake inhibitor) was tested for both experimental groups. (C) Summary of the mean amplitudes corresponding to CTL (blue) and VPA (magenta) groups. Overall, PTX (CTL: 194 cells, nctl=6, Nctl= 6; VPA: 172 cells, nvpa=6, Nvpa= 6) and NIP (CTL: 200 cells, nctl=6, Nctl=6; VPA: 188 cells, nvpa=6, Nvpa=6) reduced the mean amplitude in CTL and VPA groups. Data analyzed by Shapiro-Wilk, Mann-Whitney U, *p < 0.05, **p < 0.01, ***p < 0.001. Values are mean ± S.E.M
GABAA Receptors Modulate Spontaneous Calcium Transients
GABAergic transmission is detected by striatal astrocytes, and we tested whether GABAA receptors modulate SCT. The GABAA antagonist PTX (50 µM) reduced the frequency (Control: -77% and VPA: -63%), latency (Control: -47% and VPA: -55%) and amplitude (Control: -91% and VPA: -89%) of SCT (CTL: 194 cells; nctl=6; Nctl= 6; VPA: 172 cells; nvpa=6, Nvpa= 6; p = 0.0001) (Fig. 2B, C).
We also tested NIP (100 µM), a GABA uptake inhibitor, to increase the extracellular concentration of endogenous GABA. A significant increase in the frequency (+ 300%), but a decreased latency (-41%), of SCT was observed in striatal astrocytes (CTL: 194 cells; nctl=6; Nctl= 6). Similarly, NIP promoted a further rise in the mean frequency of SCT (+ 33%), although the mean latency and amplitude were reduced (-36% and − 30%, respectively) in the VPA group (VPA: 172 cells, nvpa=6, Nvpa= 6; p ˂ 0.01) (Fig. 2B, C).
Expression of GABAA-ρ3 Subunit is Diminished by VPA
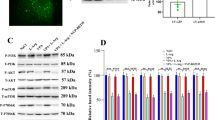
GABAA-ρ3 expression was investigated by western blot studies and a significant reduction was observed through postnatal development of the striatum (Fig. 3). The expression of GABAA-ρ3 increased linearly through development reaching its peak at P30. However, this pattern was significantly diminished in the VPA model. Our conclusion is that GABAergic signaling through cells expressing GABAA-ρ3 is reduced by VPA.
GABAA-ρ3 expression is reduced by prenatal exposure to VPA. Western blot experiments showed that GABAA-ρ3 expression increased linearly, while it was significantly reduced by VPA through development (from E16 to P30). Data were collected from at least three different litters for each developmental stage, in each group (CTL and VPA). Statistical tests used were two-way ANOVA followed by a Bonferroni test, ***p < 0.001. Data are expressed as mean ± S.E.M
Discussion
The dorsolateral striatum is linked to motor stereotypies in mammals, and astrocytes are involved in synapse structure and function [23,24,25]. VPA inhibits histone deacetylase promoting abnormal gene expression and proliferation of astrocytes [26], affecting function and maturation of synaptic circuits through neurodevelopment. Accordingly, sustained increase of GFAP expression was observed through postnatal development of the striatum and correlated with sensorimotor deficits. In agreement, reduced corticostriosomal synapses of striosomal neurons and abnormal ultrasonic vocalizations were seen in this preclinical model of ASD [4]. On the other hand, GABAergic transmission is essential for striatal function, and astrocytes express GABAA receptors [19, 27], but little is known about their role in ASD. In this study we observed sensorimotor deficits, increased expression of GFAP, augmented frequency of SCT recorded in astrocytes, and reduced expression of GABAA-ρ3, supporting a disturbed GABAA-mediated signaling in the VPA model.
