Abstract
Reduced myelin stability observed in the early stages of Alzheimer's disease leads to spatial learning and memory impairment. Exercise has been shown to protect nerves, reduce the risk of Alzheimer's disease, and strengthen synaptic connectivity. However, the underlying mechanisms of how exercise can promote myelin repair and coordinate inflammation and proliferation are still uncertain. In this study, we conducted histological and biochemical assays of cortical lysates after behavioral testing to detect pathological changes, myelin sheath thickness, and mRNA and protein levels. It is notable that D-galactose model mice exhibited elevated miRNA-34a levels, overactive astrocytes, decreased myelin staining scores, increased apoptosis, and decreased synaptic plasticity in the brain. Significantly, after eight weeks of exercise, we observed improvements in LFB scores, NeuN( +) neuron counts, and myelin basic protein (MBP) expression. Additionally, exercise promoted the expression of oligodendrocyte markers Olig2 and PDFGR-α associated with brain proliferation, and improved spatial cognitive function. Furthermore, it decreased the inflammation caused by astrocyte secretions (TNF-α, Cox-2, CXCL2). Interestingly, we also observed downregulation of miR-34a and activation of the TAN1/PI3K/CREB signaling pathway. Our data shed light on a previously unsuspected mechanism by which exercise reduces miR-34a levels and protects neuronal function and survival by preventing excessive demyelination and inflammatory infiltration in the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a spontaneous phenomenon characterized by the accumulation of cellular damage and fragmentation. It is well-recognized that aging is a key causative factor in several neurodegenerative diseases and contributes to the development of neurodegenerative pathologies [1]. Brain aging has become a primary concern due to the increasing number of elderly individuals worldwide. Alzheimer's disease (AD) is characterized by the presence of amyloid deposits and neurofibrillary tangles (NFTs), which lead to abnormal cortical neuronal loss and cognitive impairment [2]. However, the exact link and underlying mechanisms between aging and neurodegenerative diseases such as AD are still uncertain.
miRNAs are small non-coding RNAs, typically consisting of 19–24 peptides in length, thatregulate gene stability and protein expression [3]. Approximately 50% of known miRNAs are found in the brain cortex and Hippocampus. Numerous studies have confirmed that miRNAs play essential roles in inflammation, mitochondrial function, and synaptic plasticity [4]. Furthermore, it has been observed that neurodegenerative disease processes can influence the expression of miRNAs [5]. The up-regulation of miR-34a in the brain has been associated with neuronal apoptosis in age-related diseases like AD [6]. Recent, studies have also recognized the role of miR-34a in regulating neurophysiology and neuropathology [7]. However, there is limited data on the association between exercise and miR-34a signaling pathways.
In recent years, there has been a growing focus on studying the mechanism of aging and AD by examining the damage to the Hippocampus and cortex [8]. MRI images have revealedthat the cortex plays a crucial role inmemory storage and long-term memory formation. Clinical evidences have shown that demyelination, astrocyte activation, and reduced myelin sheath thickness in the brains of aging patients [9,10,11]. Additionally, multimodal imaging have demonstrated changes in brain microstructure, including a decrease in synaptic density and myelin thickness in individuals with Alzheimer's disease [12]. These findings suggesta link between myelin stability and AD, indicating that reducing demyelination and promoting myelin regeneration could be a reliable strategy for treating aging and AD and other neurodegenerative diseases. Furthermore, it was demonstrated that astrocyte hyperactivation induces neuroinflammation aggravating myelin shedding [13]. It is well recognized that AD is a neurodegenerative disease involving multiple systems such as immune, cardiovascular, and lipid metabolism. Given its complex pathological mechanisms, there is no clinical non-pharmacological intervention that can slow down the pathogenesis and pathological deterioration of AD. Therefore, identifying appropriate therapeutic therapies and potential targets is crucial for the treatment of AD.
Exercise is a non-pharmacological treatment that improves health during aging and is a valuable vehicle for diagnosing aging-related diseases. Recent studies have revealed that exercise is vital in promoting neural network connections, boosting autophagy and Neurogenesis in the brain [14], mitigating oxidative stress-induced mitochondrial dysfunction, preventing neuronal apoptosis [6], and improving behavioral performance. Despite the fact that aging and neurodegenerative disease processes are commonly associated with upregulated miR-34a and neuroinflammation, it is unclear if exercise can slow down the aging process by modulating miR-34a-mediated neuroinflammation and decreased myelin stability.
Experimental Procedures
Experimental Animals
The study protocol was approved by the the School of Medicine of Hunan Normal University. Forty-two SPF-grade 8-week-old male C57/BL6 mice (18–22 g) were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd. (Changsha, China) with the certificate number SCXK (Xiang) 2009–0004. Mice were randomly distributedinto three groups: the normal control group (NCL, n = 14), the D-gal aging model sedentary group (DAM + SED, n = 14), and the D-gal aging model exercise group (DAM + EX, n = 14). Separate cages were used to house mice in the animal room of the Hunan Provincial Key Laboratory of Physical Fitness and Exercise Rehabilitation. Water and food are available at a constant temperature of 24 °C to 26 °C in the animal room. Animal feeding follows the relevant national standards (GB14924.3–2010, China). Experiments were performed in strict accordance with the regulations of the Animal Ethics Committee throughout the process (Ethics Sect. 2021 No. 365).
Animals Handling and Drug-Dosing
Mice in the DAM + SED, DAM + EX groups were injected intraperitoneally with 150 mg/kg of D-gal once daily for 9 weeks. The NCL group mice were injected with the same dose of saline.Furthermore, DAM + EX mice were given a subsequent exercise intervention, NCL, while the DAM + SED group remained sedentary during the treatment.
Exercise Paradigms
Excessive or high-intensity exercise is harmful to the body, such as promoting ROS releaseand organ failure. Take inspiration from Sung-Eun Kim et al. 's exercise scheme [15] and make modifications. We established a treadmill training protocolwith increasing load intensity (Fig. 1A).
