Abstract
Background and Purpose: Morphine is amongst the most effective analgesics available for the management of severe pain. However, prolonged morphine treatment leads to analgesic tolerance which limits its clinical usage. Previous studies have demonstrated that melatonin ameliorates morphine tolerance by reducing neuroinflammation. However, little is known about the relationship between Toll like receptor 2 (TLR2) and neuroinflammation in morphine tolerance. The aim of this study was to explore the role of TLR2 in morphine tolerance and its connections with melatonin and Nod-like receptor protein 3 (NLRP3) inflammasome. Methods: Sprague-Dawley rats were treated with morphine for 7 days and tail-flick latency test was performed to identify the induction of analgesic tolerance. The roles of TLR2 in microglia activation and morphine tolerance were assessed pharmacologically, and the possible interactions between melatonin, TLR2 and NLRP3 inflammasome were investigated. Key Results: Morphine tolerance was accompanied by increased TLR2 expression and NLRP3 inflammasome activation in spinal cord. whereas melatonin level was down-regulated. Chronic melatonin administration resulted in a reduced TLR2 expression and NLRP3 inflammasome activation. Moreover, the analgesic effect of morphine was partially restored. Inhibition of TLR2 suppressed the microglia and NLRP3 inflammasome activation, as well as restored the spinal melatonin level while attenuated the development of morphine tolerance. Furthermore, the inhibition of microglia activation ameliorated morphine tolerance via inhibiting TLR2-NLRP3 inflammasome signaling in spinal cord. Conclusion: In this study, we directly demonstrate a TLR2-melatonin negative feedback loop regulating microglia and NLRP3 inflammasome activation during the development of morphine tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphine is used routinely for the treatment of severe painful conditions due to its potent analgesic effect. Repeated use of morphine, however, results in most cases in drug tolerance, which limits a its long-term clinical utilization [1]. For past decades, various studies have attempted to elucidate the mechanisms underlying morphine tolerance. Up to now, contributions of oxidative stress [2], mitochondrial dysfunction [3], opioid receptor desensitization [4], endocytosis [5] have been demonstrated.
Despite increasing evidence of an important function in morphine-induced analgesic tolerance, the role of neuroinflammation is up to now only poorly understood. In spinal cord, morphine can activate microglia, and induce the release of glia-derived proinflammatory cytokines, which enhance the neuronal excitability, facilitate pain transmission, and oppose morphine analgesia [6, 7]. However, the detailed mechanisms of microglia-mediated neuroinflammation remain to be defined.
Nod-like receptor proteins (NLRPs) inflammasome is a group of intracellular multimeric protein complexes, which is composed of NLRP sensor, adapter protein apoptosis-associated speck-like protein and procaspase-1 procaspase-5 or both [8, 9]. Once identifying the pathogen-associated molecular patterns (PAMPs) or damage -associated molecular patterns (DAMPs), NLRP can assemble and form inflammasomes. Formation of this complex may trigger the transformation of procaspase-1 to caspase-1 and active p10/p20 tetramer. And in turn, active caspase-1 can cleave the cytokines such as pro-interleukin-1β (pro-IL-1β) and pro-IL-18 from their immature form into their biologically active forms [10]. NLRP3 inflammasome has been demonstrated to be crucial in microglia-mediated host defense against infection. While excessive activation of microglia will lead to various auto-inflammatory conditions and contribute to the development and progression of diseases including morphine tolerance [9, 11]. It has been reported that morphine could induce spinal NLRP3 inflammasome activation through Toll-like receptor 4 (TLR4) and TGF-β-activated kinase 1 pathway [12]. And procyanidins has been demonstrated to alleviate morphine tolerance by inhibiting the activation of microglial NLRP3 inflammasome [13]. However, the molecular mechanisms by which NLRP3 inflammasome is involved in the development of morphine tolerance still need to be explored.
Melatonin (N-acetyl-5-methoxytryptamine) is the predominant neuroendocrine hormone secreted by pineal gland with marked antioxidant and metabolic properties [14]. Previous studies about melatonin mainly focused on the treatment of sleep disturbances and circadian regulations [15]. Recently, the immune-modulatory and anti-inflammatory effects of melatonin attract more and more attention [16]. It has been proved that melatonin could decrease the levels of tumor necrosis factor-α, IL-1β, IL-6, and the phosphorylation of mammalian target of rapamycin (mTOR) in the hippocampus of aging mice and attenuate isoflurane-induced cognitive impairment [17]. And nuclear factor-erythroid 2-related factor 2 (NRF2) signaling pathway could also be stimulated by melatonin to reduce LPS-induced reactive oxygen species generation in rat brain [18]. Recent study showed that exogenous melatonin could pronouncedly ameliorate airway inflammation in murine model via suppressing TLR2-NLRP3 signal [19]. TLRs are the members of pattern recognition receptors (PRRs) and expressed on cell membrane after being triggered by PAMPs and DAMPs. The activation of TLRs can induce a series of signal cascades including microglia activation [20], oxidative stress [21] and autophagy [22], etc. in response to difference stimulations [23]. Previous studies have confirmed the roles of TLR2, TLR4 and TLR5 in chronic pain [24, 25]. TLR4 has been proved to contribute to the development of morphine tolerance [12, 26, 27]. Microglia can express all the members of TLRs, in which TLR2 and TLR4 were most widely studied [28]. Once being triggered by exogenous stimulators, microglial TLR2 can activate NLRP3 inflammasome, and subsequently induce the caspase-1-mediated release of mature IL-1β and IL-18 [29]. However, the role of TLR2 in morphine tolerance remains unknown. In this study, we speculate that chronic morphine administration could increase the expression of TLR2 and subsequently activate NLRP3 inflammasome in spinal cord. We further explored the relationship between melatonin and TLR2-NLRP3 signal during the development of morphine tolerance. This study sought to provide theoretical evidences for developing the preventive and therapeutic strategies for morphine tolerance.