Sensorimotor Delay and Increased Expression of GFAP
The severity of ASD has been associated with sensorimotor impairments, and experimental data support earlier examinations of motor coordination and sensory responsivity [28,29,30]. The VPA model provides robust behavioral evidence linked to ASD [1, 2, 31,32,33,34]. Moreover, we recently tested behavioral tasks related to the striatal function such as motor skill abilities, repetitive behaviors, and flexibility to shift habits in the VPA model [35]. Briefly, enhanced motor skill learning (rotarod test) was maintained for at least 15 days. Repetitive and compulsive like behaviors were also evaluated through the marble burying test, but no changes were observed. However, a post-hoc evaluation showed a significant increase of digging episodes in the VPA group, indicating a repetitive motor behavior [35]. The aquatic Y-maze has been used to study autistic features by showing an impairment in the reversal learning test. The dorsolateral striatum is known to be involved in reversal learning and a delayed time to get the correct choice in the aquatic Y-maze test was reported, suggesting impaired decision-making [35]. In this study we used the nest-seeking test as a prognostic tool to identify sensorimotor deficits [10, 20]. Our results showed early sensorimotor delay in the VPA group on P8. Thus, this preclinical model reproduces sensorimotor deficits observed in children diagnosed with ASD [36]. On the other hand, impaired aggregation of striosomal cells into clusters was observed during earlier postnatal development in the VPA model [4]. Astrocytes are a key element for maturation and plasticity of synaptic circuits, and we observed an increased expression of GFAP through neurodevelopment of the striatum. Accordingly, the expression of GFAP also increased in the cortex, hippocampus, and cerebellum of the murine model of VPA [20, 26]. The VPA model mimics augmented expression of GFAP reported in brains of postmortem patients diagnosed with ASD [37,38,39]. These results may reflect a sustained pro-inflammatory environment, but further investigation is required. Overall, social and sensorimotor deficits observed correlate with increased expression of GFAP in the VPA model. Thus, astrocytes are another element to consider in ASD.
Calcium Signaling in Striatal Astrocytes is Impaired by VPA
Astrocyte activation is linked to intracellular calcium transients mediated by IP3R2, and mutations of ITPR2 (the corresponding gene) are correlated with ASD. Accordingly, repetitive and reduced social behaviors were reported after conditional knockout of astrocytic-specific IP3R2 (cKO- IP3R2) in the medial prefrontal cortex of the mouse [8]. The striatum receives cortical inputs, and we recorded SCT in striatal astrocytes, observing an increased frequency but reduced latency and amplitude in the VPA model. Our results support previous findings in which calcium signaling was reduced in astrocytes, after microinjection of adeno-associated viruses with an astrocyte-specific GfaABC1D promoter containing the plasma membrane calcium pump 2 (PMCA2: favors calcium extrusion). The diminished calcium signal in astrocytes (-70%) resulted in augmented grooming, an innate behavior controlled by the dorsal striatum and associated with obsessive-compulsive disorder [7]. Overall, our results show that the calcium code recorded in striatal astrocytes is disturbed in the preclinical model of autism induced by prenatal exposure to VPA.
GABAergic Signaling is Dysregulated in the VPA Model
The dorsal striatum is involved in self-grooming, and increasing GABA pharmacologically decreases this behavior [40]. Astrocytes regulate striatal MSNs via ambient GABA-mediated neuromodulation, and the GABA transporter 1 (GAT-1) regulates tonic inhibition in the synaptic circuits of the striatum, although GAT-3 becomes relevant when calcium transients are reduced, resulting in increased grooming [7]. We tested NIP, a GABA uptake inhibitor, on SCT recorded in striatal astrocytes. NIP augmented the frequency of SCT in CTL but without effect on VPA group. In contrast, the latency and amplitude of SCT were significantly reduced in both experimental groups. Altogether, our results suggest