Treadmill exercise improved phenotypic characteristics, histopathological deterioration and behavioral performancein aging mice. A Treadmill exercise program (Created by Figdraw). B Representative images of mice were observed epigenetic after 8 weeks. C Representative images of HE Staning in cortex. D Representative images of NeuN immunofluorescence in cortex and dentate gyrus. E Number of NeuN ( +) neurons in cortex. F Number of NeuN ( +) neurons in dentate gyrus. G Latency escape in each groups. H The mean swimming speed of each group of mice. I The number of palform crossings in each groups. J The percentage of time in the palform quadrant. K Total swimming distances. L Swimming trajectories of mice in space exploration experiments. Data were expressed as mean ± standard error (n = 6 or 12 per group). *P < 0.05, **P < 0.01, and ***P < 0.001vs. NCL group; #P < 0.05vs. DAM + SED group
Morris Water Maze Test
Morris Water Maze (MWM) test was used to assess spatial learning and memory [16]. The water maze device is composed of a circular pool with a diameter of 110 cm, which is filled with 23 °C water. A platform with a diameter of about 8 cm is placed in the designated quadrant, and the platform is hidden 1.5 cm below the water surface. The hidden platform (from day 1 to day 5) and probe trial (on day 6) was performed. It was considered a success of the experiment when the mice managed to ascend the platform within 60 s. Beyond 60 s, mice were guided to the platform and stayed on it for 10 s. After the positioning and navigation experiment is completed, the platform will be dismantled. The number of times the mice crossed the platform in the aquarium, swimming time, and distance in the aquarium were tracked and recorded. The swimming distance between the platform disk and the entire aquarium is 60 s. All data were recorded by the automatic camera system.
Brainsample Preparation
All mice were anesthetized with sodium pentobarbital injection (50 mg/kg) and decapitated on the following day after the behavioral assay was completed. Brain tissue was rapidly peeled off on ice boxes and placed in centrifuge tubes containing 4% paraformaldehyde for paraffin embedding for qRT-PCR, immunofluorescence, immunohistochemistry, histopathology and TUNEL assays.
Hematoxylin and Eosin (HE) Staining
To evaluate the damage to mice brain tissues, sections were stabilized in 4% paraformaldehyde solution, clarified, embedded into 4 μm paraffin sections, and stained with hematoxylin–eosin (HE). Sections were visualized with a light microscope (Motic BA210), and images were captured with Olympus cellSens standard 1.13 software.
Luxol Fast Blue (LFB) Staining
The wax blocks were cut at 10 µm slices. Sections were stained with Luxol fast blue at 58 °C. Paraffin sections were dewaxed and rehydrated to 95% ethanol, and then placed in 0.1% LFB in a 56 °C oven over overnight. The next day, excess dye was removed with 95% ethanol, rinsed with distilled water, and the sections were then fractionated with 0.05% lithium carbonate, followed by washing, dehydration, and sealing. Myelin densities on LFB-stained photographs were quantified using a procedure based on a modification of that described by Bao et al. [17]. The percentage of LFB staining area was evaluated using Image J 8.0 as a quantitative criterion to quantify myelin damage.
TUNEL Staining
Apoptotic cells in mice brain tissue were identified by TUNEL assay using In Situ Cell Death Detection Kit, Fluorescein (Roche, Rotkreuz, Switzerland). Briefly, the assay was based on the detection of DNA fragmentation caused by apoptotic signaling cascades by labeling DNA strand breaks using terminal deoxynucleotidyl transferase.
Real Time-PCR Detection
The brain tissue was removed from the -80 °C refrigerator. Total RNA was extracted from the cortex using the Ultra Pure Total RNA Extartion Kit (Simgen, Hangzhou, China). Mir-X miRNA First-Strand Synthesis Kit and PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Biomedical Technology (Wuhan) Co., Ltd., Wuhan, China) were applied to miRNA and mRNA reverse transcription reactions, respectively. The primer sequences are shown in Table 1. Products were measured by a RT-qPCR fluorescence analysis system (CFX96, Bio-Rad, Hercules, CA, USA). The relative mRNA expression was calculated by the 2 − ΔΔCt method. The U6 served as the reference gene to normalize miR-34a expression.
Protein Quantitation
The protein was separated by SDS-PAGE gel electrophoresis and subsequently transferred to the PVDF membrane. 5% skimmed milk powder closed for 1.5 h, TBST washed 4 times, 5 min each time. The membrane was incubated overnight at 4 °C with primary antibodies. The next day, the membrane was washed with TBST and incubated with the secondary antibody in a shaker for 1–2 h at room temperature. After rinsing, bands were visualized by enhanced chemiluminescence (Servicebio, Wuhan, China) with Tanon 5200 chemiluminescence imaging system (Tanon, Shanghai, China). The grayscale value of the protein band was semi-quantitatively calculated using ImageJ software, β-actin as internal reference.
Immunohistochemistry
Brain sections were subjected to standard dewaxing procedures, followed by incubation with a 3 g/L hydrogen peroxide solution at room temperature in the dark. Subsequently, high-pressure antigen retrieval was performed, and rabbit anti-rat antibodies targeting Bax (at a dilution of 1:200), GFAP (at a dilution of 1:400), and Bcl2 (at a dilution of 1:200) were applied and allowed to incubate overnight at 4 °C. Following theincubation and washing steps, secondary antibodies were added dropwise, and DAB develops color. Set up positive and negative controls, replacing primary antibodies with PBS as negative controls. Sections were photographed with an ordinary fluorescence microscope and three fields of view were randomly selected from each group to count the number of positive cells.
Prediction of Target Genes for miR-34a
The potential target genes of miR-34a were predicted by four online tools, including miRDB (http://www.mirdb.org/), miRwalk (http://mirwalk.umm.uni-heidelberg.de/), and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php), ENCORI (http://starbase.sysu.edu.cn/index.php). The miRDB, miRwalk, and miRTarBase databases were parameterized with "Search for target gene", and "Mouse" species, and the final results are exported as "miRNA Find". For ENCORI, it was necessary to execute the "miRNA-mRNA" command first, and then repeated the above operation to export the target gene results. Finally, we used Veeny 2.1.0 to visualize and analyze the overlapping genes.
mirPath for Go and KEGG Analysis
A comprehensive suite of tools is provided by mirPath (http://www.microrna.gr/miRPathv3), enabling researchers to fathom large lists of genes and their biological functions [18]. The miR-34a family and its downstream target genes were analyzed by Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis using the mirPath database. The results were visualized using the "ggplot2" package from the R project, with statistical significance set at P < 0.05.