Materials and Methods
Animals
Adult male Sprague-Dawley rats weighing 220–250 g were provided by the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, Hebei, China). All the animals were individually housed in cages to adapt to the environment for 1 weeks before experiments. The controlled conditions were 21 ± 1 ℃, 12-h light/dark cycles, and free access to food and water. The animals were randomly assigned to different group using a Research Randomizer.
All the experimental protocols and procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hebei, China. The experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the guidelines of the International Association for the Study of Pain (Zimmermann, 1983).
Intrathecal Catheterization
For drug delivery, intrathecal catheters were implanted as described previously [7]. Briefly, rats in morphine group or sham-operated group were deeply anesthetized with 1% pentobarbital sodium [60 mg/kg, intraperitoneal injection (i.p.)]. And the skin of lumbar region was shaved and disinfected. A sterile PE-10 catheter (outer diameter 0.5 mm, inner diameter 0.3 mm. Anilab Software & Instruments, Ningbo, China) filled with saline was implanted into subarachnoid cavity between L4 and L5 vertebrae. The tip of catheter was placed at the spinal lumbar enlargement level. The catheter was subcutaneously tunneled, externalized and fixed to the back of neck. Wound was sutured after disinfection with 75% (v/v) ethanol. Proper location of catheter was confirmed by a temporary motor block of both hind limbs after intrathecal injection of 10 µL of 2% lidocaine. The rats were housed individually after surgery and allowed a 7-day recovery period before the following experiments. Rats with hind limb paralysis or paresis after surgery were excluded and euthanized with overdose of pentobarbital sodium. Rats in naïve group didn’t get any operation.
Morphine Tolerance
In this study, a pure spinal mechanism was explored. Therefore, intrathecal administration was chosen to develop tolerance in analgesia. Rats were intrathecally injected with morphine (10 µg/5 µL) twice daily for consecutive 7 days to induce the tolerance to morphine.Equivalent volumes of vehicle were administered to the sham-operated rats at the same time points. The development of morphine tolerance was assessed by behavioral tests on day 1, 3, 5 and 7.
Drugs Administration
Drugs used in this study were prepared as follows. Morphine hydrochloride (10 µg/5 µL, Northeastern Pharmaceutical Group, Shenyang, China) and Minocycline hydrochloride (30 µg/5 µL, Selleckchem, USA) [30] were diluted in saline (Northeast Pharmaceutical Group, China), respectively. Melatonin (60 µg/5 µL, Selleckchem, USA) [31] and TLR2 inhibitor CU-CPT22 (3 µg/5 µL, Selleckchem, USA) [32] were dissolved in 20% (v/v) dimethyl sulfoxide (DMSO, Sigma, USA), respectively. Melatonin (60 µg/5 µL, i.t.), minocycline hydrochloride (30 µg/5 µL, i.t.), or Cu-CPT22 (3 µg/5 µL, i.t.) was injected 30 min before morphine administration, respectively, followed by 10 µL of saline for flushing. Rats in sham-operated r group received equivalent volumes of saline or20% DMSO .
Behavioral Assessment
Thermal pain thresholds in rats were measured by a tail-flick latency test before drug administration and at 30 min after morphine administration on day 1, 3, 5, and 7 [33]. Briefly, rat was placed in container to restrain its body but not head and tail, with one third of tail immersed into water. The temperature of water was adjusted to 50 ± 0.2 ℃ to maintain an average tail-flick latency of 2–4 s in naïve rats. The rapid removal of tail from water was defined as a positive response and the time of removal was recorded. To avoid the damage of tail, a cutoff time of 15 s was set. The test was repeated thrice for each rat at a 5-min interval between tests. And the mean value of three tests was considered as the final latency. The analgesic effect of morphine or the influence of different drugs to chronic morphine administration was evaluated by transforming latency to the percentage of maximum possible antinociceptive effect (%MPE), which was calculated by comparing the test latency before (baseline, BL) and after (TL) drug administration using following equation: %MPE = [(TL - BL) / (cutoff time - BL)] × 100. The behavioral assessments were conducted by the experimenter who was unaware of animal grouping.
Western Blots
Under deep anesthesia with 60 mg/kg of intraperitoneal pentobarbital sodium, the rats were sacrificed. The spinal lumbar enlargements (L3-L5) were quickly removed on ice and homogenized in ice-cold radio-immunoprecipitation assay lysis buffer combined with a mixture of proteinase inhibitors and phosphatase inhibitors, according to the manufacturer’s instructions (Boster, Wuhan, China). The lysate was subject to centrifugation at 12,000 rpm for 15 min at 4 ℃, and the supernatant was collected. The Bradford method was used to measure the protein concentration of supernatants. The extract was denatured by boiling water for 10 min and 30 µg protein per sample was loaded onto 8%, 10% or 12% sodium dodecyl sulfate polyacrylamide gel. Electrophoresis was conducted at 60 V for stacking gel and 90 V for separating gel. Then the protein was electrotransferred (250 mA) to polyvinylidene fluoride membranes (IPVH00010; Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk or 5% (v/v) bovine serum albumin dissolved in Tris-buffered saline and Tween-20 buffer for 2 h at room temperature (22–24 ℃), and subsequently incubated overnight at 4 ℃ with following primary antibodies: mouse anti-Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:10000; ABclonal, China), TLR2 (1:1000; ABclonal, China), rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1) antibody (1:500; ABclonal, China), mouse anti-Caspase-1 antibody (1:200; Santa Cruz, USA), mouse anti-IL-1β antibody (1:200; Santa Cruz, USA). After being washed in Tris-buffered saline and Tween 20 for three times, the membranes were incubated with HRP conjugated goat anti-rabbit IgG (1:5000, Aspen, China) or HRP conjugated goat anti-mouse IgG (1:5000, Aspen, China) for 2 h at room temperature. Super-Lumia enhanced chemiluminescence Plus HRP Substrate Kit (Abbkine, USA) and a computerized image analysis system (Bio-Rad, ChemiDoc XRSC, USA) were used to detect the proteins. The intensity of protein blots was quantified by using Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA).