that NIP increases GABAergic signaling and the frequency of SCT recorded in striatal astrocytes (CTL), similarly to what is observed in the VPA group. The VPA model is a strongly validated preclinical model of ASD in which several GABAergic genes are downregulated in the cortex: GAD65 (-21%), GAD67 (-77%), GABRA1 (-54%), GABRA5 (-57%), and GABRB2 (-55%) [41]. Accordingly, GABAergic signaling dysfunction is related to ASD, and previous studies reported downregulation of several GABAA subunits in postmortem studies of ASD patients [42, 43]. Polymorphisms of some GABAA receptor subunits located on chromosome 15q11-q13 (e.g., GABRB3, GABRA5 and GABRG3) are also implicated in ASD [44]. We tested whether GABAA receptors modulate SCT in striatal astrocytes and observed that PTX blocked ≥ 90% of the spontaneous events. Interestingly, the frequency of SCT, but not the amplitude or the latency, was recovered by PTX to control levels in the VPA model. Our results suggest that SCT recorded in striatal astrocytes are modulated by GABAergic signaling through GABAA receptors. This conclusion is supported by whole-cell patch-clamp recordings demonstrating functional expression of GABAA receptors, and immunofluorescence studies showing GABAA-ρ3 expression by ∼ 70% of the GFAP + cells in the striatum of the mouse [19]. Accordingly, western blot studies showed that GABAA-ρ3 expression is significantly diminished through postnatal development of the striatum in the VPA model. Moreover, other murine models of ASD have shown GABAA receptor involvement. For example, GABRB3 gene mutations resulted in a reduced tonic but augmented phasic inhibition associated with altered dendrite spine structure, resulting in repetitive behaviors, and diminished social interaction [45]. Additionally, the expression of GABAA subunits is diminished in the murine model of Rett syndrome (MeCP2+/- mouse brain) [46, 47] and the model of Fragile X syndrome (FMR1 knock-out mouse: α3, α4, β1, β2, γ1, γ2) [48,49,50]. Astrocytes are structural elements of the tripartite synapse and differentially regulate glutamatergic and GABAergic neurons, but the excitatory/inhibitory balance is disturbed by VPA [33, 51]. A recent study showed that co-culture of GABAergic neurons with astrocytes exposed to VPA resulted in a reduced frequency of miniature inhibitory postsynaptic currents (mIPSCs), suggesting a diminished function of the presynaptic terminal. Immunochemical studies confirmed this finding by showing a reduced number of vesicular GABA transporters [52]. Thus, the formation of GABAergic synapses and synaptic neurotransmission are also impaired by VPA-exposed astrocytes, thereby inducing an imbalance in neuronal signaling. Furthermore, VPA exposure promotes gliogenesis and augmented expression of GFAP [26], resulting in morphological hypertrophy and increased number of GFAP + cells in the internal granular layer of the mouse cerebellum [20]. In agreement with these studies, we also observed that prenatal exposure to VPA increased the expression of GFAP, but expression of GABAA-ρ3 was diminished through postnatal development of the striatum. These results support the abnormal GABAergic signaling recorded in striatal astrocytes.
Limitations, Future Directions and Conclusion
Neurodevelopmental disorders like ASD are usually investigated in adult animals and limited studies explore what happen at early stages of postnatal development. A technical limitation for in vivo experiments (for example at P8) is the size and weight of the miniscopes. Thus, we overcome this difficulty by performing calcium imaging studies with coronal slices of the brain containing the striatum (ex-vivo experiments), however, a direct correlation of the results with the sensorimotor test cannot be establish. Future directions should consider chemogenetic manipulation of astrocytes to test the impact on sensorimotor deficits observed in murine models of ASD. Overall, we conclude that sensorimotor deficits, increased expression of GFAP, augmented frequency of SCT recorded in astrocytes, and reduced expression of GABAA-ρ3 support a disturbed GABAA-mediated signaling in the VPA model.
Data Availability
No datasets were generated or analysed during the current study.