Protein–Protein Interaction (PPI) Network Construction
The PPI network was structured by utilizing the STRING database [19]. The confidence limit for the PPI analysis was set to 0.4 and considered statistically significant. Cytoscape 3.8.2 was applied to visualize the PPI network [20]. The Cytoscape plug-in MCODE was used to filter the significant modules in the PPI network.
Immunofluorescence Staining
Sections of 50 μm thickness were cut along the coronal plane. Immunofluorescence staining was performed to observe the expression of NeuN, PSD-95, phosphorylated AKT, and phosphorylated CREB proteins. Rabbit anti-NeuN (#MAB377, Millipore), Rabbit anti-PSD95 (ab13552, Abcam), rabbit anti-phosphorylated AKT (ab81283, Abcam), rabbit anti-phosphorylated CREB (ab254107, Abcam), and secondary anti-Alexa Fluor® 488 AffiniPure goat anti-rabbit IgG (H + L) (111–545-003, Jackson ImmunoResearch, West Grove, PA, USA) for PSD-95, p-AKT, and p-CREB were utilized in the incubation procedure. The immunofluorescence images were obtained using an automated scanning system (C13210-01; Hamamatsu Photonics, Hamamatsu, Japan).
Statistical Analysis
All data in graphs and tables expressed as mean ± standard deviation were analysed using Graph Prism 8.0 Software package. One-way analysis of variance (ANOVA) was used for comparison between groups and LSD-T test for comparison within groups. P < 0.05 was defined as the cutoff level for significant difference.
Results
Effects of Exercise on the Epigenetics and Histopathology
Figure 1B depicts the epigenetic-resulting changes observed in the mice. When compared to NCL, D-gal leads to typical features of aging such as lusterless hair, hair loss, and graying of the coat. Whereas, exercise significantly improved this deteriorating performance. In addition, treadmill exercise remarkablydecreased the cortical interstitial space, increased the number of NeuN ( +) neurons in the cortex and dentate gyrus, and improved the capacity of brain protein synthesis (Fig. 1C–F). The above results indicated that our aging model was successfully constructed and exercise significantly ameliorated nerve damage and delayed disease progression.
Exercise Improved Spatial Cognitive Performance
Throughout the whole experiment, all groups of mice exhibited a consistent decline in escape latency. Beginning on the third day, the escape latency of mice in the DAM + SED group was significantly increased compared to the NCL group. Timepoints when exercise significantly reduced escape latency occurred at the beginning of the water maze experiment on days 4, 5, and 6 (Fig. 1G). D-gal treatment significantly decreased the number of platform crossing in the DAM + SED compared with the NCL group (P < 0.05). Alternatively, treadmill exercise increased the number of platform crossings in the DAM + EX group of mice, but there was not a significant difference (Fig. 1I). In addition, exercise increased the residence time of mice in the target quadrant (P < 0.05, Fig. 1J). Notably, exercise did not dramatically affect the mean swimming speed and total swimming distance of the mice (Fig. 1H, K). These results indicated that exercise improved cognitive memory in aging mice, but did not affect swimming capability.
Exercise Reduced Demyelination and Promoted Oligodendrocyte-Mediated Cortical Myelin Regeneration
Research showed that age-related changes in neuron, such as mitochondrial alterations, an increase in oxidative stress, and disruption of paracrine junctional connections, can also affect the integrity of myelin sheaths [9]. Cortical myelin sheath (CMS) was stained using Luxor Fast Blue (LFB) and qRT-PCR for myelin basic protein (MBP). Compared with NCL, mice treated with D-gal showed a 38 ± 2% decrease in cortical LFB staining intensity and a 21 ± 1% decrease in relative MBP mRNA expression levels. Nevertheless, after exercise, the intensity of cortical LFB staining and the relative mRNA expression level of MBP were elevatedin the DAGM + EX group when compared with the DAM + SED group (P < 0.05, Fig. 2A,C).
Treadmill exercise attenuated the deterioration of demyelination and promoted myelin regeneration. A Typical LFB stained Images showing myelin sheath modifications in the cortex. B Positive expression of GFAP measured by immunohistochemistry. C Average optical density values of LFB, GFAP. The scale of the LFB average optical density corresponds to the range of 0–70%. D The relative mRNA expression levels of olig2, MBP, and PDGFR-α were detected by RT-qPCR. E, F The protein expression level of Cox-2 was detected by Western Blot. G Relative expression levels of TNF-α, CXCL2 were detected by RT-qPCR. Data were expressed as Mean ± SD (scale bar: 50 μm). *P < 0.05, **P < 0.01vs. NCL group; #P < 0.05vs. DAM + SED group
We also assayed the mRNA level of Olig2, an oligodendrocyte marker, and PDGFR-α, a marker of oligodendrocyte precursor cells (OPC) in cortical lysates. Olig-2 but not PDGFR-α mRNA relative expression was notably decreased in DAM + SED, while mRNA relative expression with both markers were significantly increased in DAM + EX (P < 0.05, Fig. 2D).
Exercise Attenuated Astrocyte Hyperactivation and Inflammatory Infiltration
Aberrant activation of astrocytes has been linked to neuronal demyelination injury [13]. We measured the level of GFAP expression, an astrocyte-specific marker, using immunohistochemistry, verifying data on LFB staining. Compared to NCL, GFAP labeling intensity increased by 41 ± 1.6% in the DAM + SED group, whereas treadmill exercise decreased the GFAP labeling intensity (P < 0.05, Fig. 2C). Furthermore, we also examined astrocyte-associated inflammatory factors. Our findings revealed that Key pro-inflammatory factors including TNF-α, Cox-2, and CXCL2 expression were increased in the DAM + SED group, while treadmill exercise reversed the inflammatory infiltration in the DAM + EX group (P < 0.05, Fig. 2E–G).