Quantitative real-time Polymerase Chain Reaction (qRT-PCR)
Under deep anesthesia with 60 mg/kg of intraperitoneal pentobarbital sodium, the rats were sacrificed and L3-L5 spinal cord segments of rats were rapidly removed. Total RNA was extracted from spinal cord by using trizol (Takara, Shiga, Japan) according to the manufacturer’s instructions and RNA concentration was quantified by a spectrophotometer (Eppendorf, Germany). One microgram of RNA was used for reverse transcription to synthesize cDNA. The mRNA expression was examined following the protocols of SYBR Premix Ex TaqTM kit (Vazyme, China) on StepOne Real-Time PCR System (Applied Biosystems, USA). The primers used in experiments are listed in Table 1. Relative expression levels were measured by 2−ΔΔCt formula. GAPDH was used for normalization.
Enzyme-linked Immunosorbent Assay (ELISA)
Melatonin level was determined by enzyme-linked immunosorbent assay. Under deep anesthesia with 60 mg/kg of intraperitoneal pentobarbital sodium, L3-L5 spinal cord segments of rats were rapidly removed and homogenized in ice-cold phosphate-buffered saline according to the mass (mg) volume (uL) ratio of 1:9. Then the lysate was centrifuged at 12,000 g for 15 min at 4 ℃. The supernatant was collected and analyzed using rat Melatonin sandwich ELISA kits (E-Bioscience, USA) according to the manufacturer’s instructions. Briefly, 100 µL of sample or calibrator was added per well. The plate was sealed and incubated at room temperature for 2 h, then washed three times and the detection antibody was added. Then the plate was sealed and incubated at room temperature for 1 h. And the read buffer was added. The photometric measurements were performed at 450 nm using a microplate reader (Varioskan Flash, Thermo Scientific, USA). The concentration of target protein was calculated according to the standard curve.
Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed with GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). The data of behavioral tests were analyzed by using two-way analysis of variance with repeated measures followed by Bonferroni test. The data of Western blots and qRT-PCR were analyzed by one-way analysis of variance with repeated measures ANOVA (treatment group × time) followed by Bonferroni test. No data point was excluded from statistical analysis in any experiments. P < 0.05 was considered statistically significant.
Results
Chronic Morphine Administration Induced drug Tolerance
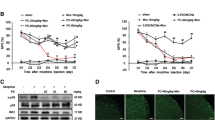
Rats were intrathecally administered with morphine (10 µg/5 µL) twice daily for consecutive7 days to induce morphine tolerance. Behavioral tests were conducted before and 30 min after drug administration on day 1, 3, 5, and 7. As shown in Fig. 1b, rats received morphine exhibited significantly higher %MPE when compared to saline-treated rats on day 1 of morphine administration. Repeated morphine administration produced a progressive decline of %MPE level from day 3 and no between-group difference was detected on day 7. There was no significant difference in %MPE level between naïve and saline-treated rats during the 7-day observation period. These results demonstrated that rats have developed morphine tolerance on day 7.
Chronic administration of morphine induced analgesic tolerance (a) A schematic profile illustrating the experimental design of the hot-water tail-flick test with a short-term morphine treatment (b) Thermal pain threshold was assessed by the hot-water tail-flick test and represented as the percentage of maximal possible antinociceptive effect (%MPE). The %MPE in rats treated with morphine (10 µg/ 5 µl, twice daily, intrathecally) on days 5 and 7 were dramatically decreased when compared with the baseline on day 1. (**p < 0.01, ****p < 0.0001 vs. NS group. n = 6 per group). n = number of animals. MT, morphine treatment; NS, normal saline; NA, naive
Spinal Microglia and NLRP3 Inflammasome were Activated in morphine-tolerant rats
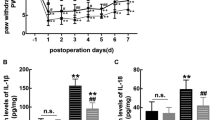
Given the importance of microglia activation in neuroinflammation, we first examined the expression of microglia marker Iba1 and TLR2 in spinal cord by western blots and qRT-PCR. As shown in Fig. 2a-d, the mRNA and protein levels of Iba1 and TLR2 were significantly increased on day 7 of morphine administration. We next tested the expression of NLRP3 inflammasome and the effectors in spinal cord. The results in Fig. 2e-g showed that the morphine significantly increased the mRNA levels of NlRP3, IL-1β and Caspase-1 in morphine-tolerant rats. At the same time, the spinal protein levels of P20 and IL-1β, the active forms of procaspase-1 and pro-IL-1β, respectively, were both up-regulated (Fig. 2h and i) These findings suggest the activation of the spinal NLRP3 inflammasome and its effectors induced by chronic morphine treatment.
Expression of spinal Iba1, TLR2 and NLRP3 inflammasome in morphine-tolerant rats (a-d) The mRNA and protein expression of IBA1 and TLR2 were increased measured by qRT-PCR and western blot.(****p < 0.0001 ,**p < 0.01, *p < 0.05 vs. NS group, n = 4 per group for qRT-PCR, n = 5 per group for western blot) (e-g) The mRNA expression of NLRP3, Caspase-1 and IL-1β were increased measured by qRT-PCR.( (****p < 0.0001,vs. NS group, n = 4 per group) (h and i) The protein expression of matured caspase1, P20, and IL-1β were increased measured by western blot. (***p < 0.001, **p < 0.01vs. NS group, n = 5 per group for western blot.) n = number of animals. M, morphine; MT, morphine treatment; NS, normal saline; NA, naive
TLR2 Activation in Spinal cord Contributed to the Development of Morphine Tolerance
To identify whether TLR2 participates in the development of morphine tolerance, a TLR2 antagonist CU-CPT22 was intrathecally injected (3 µg/5 µL) 30 min before morphine administration twice daily for 7 days. Behavioral tests showed that CU-CPT22 itself had no effect on the baseline tail-flick responses of rats. And from day 5 to 7, the %MPE in rats treated with morphine and CU-CPT22 was significantly higher than those in morphine-treated rats (Fig. 3a), demonstrating that the development of morphine tolerance was partially prevented by CU-CPT22. We next examined the effect of CU-CPT22 on TLR2 expression using Western blots. The results in Fig. 3b showed that the increased expression of TLR2 protein induced by repeated morphine administration was inhibited by CU-CPT22. These results indicate that the activation of TLR2 contributed to the development of morphine tolerance.