References
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally to valproic acid: animal model of autism. Neuropsychopharmacology 30:80–89. https://doi.org/10.1038/sj.npp.1300518
Roullet FI, Wollaston L, deCatanzaro D, Foster JA (2010) Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience 170:514–522. 10.1016/j. neuroscience.2010.06.069
Thabault M, Turpin V, Maisterrena A, Jaber M, Egloff M, Galvan L (2022) Cerebellar and striatal implications in Autism Spectrum disorders: from clinical observations to animal models. Int J Mol Sci 23:2294. https://doi.org/10.3390/ijms23
Kuo HY, Liu FC (2017) Valproic acid induces aberrant development of striatal compartments and corticostriatal pathways in a mouse model of autism spectrum disorder. FASEB J 31:4458–4471. https://doi.org/10.1096/fj.201700054R
Khakh BS (2019) Astrocyte-neuron interactions in the striatum: insights on identity, form, and function. Trends Neurosci 42:617–630. https://doi.org/10.1016/j.tins.2019.06.003
Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. https://doi.org/10.1126/science.aaa7945
Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G et al (2018) Reducing astrocyte Calcium Signaling. Vivo Alters Striatal Microcircuits Causes Repetitive Behav Neuron 99:1170–1187e9. https://doi.org/10.1016/j.neuron.2018.08.015
Wang Q, Kong Y, Wu DY, Liu JH, Jie W, You QL et al (2021) Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat Commun 12:3321. https://doi.org/10.1038/s41467-021-23843-0
Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK et al (2001) GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33:72–86. https://doi.org/10.1002/1098-1136(20010101)33:1%3C72::AID-GLIA1007%3E3.0.CO;2-A
Varman DR, Soria-Ortíz MB, Martínez-Torres A, Reyes-Haro D (2018) GABAρ3 expression in lobule X of the cerebellum is reduced in the valproate model of autism. Neurosci Lett 687:158–163. https://doi.org/10.1016/j.neulet.2018.09.042
Kim KC, Kim P, Go HS, Choi CS, Park JH, Kim HJ et al (2013) Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J Neurochem 124:832–843. https://doi.org/10.1111/jnc.12147
Perez-Pouchoulen M, Miquel M, Saft P, Brug B, Toledo R, Hernandez ME et al (2016) Prenatal exposure to sodium valproate alters androgen receptor expression in the developing cerebellum in a region and age specific manner in male and female rats. Int J Dev Neurosci 53:46–52. https://doi.org/10.1016/j.ijdevneu.2016.07.001
Melancia F, Schiavi S, Servadio M, Cartocci V, Campolongo P, Palmery M et al (2018) Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br J Pharmacol 175:3699–3712. https://doi.org/10.1111/bph.14435
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(76):248–254. https://doi.org/10.1016/0003-2697
Schnell C, Shahmoradi A, Wichert SP, Mayerl S, Hagos Y, Heuer H, Rossner MJ, Hülsmann S (2015) The multispecific thyroid hormone transporter OATP1C1 mediates cell-specific sulforhodamine 101-labeling of hippocampal astrocytes. Brain Struct Funct 220:193–203. https://doi.org/10.1007/s00429-013-0645-0
Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F (2004) Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1:31–37. https://doi.org/10.1038/nmeth706
Hoogland TM, Kuhn B, Göbel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS (2009) Radially expanding transglial calcium waves in the intact cerebellum. Proc Natl Acad Sci U S A 106:3496–3501. https://doi.org/10.1073/pnas.0809269106
Reyes-Haro D, González-González MA, Pétriz A, Rosas-Arellano A et al (2013) γ-Aminobutyric acid-ρ expression in ependymal glial cells of the mouse cerebellum. J Neurosci Res 91:527–534. https://doi.org/10.1002/jnr.23183
Reyes-Haro D, Hernández-Santos JA, Miledi R, Martínez-Torres A (2017) GABAρ selective antagonist TPMPA partially inhibits GABA-mediated currents recorded from neurones and astrocytes in mouse striatum. Neuropharmacology 113:407–415. https://doi.org/10.1016/j.neuropharm.2016.10.024