Exercise Alleviated Mitochondria-Dependent Neuronal Apoptosis
Elevated oxidative stress leads to apoptosis of neurons in brain [21]. To analyze the effect of treadmill exercise on neuronal apoptosis, we applied the TUNEL assay. As illustrated in Fig. 4A, TUNEL ( +) cells increased by 24 ± 1.5% following D-gal treatment compared to NCL. However, the data also indicated that aerobic exercise reduced apoptotic neurons in the DAM + EX group (P < 0.05, Fig. 3A, C).
Treadmill exercise alleviated neuronal apoptosis in the cortex from aging mice. A TUNEL staining of cortical sections of mice. B Bax ( +) and Bcl-2 ( +) cells were labeled by immunohistochemistry. C TUNEL-positive cell counts for each group of mice, Number of Bax and Bcl-2-positive cells in sections of three groups of mice. Data were expressed as Mean ± SD (n = 3 per group, scale bar: 50 μm). *P < 0.05, **P < 0.01vs. NCL group; #P < 0.05vs. DAM + SED group
In addition, we also measured the expression of apoptosis-related proteins using immunohistochemistry. As presented in Fig. 3B, Bax ( +) cells were strikinglyincreased in DAM + SED, while cells with Bcl-2 ( +) were increased. However, treadmill exercise significantly reversed this trend (P < 0.05, Fig. 3B, C).
Exercise Enhanced Synaptic Plasticity in the Cerebral Cortex
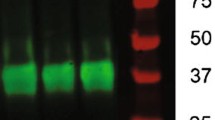
Previous studies have demonstrated the beneficial effects of treadmill exercise on neurons, and it is well-known that synapses are the cornerstone of learning and memory abilities [8]. To further evaluate the effect of myelin thickness restoration on neuronal function in the mice brain, we quantified the expression of synaptic plasticity-related proteins. BDNF, a key brain-derived neurotrophic factor, has been shown to protect neurons and promote neurogenesis and synaptic plasticity during Alzheimer's disease. To evaluate the effects of exercise on synaptic connectivity after D-gal treatment, Brain-derived neurotrophic factor (BDNF), PSD-95 (Postsynaptic membrane dense material), GAP43 (the crucial component of axonal outgrowth), Synaptophysin (SYP, presynaptic marker) were examined by immunofluorescence and Western Blot. As depicted in Fig. 4A–D, the relative expression levels of PSD-95, BDNF, and GAP-43 were decreased in the DAM + SED (P < 0.05, P < 0.01), whereas SYP levels remained unchanged. Surprisingly, exercise increased the expression levels of SYP, PSD-95, BDNF, and GAP-43 (P < 0.05, P < 0.01). These findings suggested that exercise may be positively associated with increasing synaptic plasticity.
Exercise enhanced synaptic plasticity by increasing the expression of PSD-95, BDNF, GAP43, and SYP in mice. A Immunofluorescence for labeling PSD95. B Fluorescence intensity of PSD95. C Representative protein bands of BDNF, GAP43, SYP detected by Western Blot. D Relative expression levels of BDNF, GAP43, SYP Assayed in cortical lysates. All data were expressed as Mean ± SD (n = 3 per group, scale bar: 20 μm). Go and KEGG enrichment analysis of miR-34a target genes was performed using the mirPath database. E Go term analysis of the miR‐34a‐5p target genes. F, G Biological pathways (KEGG database) shared by miR-34a-5p target genes as annotated by the KEGG pathway database. H Expression of miR-34a in three groups of mice assayed by RT-qPCR. G *P < 0.05, **P < 0.01vs. NCL group; #P < 0.05, ##P < 0.01vs. DAM + SED group
Results of GO and KEGG Enrichment Analysis of miR-34a
The results of GO analysis of miR-34a-5p target genes were shown in Fig. 4E. We observed significant enrichment in terms related to biological processes and cellular components. Specifically, the enriched functional processes included biosynthetic processes, cell death, protein complexes, and immunological synapses. In KEGG analysis, we found that the KEGG enrichment pathway was strongly involved in cell growth, development and regeneration as well as the nervous system. Among the numerous significantly enriched pathways, some play a major role in neurological damage during aging and neurodegenerative diseases, such as TNF-a, Notch, and NF-KB signaling pathways (Fig. 4F, G). All items for GO and KEGG analysis were shown in Table S1.
Treadmill Exercise SuppressedmiR-34a in the Brains of Model Mice
To investigate the functional role of miR-34a in regulating brain aging-related lesions, we initially examined the changes in miR-34a expression in the brain after D-gal treatment. Obvious upregulation of miR-34a was observed in the cortex, an in vivo model of aging.
In contrast, it has been previously reported that miR-34a expression decreased in AD model mice's brain tissue after exercise training [6]. In the present study, we further investigated the regulatory effect of exercise on miR-34a in a mice regular treadmill exercise model. Interestingly, we observed that treadmill exercise significantly suppressed miR-34a expression (P < 0.05, Fig. 4H). These data suggested that exercise can effectively inhibit miR-34a expression, prompting us to further explore the downstream targets and signaling pathways associated with miR-34a's involvement in ameliorating brain function.
Translocation-Associated Notch Protein (TAN1) is a Target for miR-34a
We employed four murine-derived database algorithms (Targetscan, ENCORI, miRDB, and miRwalk) to predict the target genes of miR-34a-5p. This analysis aimed to elucidate the molecular mechanisms underlying the amelioration of demyelination and apoptosis by miR-34a-5p. By determining the overlap of the predicted results from all sites, as shown in Fig. 5A, it was found that six genes (TAN1, Sirt1, Foxp1, Sema4b, Bcl2, and Ywhag) were potentially susceptible to miR-34a-5p targeting. We also analyzed the binding sites of the potential candidate gene TAN1 and found conserved sites (Fig. 5B).