Inhibiting TLR2 Suppressed the Activation of Spinal Microglia and NLRP3 Inflammasome and Partially Restored the Expression of Melatonin in morphine-tolerant rats
To further investigate the intrinsic mechanism of TLR2 activation in morphine tolerance, we examined the effect of CU-CPT22 on the activation of spinal microglia and NLRP3 inflammasome. The results in Fig. 3c and f showed that CU-CPT22 pretreatment could inhibit the upregulation of Iba1 protein and the increased levels of spinal NLRP3, P20 and IL-1β induced by chronic morphine administration, respectively. Furthermore, the results of ELISA showed that chronic morphine administration resulted in a decrease of melatonin levels in spinal cord, which could be partially restored by CU-CPT22. The basal level of melatonin, however, was not affected by CU-CPT22 (Fig. 3g). These indicate that TLR2 could increase spinal microglia, activate NLRP3 inflammasome and down-regulate the expression of melatonin.
Spinal TLR2 participated in the development of morphine tolerance via activating microglia and NLRP3 inflammasome down-regulating the expression of melatonin The TLR2 inhibitor CU-CPT22 (3 µg/5 uL) was intrathecally injected 30 min before morphine administration twice daily for 7 days. (a) The %MPE in rats receiving TLR2 inhibitor CU-CPT22 30 min before morphine administration were higher than those in morphine-tolerant rats from day 5 to 7. (###p < 0.001, ####p < 0.0001 vs. Morphine + Vehicle group, ****p < 0.0001, **p < 0.01 vs. NS + Vehicle group, n = 5 per group). (b-f) Pretreatment with CU-CPT22 reduced increased protein expressions of (b) TLR2, (c) Iba1, (e) P20, (f)IL-1β measured by western blot and reduced increased mRNA expression of (d) NLRP3 measured by qRT-PCR. (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. Morphine + Vehicle group; *p < 0.05, ***p < 0.001, ****<0.0001 vs. NS + Vehicle group, n = 4 per group for qRT-PCR , n = 5 per group for western blot.) (g) morphine inhibited melatonin expression, while CU-CPT22 partially restored the melatonin secretion in morphine-tolerant rats measured by ELISA (**p < 0.01 vs. NS + Vehicle group, ##p < 0.01 vs. Morphine + Vehicle group, n = 4 per group)%MPE, the percentage maximal possible antinociceptive effect; n = number of animals. MT, morphine treatment; M, morphine; NS, normal saline;
Melatonin Prevented the Development of Morphine Tolerance via Inhibiting TLR2-NLRP3 Signal and Microglia Activation
Given the down-regulation of melatonin in morphine-tolerant rats, we investigated if melatonin might prevent the development of morphine tolerance. Here, exogenous melatonin was intrathecally injected 30 min before morphine administration twice daily for 7 days. As showed in Fig. 4a, behavioral tests showed that melatonin itself did not exhibit an analgesic effect. From day 5 to 7, however, the %MPE in rats treated with morphine and in addition with melatonin was significantly higher than those in morphine-treated rats, demonstrating that pretreatment with melatonin can prevent the development of morphine tolerance.
Previous study reported that melatonin could inhibit the activation of TLR2 in allergic airway inflammation [19]. Therefore, the effect of melatonin on TLR2 expression in spinal cord was investigated in the context of morphine tolerance. As shown in Fig. 4b and c, the expression of spinal TLR2 mRNA and protein did not change after repeated administration of melatonin alone. The increase of TLR2 induced by morphine, however, was remarkably reduced by melatonin.
We next investigated the influence of exogenous melatonin to the increase of spinal microglia and the activation of NLRP3 inflammasome using Western blots and qRT-PCR. The results showed that melatonin pretreatment could decrease the upregulation of Iba1 protein and the increased levels of spinal NLRP3, P20 and IL-1β induced by chronic morphine administration, while melatonin alone had no effect (Fig. 4d- g). Taken together, these results demonstrate that melatonin attenuated morphine tolerance via suppressing TLR2-NLRP3 signal and the ameliorating the activation of spinal microglia.