Soria-Ortiz MB, Reyes-Ortega P, Martínez-Torres A, Reyes-Haro D (2021) A functional signature in the developing cerebellum: evidence from a preclinical model of Autism. Front Cell Dev Biol 9:727079. https://doi.org/10.3389/fcell.2021.727079
Reyes-Haro D, Müller J, Boresch M, Pivneva T, Benedetti B, Scheller A et al (2010) Neuron-astrocyte interactions in the medial nucleus of the trapezoid body. J Gen Physiol 135:583–594. https://doi.org/10.1085/jgp.200910354
Labrada-Moncada FE, Martínez-Torres A, Reyes-Haro D (2020) GABAA receptors are selectively expressed in NG2 glia of the cerebellar white matter. Neuroscience 433:132–143. https://doi.org/10.1016/j.neuroscience.2020.03.003
Lingawi NW, Balleine BW (2012) Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci 32:1073–1081. https://doi.org/10.1523/JNEUROSCI.4806-11.2012
Fuccillo MV (2016) Striatal Circuits as a common node for Autism Pathophysiology. Front Neurosci 10:27. https://doi.org/10.3389/fnins.2016.00027
Petrelli F, Pucci L, Bezzi P (2016) Astrocytes and Microglia and their potential link with Autism Spectrum disorders. Front Cell Neurosci 10:21. https://doi.org/10.3389/fncel.2016.00021
Mony TJ, Lee JW, Kim SS, Chun W, Lee HJ (2018) Early postnatal valproic acid exposure increase the protein level of astrocyte markers in Frontal Cortex of Rat. Clin Psychopharmacol Neurosci 16:214–217. https://doi.org/10.9758/cpn.2018.16.2.214
Rosas-Arellano A, Machuca-Parra AI, Reyes-Haro D, Miledi R, Martínez-Torres A (2012) Expression of GABAρ receptors in the neostriatum: localization in aspiny, medium spiny neurons and GFAP-positive cells. J Neurochem 122:900–910. https://doi.org/10.1111/j.1471-4159.2011.07621.x
Brisson J, Warreyn P, Serres J, Foussier S, Adrien-Louis J (2012) Motor anticipation failure in infants with autism: a retrospective analysis of feeding situations. Autism 16:420–429. https://doi.org/10.1177/1362361311423385
Sacrey LAR, Bennett JA, Zwaigenbaum L (2015) Early infant development and intervention for autism spectrum disorder. J Child Neurol 30:1921–1929. https://doi.org/10.1177/0883073815601500
Hannant P, Cassidy S, Tavassoli T, Mann F (2016) Sensorimotor Difficulties Are Associated with the severity of Autism Spectrum conditions. Front Integr Neurosci 10:28. https://doi.org/10.3389/fnint.2016.00028
Favre MR, Barkat TR, LaMendola D, Khazen G, Markram H, Markram K (2013) General developmental health in the VPA-rat model of autism. Front Behav Neurosci 7:88. https://doi.org/10.3389/fnbeh.2013.00088
Kazlauskas N, Campolongo M, Lucchina L, Zappala C, Depino AM (2016) Postnatal behavioral and inflammatory alterations in female pups prenatally exposed to valproic acid. Psychoneuroendocrinology 72:11–21. https://doi.org/10.1016/j.psyneuen.2016.06.001
Wang R, Tan J, Guo J, Zheng Y, Han Q, So KF et al (2018) Aberrant development and synaptic transmission of cerebellar cortex in a VPA induced mouse autism model. Front Cell Neurosci 12:500. https://doi.org/10.3389/fncel.2018.00500
Tartaglione AM, Schiavi S, Calamandrei G, Trezza V (2019) Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 159:107477. https://doi.org/10.1016/j.neuropharm.2018.12.024
Hernandez A, Delgado-González E, Durairaj RV, Reyes-Haro D, Martínez-Torres A, Espinosa F (2023) Striatal synaptic changes and behavior in adult mouse upon prenatal exposure to valproic acid. Brain Res 1815:148461. https://doi.org/10.1016/j.brainres.2023.148461
Lloyd M, MacDonald M, Lord C (2013) Motor skills of toddlers with autism spectrum disorders. Autism 17:133–146. https://doi.org/10.1177/1362361311402230/
Laurence JA, Fatemi SH (2005) Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum 4:206–210. https://doi.org/10.1080/14734220500208846
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57:67–81. https://doi.org/10.1002/ana.20315
Edmonson C, Ziats MN, Rennert OM (2014) Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism 5:3. https://doi.org/10.1186/2040-2392-5-3