Exercise activated the TAN1/PI3K/AKT/CREB signaling pathway. A Venn diagram showing the Genes predicted by four different algorithms to target miR-34a. B Putative binding sequence of miR-34a-5p in the 3′UTR of Notch1 by TargetScan. C Protein protein interaction network analysis of TAN1. D Representative images of Western Blot of TAN1, PTEN, PI3K, p-PI3K, AKT, p-AKT, CREB. E The protein levels of TAN1, PTEN, PI3K, p-PI3K, AKT, p-AKT, CREB assessed in cortical lysates. All data were expressed as Mean ± SD (n = 3 per group). *P < 0.05, **P < 0.01vs. NCL group; #P < 0.05vs. DAM + SED group
The TAN1/PI3K/CREB Signaling Axis Essential for Functional Preservation from Exercise
To further investigate the potential mechanism by which exercise inhibits the progression of aging-associated lesions by regulating miR-34a, we performed the protein interaction network analysis using String. Through this analysis, we identified a tight association between TAN1 and PTEN, an crucial inhibitor of the PI3K pathway (Fig. 5C). Here, our study demonstrated that D-gal induction resulted in elevated PTEN protein expression and significantly decreased p-PI3K/PI3K, p-AKT/AKT ratio, and CREB protein expression in mice. Notably, treadmill exercise significantly decreased PTEN protein expression, increased p-PI3K/PI3K, p-AKT/AKT ratio, and CREB protein expression, and promoted CREB phosphorylation as well as nuclear localization, which was further verified by subsequent immunofluorescence (P < 0.05, Fig. 5D, E). The above results confirmed that exercise contributed to the activation of the PI3K/AKT/CREB signaling pathway.
Exercise Promoted CREB Phosphorylation and Cytosolic Translocation
CREB phosphorylation and nuclear translocation promote myelination and neuronal survival. Therefore, immunofluorescence was used to verify whether nucleocytoplasmic shuttling occurs with p-CREB. Our findings revealed that D-gal remarkably reduced the accumulation in the nucleus of p-CREB to maintain its activity. Nevertheless, treadmill exercise reversed D-gal-induced deficits in p-CREB phosphorylation and nuclear localization (P < 0.05, Fig. 6A, B).
Treadmill exercise enhanced CREB phosphorylation and nucleus accumulation. A Representative immunofluorescence images of p-CREB (red), p-AKT (green), and DAPI (blue) in the cortex. B Average fluorescence density of p-CREB in nucleis. **P < 0.01vs. NCL group; #P < 0.05vs. DAM + SED group. All data were expressed as Mean ± SD (n = 3 per group, scale bar: 20 μm). C Diagram for exercise to alleviate D-gal-induced demyelination of aging mice brain cells through down-regulation of miR-34a (Created by Figdraw). License ID: IOASY9a4a4. Treadmill exercise activates the TAN1/PI3K/AKT/CREB signaling pathway by down-regulating miR-34a levels in the brains of senescent mice, reduces the abnormal activation of astrocytes, improves demyelination and synaptic plasticity, and inhibits neuronal apoptosis, thereby alleviating senescence-like lesions and cognitive dysfunction
Discussion
Currently, there is no non-pharmacological approach to intervene in the treatment of Alzheimer's disease, highlighting the urgent need to explore new potential targets for the development of target-driven therapies. Exercise and its regulated molecules have shown protective effects against age-related brain damage in neurodegenerative diseases [22]. Among a few dysregulated miRNAs, miR-124, miR-125b, miR-34a, miR-155, and miR-21 have been found to exhibit significant alterations in biological fluids and biopsy specimens from AD patients [23]. Nevertheless, the mechanisms of whether exercise would be involved in regulating miR-34a expression and how it affects the altered pathological characteristics of AD remain unclear. In the present study, we demonstrated that treadmill exercise reduced apoptosis, promoted myelin formation, synaptic regeneration, and improved cognitive function in aged mice. These effects were associated with the downregulation of miR-34a-related pathways.
The brain is the organ most susceptible to aging, is often affected in terms of cognitive memory capacity, information processing, and transmission [24]. Numerous studies have proved that the D-gal-induced animal AD model is primarily caused by high doses of D-gal, which leads to glucose metabolism disorders in animals. Consequently, the model exhibits key features of aging, such as cognitive decline, oxidative stress, mitochondrial damage, neuronal loss, etc. [6]. This animal model effectively simulates both the pathological and behavioral changes observed in AD patients, making it commonly utilized in studying the pathogenesis of AD, as well as in drug discovery and development [25]. In ourstudy, we observed that a severe deterioration of cortical pathology in aging mice, characterized by a significant decrease in NeuN ( +) cells. Furthermore, our findings suggest that D-gal treatment impairs behavioral performance in aging mice, further supporting the notion that cognitive decline accompanies aging, consistent with previous studies.
Frontal lobe lesions and astrocyte and oligodendrocyte synergistic axes in Alzheimer affect myelin catabolism. Furthermore, numerous studies have indicated that myelin stability deteriorates with aging, and age-related demyelination is associated with increased apoptosis and dysfunctional nerve regeneration [17]. A study demonstrated that exercise can prevent cognitive deficits and demyelination in APP/PS1 transgenic AD mice [26]. Therefore, the present experiment used LFB staining and qRT-PCR techniques to observe the demyelination among the different groups. As we expected, LFB staining showed significant demyelination in the DAM + SED group. MBP is currently the only known myelin structural protein necessary for the formationof myelin sheaths [26]. Aerobic exercise attenuates abnormal myelination and oligodendrocyte differentiation while promoting brain MBP expression in 3xTg-AD mice [27]. Similarly, the relative expression of MBP mRNA was decreased in the DAM + SED group compared to NCL. These findings indicated that aging may lead to memory and cognitive deficits, resulting in decreased MBP expression and myelin sheaths thickness in brain tissues, which is consistent with Dan Qiu et al. [26]. Numerous data suggest that physical exercise is important in maintaining homeostasis in the brain environment and ameliorating aging-related pathologies [28, 29]. Additionally, a recent study has confirmed that running exercise can improve cognitive performance and delay the pathological progression of demyelination of brain nerve fibers in APP/PS1 mice [26]. Rather, our findings also demonstrated that myelin thickness and MBP expression levels were significantly higher in the DAM + EX group than those in the DAM + SED group, providing further support for the beneficial effects of exercise in delaying demyelinating lesions.