Supplement of exogenous melatonin improved the morphine tolerance via suppressing TLR2-NLRP3 signal and the activation of spinal microglia (a) Melatonin (60 ug/5 uL) was intrathecally injected 30 min before morphine administration twice daily for 7 days. The %MPE in rats receiving melatonin 30 min before morphine administration were higher than those in morphine-tolerant rats from day 5 to 7. (##P < 0.01 vs. Morphine + Vehicle group, ****p < 0.0001, **p < 0.01 vs. NS + Vehicle group, n = 5 per group.) (b-g) Pretreatment with melatonin reduced increased protein expressions of (c) TLR2, (d) Iba1, (f) P20, (g)IL-1β measured by western blot and reduced increased mRNA expression of (b) TLR2 and (e) NLRP3 measured by qRT-PCR. (#p<0.05, ##p < 0.01, ####p < 0.0001 vs. Morphine + Vehicle group; **p < 0.01, ****<0.0001 vs. NS + Vehicle group, n = 4 per group for qRT-PCR ,n = 5 per group for western blot.) n = number of animals. MT, morphine treatment; M, morphine; NS, normal saline;
Inhibition of Microglial Activation Attenuated Morphine Tolerance by Inhibiting the Activation of TLR2 and NLRP3 Inflammasome
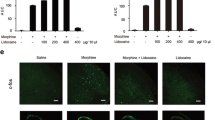
To clarify the regulatory effect of microglial TLR2 on the activation of NLRP3 inflammasome in morphine tolerance, minocycline hydrochloride was intrathecally injected 30 min before morphine administration twice daily for 7 days. The results of behavioral tests showed that minocycline itself did not affect the %MPE of rats. Repeated administration of minocycline, however, significantly attenuated the development of morphine tolerance, as indicated by the significant higher %MPE on day 5 and day 7 in rats treated with morphine and minocycline as compared to that of rats treated with morphine alone (Fig. 5a). Moreover, minocycline pretreatment resulted in a reduced increased protein level of Iba1 in spinal cord induced by morphine, suggesting a reduced spinal microglia activation by morphine under these conditions (Fig. 5b). Investigation of TLR2 and NLRP3 mRNA levels and P20 and IL-1β protein levels revealed that minocycline treatment alone had no significant effect of the respective expression level, as compared to the saline treated control group. The increased expression of TLR2, NLRP3, P20 and IL-1β induced by repeated morphine administration, however, was inhibited by minocycline (Fig. 5c and f). These results further support the hypothesis that activation of the NLRP3 inflammasome in microglia, via TLR2 contributes to morphine tolerance in rats.
Inhibition of microglial activation attenuate morphine tolerance by inhibiting activation of TLR2 and NLRP3 inflammasome Minocycline (30 ug/5 uL) was intrathecally injected 30 min before morphine administration twice daily for 7 days. (a) Pretreatment with minocycline attenuated the development of morphine tolerance. (**p < 0.01, ****p < 0.0001 vs. Morphine group, #p < 0.05, ###p < 0.001, ####p < 0.0001, vs. NS group, n = 5 per group.) (b-f) Pretreatment with minocycline reduced increased protein expressions of (b)Iba1, (e) P20, (f)IL-1β measured by western blot and reduced increased mRNA expression of (c) TLR2 and (d) NLRP3 measured by qRT-PCR. (#p < 0.05, ##p < 0.01, ###p <0.001 vs. Morphine + Vehicle group; *p < 0.05, **p < 0.01 vs.NS + Vehicle group, n = 4 per group for qRT-PCR, n = 5 per group for western blot.) n = number of animals. M, morphine;MT, morphine treatment; NS, normal saline;
Discussion
In this study, we found that the activation of TLR2 participates in the mechanism of morphine tolerance. Chronic morphine administration down-regulated the expression of melatonin in spinal cord, whereas exogenous melatonin could attenuate the development of morphine tolerance. Furthermore, the activation of TLR2 could be partially inhibited by exogenous melatonin and in turn spinal melatonin administration could be restored by TLR2 inhibitor in morphine-tolerant rats. The activation of spinal microglia and NLRP3 inflammasome induced by morphine could be depressed by TLR2 inhibitor or exogenous melatonin. Inhibiting microglia activation could block morphine-activated NLRP3 inflammasome via partially inhibiting the activation of TLR2. In summary, we report on a possible feedback loop between TLR2 and melatonin that might contribute to the control spinal microglial activation and NLRP3 inflammasome induction in morphine-tolerant rats.
TLRs family is a group of PRRs, which are closely related to various diseases in CNS. It has been reported previously that spinal TLR2 expression is significantly increased in a rat neuropathic pain model [34, 35] and Botulinum toxin type A could alleviate neuropathic pain via down-regulating the expression of TLR2, thereby suppressing the release of proinflammatory cytokines such as IL-1β, IL-18, and IL-6 from microglia [36]. Considering the important relationship between chronic pain and morphine tolerance, recent studies focused on the role of TLR2 in the development of morphine tolerance. It has been reported that chronic morphine treatment-induced gut dysbiosis led to gut barrier disruption and bacterial translocation, initiating local gut inflammation through TLR2/4 activation, which resulted in the activation of proinflammatory cytokines [37]. Consistent with in involvement of TLR2 and TLR4 in morphine tolerance, morphine analgesic tolerance was prevented in TLR2 -/- and TLR4 -/- mice [38]. We now show, that the expression of TLR2 was also increased in spinal cord after 7 days of morphine administration, while TLR2 inhibitor could alleviate the development of analgesic tolerance. These results show that TLR2 plays an important role in the development of morphine tolerance.
TLR2 has been previously reported to play a role in various nervous system diseases mainly by inducing neuroinflammation [39, 40]. Microglia, as the main inflammatory cell in CNS, has been proven to be closely related to morphine tolerance [41, 42]. Our study also suggests an increase in activation of spinal microglia in morphine tolerant-rats, mainly manifested by increased expression of the microglia activation marker Iba1. The inhibitory effect of minocycline on microglia activation has been reported in previous studies [43, 44] In this study, minocycline was intrathecally injected before morphine administration. Here, minocycline significantly diminished the development of morphine tolerance and inhibit the expression of Iba1. We demonstrated further on, that the TLR2 inhibitor CU-CPT22 also down-regulated the expression of Iba1 in spinal cord. Taken together, these data suggest that TLR2 mediates the development of morphine tolerance, at least in part by activating microglia.