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17:45–59. https://doi.org/10.1038/nrn.2015.8
Chau DK, Choi AY, Yang W, Leung WN, Chan CW (2017) Downregulation of glutamatergic and GABAergic proteins in valproric acid associated social impairment during adolescence in mice. Behav Brain Res 316:255–260. https://doi.org/10.1016/j.bbr.2016.09.003
Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD (2009) GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord 39:223–230. https://doi.org/10.1007/s10803-008-0646-7
Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD (2009) Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum 8:64–69. https://doi.org/10.1007/s12311-008-0075-3
Noroozi R, Taheri M, Ghafouri-Fard S, Bidel Z, Omrani MD, Moghaddam AS et al (2018) Meta-analysis of GABRB3 gene polymorphisms and susceptibility to Autism Spectrum Disorder. J Mol Neurosci 65:432–437. https://doi.org/10.1007/s12031-018-1114-2
Vien TN, Modgil A, Abramian AM, Jurd R, Walker J, Brandon NJ et al (2015) Compromising the phosphodependent regulation of the GABAAR β3 subunit reproduces the core phenotypes of autism spectrum disorders. Proc Natl Acad Sci U S A 112:14805–14810. https://doi.org/10.1073/pnas.1514657112
Jin X, Cui N, Zhong W, Jin XT, Jiang C (2013) GABAergic synaptic inputs of locus coeruleus neurons in wild-type and Mecp2-null mice. Am J Physiol Cell Physiol 304:C844–857. https://doi.org/10.1152/ajpcell.00399.2012
Oyarzabal A, Xiol C, Castells AA, Grau C, O’Callaghan M, Fernández G et al (2020) Comprehensive Analysis of GABAA-A1R Developmental alterations in Rett Syndrome: setting the focus for therapeutic targets in the Time Frame of the Disease. Int J Mol Sci 21:518. https://doi.org/10.3390/ijms21020518
D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA et al (2006) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121:238–245. https://doi.org/10.1016/j.brainres.2006.08.115
Adusei DC, Pacey LK, Chen D, Hampson DR (2010) Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59:167–171. https://doi.org/10.1016/j.neuropharm.2010.05.002
Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V et al (2008) Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry 63:963–973. https://doi.org/10.1016/j.biopsych.2007.09.008
Banerjee A, García-Oscos F, Roychowdhury S, Galindo LC, Hall S, Kilgard MP et al (2013) Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. Int J Neuropsychopharmacol 16:1309–1318. https://doi.org/10.1017/S1461145712001216
Takeda K, Watanabe T, Oyabu K, Tsukamoto S, Oba Y, Nakano T et al (2021) Valproic acid-exposed astrocytes impair inhibitory synapse formation and function. Sci Rep 11:23. https://doi.org/10.1038/s41598-020-79520-7
Acknowledgements
The authors recognize proofreading of the manuscript by Jessica Norris. We acknowledge R. Arellano, A. Martínez-Torres and E. Garay for academic and technical support. We thank the technical support of E. N. Hernández-Ríos, M. Mendoza-Baltazar, A. Castilla, M.C. Mendoza-López, N. Aranda, M. García-Servín.
Funding
This work was supported by Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT-319209) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica -Dirección General de Asuntos de Personal Académico -Universidad Nacional Autónoma de México (PAPIIT-DGAPA-UNAM IN209121 and IN214324) to DR-H.
Author information
Authors and Affiliations
Contributions
H.S-B., D.R.V. and D.R-H. designed experiments. H.S-B. and D.R.V. performed research. H.S-B., D.R.V and D.R-H. contributed data analysis. H.S-B. and D.R-H. wrote the manuscript. Resources and funding acquisition by D.R-H. All authors readed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saavedra-Bonilla, H., Varman, D.R. & Reyes-Haro, D. Spontaneous Calcium Transients Recorded from Striatal Astrocytes in a Preclinical Model of Autism. Neurochem Res (2024). https://doi.org/10.1007/s11064-024-04218-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-024-04218-5