While the hippocampus has been the focus of memory research for the past six decades, recent studies have confirmed that cortical integration serves as the foundation of spatial cognition. The cortex integrates information before transmitting it to the hippocampus for processing and the formation of memories, which is known as the cortical-hippocampal network [30]. It is widely known that synapses are fundamental to cognitive function and myelin plays a role in the excitatory transmission of nerve fiber axons [31]. Previous studies have demonstrated that exercise enhances neuronal synapse density in AD mice [8]. Here, we further investigated the effects of exercise on brain PSD-95, SYP, GAP-43, and BDNF, providing additional evidence for the neuroprotective effects of treadmill exercise on the brains of D-gal-induced mice.
Myelin abnormalities, oligodendrocyte damage, and concomitant glia activation are commonly observed in demyelinating diseases of the central nervous system (CNS). A type of cell that plays a role in regulating neuroinflammation is the astrocyte. Astrocyte activation is primarily characterized by the acquisition of a response phenotype, which leads to excitotoxicity, loss of synaptic plasticity, and dysregulation of calcium regulation [32]. In another study, it was found that prednisone alleviated astrocyte activation and attenuated demyelinating lesions, directly illustrating the inverse relationship between the LFB index and the strength of the GFAP immune response [33]. Several studies have reported that Aβ deposition in the brains of patients in the early stages of AD and animal models of AD is associated with aberrant activation of microglia and astrocytes, as well aselevated expression of their markers, IBA-α, GFAP [27, 34]. Overactivation of astrocytes can accelerate demyelinating injury of nerve fibers [35]. Meanwhile, astrocyte depletion in brassinosterone-induced demyelination results in reduced microglia/macrophage activation, leading to the persistence of myelin debris and reduced myelin regeneration [36]. It is optimal to maintain GFAP at moderate levels, and combining the existing conditions, we hypothesized that the inverse relationship between GFAP and LFB indicators may be related to a specific inflammatory response caused by astrocyte overactivation. Therefore, we examined GFAP and representative products of astrocyte activation including TNF-α, Cox-2, and CXCL2. Interestingly, treadmill exercise can achieve its neuroprotective function by effectively inhibiting astrocyte activation and the subsequent secretion of inflammatory factors TNF-α, Cox-2, and CXCL2 in D-gal-treated cortex.
Nevertheless, there is a paucity of studies on changes in miRNAs profiles in human AD models and a deficiencyof studies translating the regulation of miRNAs into potential therapeutics. Consequently, it is crucial to expedite research on neuronal and miRNAs, primarily those linked to myelin disorders, as demyelinating lesions are connected with AD susceptibility, cognitive deficits, and pathology [23]. Previous studies have shown the upregulation of miR-34a in various age-related neurodegenerative diseases, including AD [37, 38]. More conclusive proof comes from a study showing that Sera miR-34a, miR-29b, and miR-181c serve as a potential novel diagnostic biomarker panel for Alzheimer's in the Egyptian population [39]. Additionally, a study demonstrated that over-expression of miR-34a triggers cognitive impairment and Alzheimer's disease-like pathology [6]. Based on the above findings, we detected the expression of miR-34a in the brain of model mice. Interestingly, our findings indicated that miR-34a expression was significantly elevated in the DAM + SED group, which is compatible with previous studies, suggesting that elevated miR-34a is involved in the pathological events of brain aging. Strikingly, exercise intervention notably reversed the D-gal-induced up-regulation of miR-34a, promoting us to further investigate the downstream genes of miR-34a in the process of exercise therapy for ameliorating brain aging. Nonetheless, the underlying mechanisms still deserve to be explored thoroughly, especially the signaling pathways involved in the regulation of miR-34 in AD pathology.
We demonstrated again using online bioanalytical databases that TAN1 is downstream of miR-34a. Reduced TAN1 signaling in mice cortex and hippocampus is associated with aging, a natural process of neurodegeneration [40]. A study revealed that the TAN1 signaling pathway was repressed in the cortex of Aβ 42-induced Alzheimer model rats [41]. Studies have indicated that TAN1could ameliorate ischemia–reperfusion injury and cardiomyocyte apoptosis by down-regulating PTEN, thereby deregulating the inhibition of the PI3K/AKT signaling pathway [42, 43]. The phosphorylation AKT promotes the CREB phosphorylation and nuclear transcription of CREB, which is associated with enhanced synaptic plasticity, inhibition of neuronal apoptosis, and delayed demyelinating lesions [44, 45]. In addition, it has been found that exercise improves synaptic plasticity and improves spatial cognition by modulating AKT, CREB, and BDNF [46]. In the present study, treadmill exercise significantly increased the relative expression of TAN1, p-PI3K/PI3K, p-AKT/AKT, and CREB and decreased the expression of PTEN in mice. Moreover, exercise increased CREB phosphorylation and nucleus clustering. The above findings indicated that delayed demyelination after exercise may be associated with activation of the PI3K/AKT/CREB signaling pathway.
There is a strong correlation between oligodendrocyte apoptosis and demyelinating lesions in aging [47]. Cell death and survival responses are mediated by PI3K/AKT/CREB signaling. Exercise appears to have an anti-apoptotic effect on hippocampal neurons, as our earlier research has shown, though its effect on the cortex is unknown [48]. The current investigation verified that the DAM + EX group's TUNEL ( +) counts were considerably reduced by treadmill exercise. To learn more about the apoptotic pathways, it was quantified using Bcl-2 and Bax immunohistochemistry. The findings demonstrated a significant increase in cortical Bax ( +) counts and a decrease in Bcl-2 ( +) counts following D-gal treatment. On the other hand, treadmill exercise reversed this trend. By preventing oligodendrocyte precursor cell (OPC) migration and differentiation and causing oligodendrocyte death, astrocytes aid in the process of demyelination [49]. Myelin synthesis and renewal are greatly aided by oligodendrocytes [50]. The field's attention has recently been drawn to the roles played by oligodendrocyte-specific markers Olig2 and PDGF-α in controlling myelination [24, 51]. Here, we report that aging mice express less PDGFR-α and Olig2. On the other hand, Olig2 and PDGFR-α expression in aging mice decreased, a trend that treadmill training reversed. We hypothesized that the miR-34a/TAN1/PI3K/AKT/CREB pathway plays a significant role in improving myelin thickness and preventing apoptosis. The above results showed that training increases myelinating and decreases apoptosis.