NLRP3 inflammasome, as the high-profile neuroinflammatory mechanism has also been confirmed to be associated with morphine tolerance. Previous study found that chronic morphine activation resulted in activation of the microglia NLRP3 inflammasome, and ultimately to an increase in the release of IL-1β, which mediated central sensitization and eventually morphine tolerance [12]. Consistently, knockdown or pharmacological inhibition of the NLRP3 effectively diminished the development of morphine tolerance by abolishing morphine-induced inflammasome activation, proving the important role of NLRP3 inflammasome in morphine tolerance [45]. Here, we confirm the increase of NLRP3 inflammasome activity in morphine-tolerant rats, by demonstrating an increased expression of NLRP3, P20 and IL-1β in spinal cord. Since NLRP3 inflammasome can be expressed in a variety of cells, including microglia, astrocytes and even neurons, the source of activated spinal NLRP3 inflammasome in morphine tolerance needs to be further studied [46]. Although previous study reported NLRP3 inflammasome activated by chronic morphine treatment is mainly in microglia, other studies also show that the up-regulation of NLRP3 inflammasome in spinal neurons is an important mechanism for the maintenance of cancer pain [47]. Therefore, we cannot rule out the possibility of the involvement of astrocytes or neurons NLRP3 inflammasomes in morphine tolerance, and the glia-neuron interactions that may be involved may warrant further investigation. Previous studies showed activated TLR2-NLRP3 inflammasome pathway is involved in various physiological and pathological processes [48, 49]. In this study, we found CU-CPT22 inhibited NLRP3 inflammasome activation, ultimately leading to reduced mature IL-1β release in morphine tolerant-rats. Taken overall, our results suggest that spinal TLR2 mediated morphine tolerance most likely through the activation of microglia and NLRP3 inflammasome. As for the cellular localization of spinal TLR2, previous studies have reported that TLR2 can be expressed by a variety of immune cells and mainly expressed in glial cells including microglia and astrocytes in CNS [41, 42]. In this study, we found that inhibiting the activation of microglia can down-regulated the expression of TLR2 in spinal cord. The expression of TLR2 in microglia is up-regulated after chronic morphine, which in turn induces the activation of NLRP3 inflammasome and ultimately leads to the development of morphine tolerance. However, due to the technical limitation, we didn’t explore whether TLR2 was specifically activated in microglia. Given that minocycline did not completely down-regulate the expression of TLR2, and the important role of TLR2 in astrocyte activation [50], the effect and extent of TLR2 activation in astrocytes in morphine tolerance might also need further studies.
Melatonin is commonly considered as a powerful antioxidant which can scavenge free radicals, enhance the activity of endogenous antioxidant enzymes and other antioxidants [51]. Increasing evidence supports the anti-inflammatory effects of melatonin [52, 53] and its effect on morphine tolerance. Previous studies showed that exogenous melatonin supplementation could inhibit neuroinflammation and alleviate or even reverse morphine tolerance through a variety of ways, including inhibiting the activation of microglia, heat shock protein 27[31], N-methyl-D-aspartate receptors [54] and NLRP3 inflammasome [45]. In this study, we found that chronic morphine treatment led to the down-regulation of spinal melatonin expression, while exogenous melatonin could alleviate morphine tolerance via inhibiting the activation of microglia and NLRP3 inflammasome, as shown by the down-regulation of spinal Iba1, NLRP3, P20 and IL-1β expression. These results demonstrated the important role of melatonin in delaying morphine tolerance. Previous study reported that melatonin could restore the upregulated TLR2 expression in gastric mucosa of the infected mice and suppress the production of inflammatory factors [55]. In chronic airway inflammation, the up-regulation of TLR2 could inhibit the synthesis of endogenous melatonin and thus induce airway inflammation [19]. We proved that TLR2 inhibitor could only partially restored the melatonin level in spinal cord. These suggest that the decrease in melatonin release might not be directly caused by morphine, but by TLR2 activation in spinal cord. And the activation of TLR2 induced by chronic morphine treatment might be partially due to the decrease of spinal melatonin. Activated TLR2 and down-regulation of melatonin may co-mediate the activation of microglia and NLRP3 inflammasome during the development of morphine tolerance.
In summary, it could be reasonably inferred that there may be a TLR2-melatonin negative feedback loop which regulates microglia and NLRP3 inflammasome activation during the development of morphine tolerance. The mechanisms involved in the interaction between TLR2 and melatonin require further exploration. And TLR2 inhibitor and melatonin may be the promising therapeutic medicines for morphine tolerance.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fields HL (2011) The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69(4):591–594. https://doi.org/10.1016/j.neuron.2011.02.001
Abdel-Zaher AO, Mostafa MG, Farghaly HS, Hamdy MM, Abdel-Hady RH (2013) Role of oxidative stress and inducible nitric oxide synthase in morphine-induced tolerance and dependence in mice. Effect of alpha-lipoic acid. Behav Brain Res 247:17–26. https://doi.org/10.1016/j.bbr.2013.02.034
Kong H, Jiang CY, Hu L, Teng P, Zhang Y, Pan XX, Sun XD, Liu WT (2019) Morphine induces dysfunction of PINK1/Parkin-mediated mitophagy in spinal cord neurons implying involvement in antinociceptive tolerance. J Mol Cell Biol 11(12):1056–1068. https://doi.org/10.1093/jmcb/mjz002
Dang VC, Christie MJ (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165(6):1704–1716. https://doi.org/10.1111/j.1476-5381.2011.01482.x
Chao PK, Chang HF, Ou LC, Chuang JY, Lee PT, Chang WT, Chen SC, Ueng SH, Hsu JT, Tao PL, Law PY, Loh HH, Yeh SH (2019) Convallatoxin enhance the ligand-induced mu-opioid receptor endocytosis and attenuate morphine antinociceptive tolerance in mice. Sci Rep 9(1):2405. https://doi.org/10.1038/s41598-019-39555-x
Eidson LN, Murphy AZ (2019) Inflammatory mediators of opioid tolerance: implications for dependency and addiction. Peptides 115:51–58. https://doi.org/10.1016/j.peptides.2019.01.003
Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 24(33):7353–7365. https://doi.org/10.1523/JNEUROSCI.1850-04.2004
Zhang H, Li F, Li WW, Stary C, Clark JD, Xu S, Xiong X (2016) The inflammasome as a target for pain therapy. Br J Anaesth 117(6):693–707. https://doi.org/10.1093/bja/aew376
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426. https://doi.org/10.1016/s1097-2765(02)00599-3
Kim JJ, Jo EK (2013) NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci 28(10):1415–1423. https://doi.org/10.3346/jkms.2013.28.10.1415
Wang H, Huang M, Wang W, Zhang Y, Ma X, Luo L, Xu X, Xu L, Shi H, Xu Y, Wang A, Xu T (2021) Microglial TLR4-induced TAK1 phosphorylation and NLRP3 activation mediates neuroinflammation and contributes to chronic morphine-induced antinociceptive tolerance. Pharmacol Res 165:105482. https://doi.org/10.1016/j.phrs.2021.105482
Cai Y, Kong H, Pan YB, Jiang L, Pan XX, Hu L, Qian YN, Jiang CY, Liu WT (2016) Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J Neuroinflammation 13(1):53. https://doi.org/10.1186/s12974-016-0520-z
Zhang HM, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57(2):131–146. https://doi.org/10.1111/jpi.12162
Zisapel N (2018) New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 175(16):3190–3199. https://doi.org/10.1111/bph.14116
Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade. J Pineal Res 65(4):e12525. https://doi.org/10.1111/jpi.12525
Yuan H, Wu G, Zhai X, Lu B, Meng B, Chen J (2019) Melatonin and rapamycin attenuate Isoflurane-Induced Cognitive Impairment through Inhibition of Neuroinflammation by suppressing the mTOR Signaling in the Hippocampus of aged mice. Front Aging Neurosci 11:314. https://doi.org/10.3389/fnagi.2019.00314
Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S (2019) Melatonin attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol 10:1511. https://doi.org/10.3389/fimmu.2019.01511
Wu HM, Zhao CC, Xie QM, Xu J, Fei GH (2020) TLR2-Melatonin feedback Loop regulates the activation of NLRP3 inflammasome in Murine allergic airway inflammation. Front Immunol 11:172. https://doi.org/10.3389/fimmu.2020.00172
Schilling S, Chausse B, Dikmen HO, Almouhanna F, Hollnagel JO, Lewen A, Kann O (2021) TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner. Brain Behav Immun 96:80–91. https://doi.org/10.1016/j.bbi.2021.05.013
Gill R, Tsung A, Billiar T (2010) Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med 48(9):1121–1132. https://doi.org/10.1016/j.freeradbiomed.2010.01.006
Kopiasz L, Dziendzikowska K, Gajewska M, Oczkowski M, Majchrzak-Kuligowska K, Krolikowski T, Gromadzka-Ostrowska J (2021) Effects of Dietary Oat Beta-Glucans on Colon Apoptosis and Autophagy through TLRs and Dectin-1 Signaling Pathways-Crohn’s Disease Model Study. Nutrients 13(2). https://doi.org/10.3390/nu13020321
Milanesi S, Verzola D, Cappadona F, Bonino B, Murugavel A, Pontremoli R, Garibotto G, Viazzi F (2019) Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J Cell Physiol 234(7):10868–10876. https://doi.org/10.1002/jcp.27929
Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR (2015) Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 21(11):1326–1331. https://doi.org/10.1038/nm.3978
Lacagnina MJ, Watkins LR, Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol Ther 184:145–158. https://doi.org/10.1016/j.pharmthera.2017.10.006
Chen J, Wang G, Sun T, Ma C, Huo X, Kong Y (2021) Involvement of TCF7L2 in generation of morphine-induced antinociceptive tolerance and hyperalgesia by modulating TLR4/ NF-kappaB/NLRP3 in microglia. Toxicol Appl Pharmacol 416:115458. https://doi.org/10.1016/j.taap.2021.115458
Qu J, Tao XY, Teng P, Zhang Y, Guo CL, Hu L, Qian YN, Jiang CY, Liu WT (2017) Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. J Neuroinflammation 14(1):228. https://doi.org/10.1186/s12974-017-0997-0
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924. https://doi.org/10.4049/jimmunol.173.6.3916
Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, Ye B, Lian Q, Zhuo W, Si J, Chen S, Wang L (2021) Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunol Res 9(10):1111–1124. https://doi.org/10.1158/2326-6066.CIR-20-1019
Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22(1):114–123. https://doi.org/10.1016/j.bbi.2007.07.014
Lin SH, Huang YN, Kao JH, Tien LT, Tsai RY, Wong CS (2016) Melatonin reverses morphine tolerance by inhibiting microglia activation and HSP27 expression. Life Sci 152:38–43. https://doi.org/10.1016/j.lfs.2016.03.032
Lin F, Shan W, Zheng Y, Pan L, Zuo Z (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem 158(2):328–341. https://doi.org/10.1111/jnc.15368
Liu D, Zhou Y, Peng Y, Su P, Li Z, Xu Q, Tu Y, Tian X, Yang H, Wu Z, Mei W, Gao F (2018) Endoplasmic reticulum stress in spinal cord contributes to the development of Morphine Tolerance. Front Mol Neurosci 11:72. https://doi.org/10.3389/fnmol.2018.00072
Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J (2016) Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model, Neural Plast (2016) 5238730.https://doi.org/10.1155/2016/5238730
Yang H, Wu L, Deng H, Chen Y, Zhou H, Liu M, Wang S, Zheng L, Zhu L, Lv X (2020) Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-kappaB signaling pathway in spinal microglia. J Neuroinflammation 17(1):154. https://doi.org/10.1186/s12974-020-1731-x