To summarize, TAN1/PI3K/AKT/CREB pathway, which is mediated by miR-34a and plays a protective role in the cognitive deficits linked to D-gal, is what triggers exercise (Fig. 6C). The underlying mechanisms may be related to the fact that exercise promotes increased myelin thickness, and inhibits astrocyte hyperactivation, apoptosis, and down-regulation of miR-34a, which lays the theoretical foundation and support for the use of exercise as a non-toxic treatment against aging and its correlated neurodegenerative diseases.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- miR-34a:
-
MicroRNA-34a
- D-Gal:
-
D-galactose
- AD:
-
Alzheimer's disease
- NFTs:
-
Neurofibrillary tangles
- LFB:
-
Luxol Fast Blue
- MWM:
-
Morries water maze
- Cox-2:
-
Cyclooxygenase-2
- CXCL2:
-
C-X-C Motif Chemokine Ligand 2
- PDGFR:
-
Platelet derived growth factor receptor alpha
- MBP:
-
Myelin basic protein
- TAN1:
-
Translocation-associated notch protein TAN-1
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome ten
References
Hou YJ, Dan XL, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15:565–581
Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7:137–152
Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S (2008) MicroRNA expression in the adult mouse central nervous system. RNA 14:432–444
Hebert SS, De Strooper B (2009) Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 32:199–206
Tan L, Yu JT, Tan L (2015) Causes and consequences of microRNA dysregulation in neurodegenerative diseases. Mol Neurobiol 51:1249–1262
Kou XJ, Li J, Liu XR, Chang JR, Zhao QX, Jia SH, Fan JJ, Chen N (2017) Swimming attenuates D-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J Appl Physiol 122:1462–1469
Chua CEL, Tang BL (2019) miR-34a in neurophysiology and neuropathology. J Mol Neurosci 67:235–246
Jian Y, Yuan SL, Yang JL, Lei Y, Li X, Liu WF (2022) Aerobic exercise alleviates abnormal autophagy in brain cells of APP/PS1 mice by upregulating adipoR1 levels. Int J Mol Sci 23:9921
Correale J, Ysrraelit MC (2022) Multiple sclerosis and aging: the dynamics of demyelination and remyelination. ASN Neuro. https://doi.org/10.1177/17590914221118502
Nogueras-Ortiz CJ, Mahairaki V, Delgado-Peraza F, Das D, Avgerinos K, Eren E, Hentschel M, Goetzl EJ, Mattson MP, Kapogiannis D (2020) Astrocyte- and neuron-derived extracellular vesicles from Alzheimer’s disease patients effect complement-mediated neurotoxicity. Cells-Basel. 9:1618
Iliadou P, Bakirtzis C, Ioannidis P, Possin K, Zygouris S, Sintila SA, Grigoriadis N, Aretouli E (2022) Neuropsychological correlates of cerebellar volumes in multiple sclerosis: an MRI volumetric analysis study. J Integr Neurosci 21:13
Moallemian S, Salmon E, Bahri MA et al (2023) Multimodal imaging of microstructural cerebral alterations and loss of synaptic density in Alzheimer’s disease. Neurobiol Aging 132:24–35
Esser S, Gopfrich L, Bihler K, Kress E, Nyamoya S, Tauber SC, Clarner T, Stope MB, Pufe T, Kipp M et al (2018) Toll-like receptor 2-mediated glial cell activation in a mouse model of cuprizone-induced demyelination. Mol Neurobiol 55:6237–6249
Isaev NK, Stelmashook EV, Genrikhs EE (2019) Neurogenesis and brain aging. Rev Neurosci 30:573–580
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS (2010) Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol 45:357–365
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Bao CC, He CQ, Shu B, Meng T, Cai QY, Li BC, Wu GY, Wu B, Li HL (2021) Aerobic exercise training decreases cognitive impairment caused by demyelination by regulating ROCK signaling pathway in aging mice. Brain Res Bull 168:52–62
Tastsoglou S, Skoufos G, Miliotis M, Karagkouni D, Koutsoukos I, Karavangeli A, Kardaras FS, Hatzigeorgiou AG (2023) DIANA-miRPath v4.0 expanding target-based miRNA functional analysis in cell-type and tissue contexts. Nucleic Acids Res. https://doi.org/10.1093/nar/gkad431
Singhal N, Sharma A, Kumari S, Garg A, Rai R, Singh N, Kumar M, Goel M (2020) Biophysical and biochemical characterization of nascent polypeptide-associated complex of picrophilus torridus and elucidation of its interacting partners. Front Microbiol 11:915
Parsa FG, Nobili S, Karimpour M, Aghdaei HA, Nazemalhosseini-Mojarad E, Mini E (2022) Fanconi anemia pathway in colorectal cancer: a novel opportunity for diagnosis. Prognosis Therapy. J Pers Med. 12:396
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 42:S125–S152
Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Moran M, Emanuele E, Joyner MJ, Lucia A (2015) Exercise attenuates the major hallmarks of aging. Rejuv Res 18:57–89
Garcia G, Pinto S, Ferreira S (2022) emerging role of miR-21–5p in neuron-glia dysregulation and exosome transfer using multiple models of Alzheimer’s disease. Cells 11:3377
Lee JS, Park YH, Park S, Yoon U, Choe Y, Cheon BK, Hahn A, Cho SH, Kim SJ, Kim JP et al (2019) Distinct brain regions in physiological and pathological brain aging. Front Aging Neurosci. 11:147
Sun CC, Yin ZP, Chen JG et al (2021) Dihydromyricetin improves cognitive impairments in d-galactose-induced aging mice through regulating oxidative stress and inhibition of acetylcholinesterase. Mol Nutr Food Res 66:e2101002
Zhang L, Chao FL, Luo YM et al (2017) Exercise prevents cognitive function decline and demyelination in the white matter of APP/PS1 transgenic AD mice. Curr Alzheimer Res 14(6):645–655
Mahan TE, Wang C, Bao X, Choudhury A, Ulrich JD, Holtzman DM (2022) Selective reduction of astrocyte apoE3 and apoE4 strongly reduces a beta accumulation and plaque-related pathology in a mouse model of amyloidosis. Mol Neurodegener 17:1–20
Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA (2009) Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol 94:1153–1160
Sujkowski A, Hong LK, Wessells RJ, Todi SV (2022) The protective role of exercise against age-related neurodegeneration. Ageing Res Rev 74:101543
Christensen AJ, Ott T, Kepecs A (2022) Cognition and the single neuron: How cell types construct the dynamic computations of frontal cortex. Curr Opin Neurobiol 77:102630
Marangon D, Boccazzi M, Lecca D, Fumagalli M (2020) Regulation of oligodendrocyte functions: targeting lipid metabolism and extracellular matrix for myelin repair. J Clin Med 9:470
Stogsdill JA, Harwell CC, Goldman SA (2023) Astrocytes as master modulators of neural networks: synaptic functions and disease-associated dysfunction of astrocytes. Ann Ny Acad Sci 1525:41–60
Yu H, Wu M, Lu G et al (2018) Prednisone alleviates demyelination through regulation of the NLRP3 inflammasome in a C57BL/6 mouse model of cuprizone-induced demyelination. Brain Res 1678:75–84
Yang YY, Garcia-Cruzado M, Zeng HR, Camprubi-Ferrer L, Bahatyrevich-Kharitonik B, Bachiller S, Deierborg T (2023) LPS priming before plaque deposition impedes microglial activation and restrains A ss pathology in the 5xFAD mouse model of Alzheimer ’ s disease. Brain Behav Immun 113:228–247
Silvestroff L, Bartucci S, Pasquini J, Franco P (2012) Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination. Exp Neurol 235:357–367
Allnoch L, Baumgärtner W, Hansmann F (2019) Impact of astrocyte depletion upon inflammation and demyelination in a murine animal model of multiple sclerosis. Int J Mol Sci. https://doi.org/10.1093/nar/gkad431
Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Liu R, Thannickal VJ, Liu G (2018) miR-34a promotes fibrosis in aged lungs by inducing alveolar epithelial dysfunctions. Am J Physiol-Lung C 314:L332–L332
Raucci A, Macri F, Castiglione S, Badi I, Vinci MC, Zuccolo E (2021) MicroRNA-34a: the bad guy in age-related vascular diseases. Cell Mol Life Sci 78:7355–7378
Abuelezz NZ, Nasr FE, Abdel Aal WM et al (2022) Sera miR-34a, miR-29b and miR-181c as potential novel diagnostic biomarker panel for Alzheimers in the egyptian population. Exp Gerontol 169:111961
Placanica L, Zhu L, Li YM (2009) Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS ONE 4:e5088
Wang P, Zhang S, Hu C et al (2023) Regulatory role of melatonin in Notch1 signaling pathway in cerebral cortex of Aβ 1–42 -induced Alzheimer’s disease rat model. Mol Biol Rep 50(3):2463–2469
Yu LM, Li Z, Dong X, Xue XD, Liu Y, Xu S, Zhang J, Han JS, Yang Y, Wang HS (2018) Polydatin protects diabetic heart against ischemia-reperfusion injury via notch1/hes1-mediated activation of pten/akt signaling. Oxid Med Cell Longev. https://doi.org/10.1155/2018/2750695
Zhang J, Li BR, Zheng ZQ, Kang T, Zeng MH, Liu YH, Xia BH (2015) Protective effects of notch1 signaling activation against high glucose-induced myocardial cell injury: analysis of its mechanisms of action. Int J Mol Med 36:897–903
Bi JY, Zhang HY, Lu J, Lei WF (2016) Nobiletin ameliorates isoflurane-induced cognitive impairment via antioxidant, anti-inflammatory and anti-apoptotic effects in aging rats. Mol Med Rep 14:5408–5414
Pak ME, Jung DH, Lee HJ, Shin MJ, Kim SY, Shin YB, Yun YJ, Shin HK, Choi BT (2018) Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Exp Neurol 300:222–231
Aguiar AS, Castro AA, Moreira EL et al (2011) Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev 132(11–12):560–567
Caprariello AV, Mangla S, Miller RH, Selkirk SM (2012) Apoptosis of oligodendrocytes in the central nervous system results in rapid focal demyelination. Ann Neurol 72:395–405
Liu Y, Guo W, Hong S (2023) Aerobic exercise mitigates hippocampal neuronal apoptosis by regulating DAPK1/CDKN2A/REDD1/FoXO1/fasL signaling pathway in D-galactose-induced aging mice. FASEB J 37:e23205
Lombardi M, Parolisi R, Scaroni F et al (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol 138(6):987–1012
Chen JF, Wang F, Huang NX, Xiao L, Mei F (2022) Oligodendrocytes and myelin: active players in neurodegenerative brains? Dev Neurobiol 82:160–174
Sy M, Brandt AU, Lee SU, Newton BL, Pawling J, Golzar A, Rahman AMA, Yu ZX, Cooper G, Scheel M et al (2020) N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J Biol Chem 295:17413–17424
Acknowledgements
The animal study protocol was approved by the Biomedical Research Ethics Committee of Hunan Normal University (Ethics Section 2021 No. 365).
Funding
This research was funded by National Natural Science Foundation of China, grant number: 32371182.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this research and preparation of the manuscript. YL, WG, LZ, and (Chang-fa Tang) CT conceived and designed the experiments and wrote the manuscript. YL, XM, SD, and CT performed the experiments and analyzed the data; YL, WS edited and revised manuscript. YL is the first author to the work. All authors have been involved in the final approval of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Meng, XK., Shao, Wz. et al. miR-34a/TAN1/CREB Axis Engages in Alleviating Oligodendrocyte Trophic Factor-Induced Myelin Repair Function and Astrocyte-Dependent Neuroinflammation in the Early Stages of Alzheimer's Disease: The Anti-Neurodegenerative Effect of Treadmill Exercise. Neurochem Res 49, 1105–1120 (2024). https://doi.org/10.1007/s11064-024-04108-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04108-w