Wang X, Tian S, Wang H, Liu P, Zheng H, Wu L, Liu Q, Wu W (2020) Botulinum toxin type a alleviates neuropathic pain and suppresses inflammatory cytokines release from microglia by targeting TLR2/MyD88 and SNAP23. Cell Biosci 10(1):141. https://doi.org/10.1186/s13578-020-00501-4
Zhang L, Meng J, Ban Y, Jalodia R, Chupikova I, Fernandez I, Brito N, Sharma U, Abreu MT, Ramakrishnan S, Roy S (2019) Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A 116(27):13523–13532. https://doi.org/10.1073/pnas.1901182116
Thomas JHL, Lui L, Abell A, Tieu W, Somogyi AA, Bajic JE, Hutchinson MR (2022) Toll-like receptors change morphine-induced antinociception, tolerance and dependence: studies using male and female TLR and signalling gene KO mice. Brain Behav Immun 102:71–85. https://doi.org/10.1016/j.bbi.2022.02.001
Kwilasz AJ, Todd LS, Duran-Malle JC, Schrama AEW, Mitten EH, Larson TA, Clements MA, Harris KM, Litwiler ST, Wang X, Van Dam AM, Maier SF, Rice KC, Watkins LR, Barrientos RM (2021) Experimental autoimmune encephalopathy (EAE)-induced hippocampal neuroinflammation and memory deficits are prevented with the non-opioid TLR2/TLR4 antagonist (+)-naltrexone. Behav Brain Res 396:112896. https://doi.org/10.1016/j.bbr.2020.112896
Wang L, Yang HY, Zang CX, Shang JM, Liu H, Zhang ZH, Yuan FY, Ju C, Li FY, Bao XQ, Zhang D (2021) TLR2 potentiates SR-Marco-Mediated neuroinflammation by interacting with the SRCR Domain. Mol Neurobiol 58(11):5743–5755. https://doi.org/10.1007/s12035-021-02463-1
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65(1):223–254. https://doi.org/10.1124/pr.112.005942
Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, Kielian T (2008) Microglia and astrocyte activation by toll-like receptor ligands: modulation by PPAR-gamma agonists. PPAR Res 2008:453120. https://doi.org/10.1155/2008/453120
Gong X, Chen Y, Chang J, Huang Y, Cai M, Zhang M (2018) Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J Neuroinflammation 15(1):262. https://doi.org/10.1186/s12974-018-1301-7
Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P (2017) Microglia activation and Schizophrenia: Lessons from the Effects of Minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr Bull 43(3):493–496. https://doi.org/10.1093/schbul/sbw088
Liu Q, Su LY, Sun C, Jiao L, Miao Y, Xu M, Luo R, Zuo X, Zhou R, Zheng P, Xiong W, Xue T, Yao YG (2020) Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol 34:101560. https://doi.org/10.1016/j.redox.2020.101560
Derangula K, Javalgekar M, Kumar Arruri V, Gundu C, Kumar Kalvala A, Kumar A (2022) Probucol attenuates NF-kappaB/NLRP3 signalling and augments Nrf-2 mediated antioxidant defence in nerve injury induced neuropathic pain. Int Immunopharmacol 102:108397. https://doi.org/10.1016/j.intimp.2021.108397
Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM (2021) Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun 91:142–158. https://doi.org/10.1016/j.bbi.2020.09.017
Tian L, Yan J, Li K, Zhang W, Lin B, Lai W, Bian L, Liu H, Xi Z, Liu X (2021) Ozone exposure promotes pyroptosis in rat lungs via the TLR2/4-NF-kappaB-NLRP3 signaling pathway. Toxicology 450:152668. https://doi.org/10.1016/j.tox.2020.152668
Duffy EB, Periasamy S, Hunt D, Drake JR, Harton JA (2016) FcgammaR mediates TLR2- and syk-dependent NLRP3 inflammasome activation by inactivated Francisella tularensis LVS immune complexes. J Leukoc Biol 100(6):1335–1347. https://doi.org/10.1189/jlb.2A1215-555RR
Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR (2011) Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc 110(8):487–494. https://doi.org/10.1016/S0929-6646(11)60074-0
Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, Zagrean AM (2020) Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 10(9). https://doi.org/10.3390/biom10091211
Gonzalez-Gonzalez A, Garcia Nieto E, Gonzalez A, Sanchez-Fernandez C, Alonso-Gonzalez C, Menendez-Menendez J, Gomez-Arozamena J, Cos S (2019) Martinez-Campa, Melatonin Modulation of Radiation and Chemotherapeutics-induced changes on differentiation of breast fibroblasts. Int J Mol Sci 20(16). https://doi.org/10.3390/ijms20163935
Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L (2020) Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther 11(1):259. https://doi.org/10.1186/s13287-020-01756-x
Song L, Wu C, Zuo Y (2015) Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol 15:12. https://doi.org/10.1186/1471-2253-15-12
Luo J, Song J, Zhang H, Zhang F, Liu H, Li L, Zhang Z, Chen L, Zhang M, Lin D, Lin M, Zhou R (2018) Melatonin mediated Foxp3-downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. pylori infected mice. Int Immunopharmacol 64:116–122. https://doi.org/10.1016/j.intimp.2018.08.034
Funding
We would like to thank the National Natural Science Foundation of China (Grant no. 81974168) for their support in this research.
Author information
Authors and Affiliations
Contributions
Xiaoling Peng wrote the main manuscript text. Jihong Wang and Zheng Li prepared Figs. 1 and 2. Xiaoqian Jia, Anqi Zhang and Jie Ju prepared Figs. 3, 4 and 5. Volker Eulenburg and Feng Gao revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflict of financial interest or benefit. Informed consent was obtained from all individual participants included in the study.
Ethical Approval
The animal research was approved by the Animal Experimental Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Ethics Approval Number: TJH-202007007).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, X., Wang, J., Li, Z. et al. Toll-like Receptor 2-Melatonin Feedback Loop Regulates the Activation of Spinal NLRP3 Inflammasome in Morphine-Tolerant Rats. Neurochem Res 48, 3597–3609 (2023). https://doi.org/10.1007/s11064-023-03998-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03998-6