Abstract
Spinal cord injuries (SCI) are complex and cause complex neurological disorders with serious implications for the health of society. Excessive neuroinflammation is one of the pathogenesis of trauma-related central nervous system (CNS) dysfunction. The initiation of inflammatory response mainly stems from neuronal necrosis in the central nervous system. The therapeutic effects and underlying mechanisms of zinc targeting neurons were investigated in vivo and in vitro using protein chips, western blotting, reactive oxygen species (ROS) activity assays, ELISA, RT-qPCR, and immunostaining. In this study, we found that zinc promotes functional recovery. Specifically, we found that zinc increased neuronal survival and suppressed lesion size and focal apoptosis levels in vivo. Zinc administration confers neuroprotection by inhibiting NLRP3 inflammasome-associated cytokine levels probed with a protein chip. Furthermore, we found that zinc promoted SIRT3-mediated induction of autophagy, which abrogated inflammatory responses and mitochondrial ROS production in the injured spinal cord and cultured neurons. These findings suggest that zinc improves neuroinflammation and improves dyskinesia after SCI. In conclusion, zinc may be a potential therapeutic immunomodulatory challenge for the treatment of trauma-related CNS dysfunction.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is one of the most prevalent traffic accident complications worldwide [1], characterized by sensory and/or motor dysfunction [2]. Most clinical treatments are based on high-dose glucocorticoid shock and have many side effects [3]. Therefore, novel strategies are needed for the therapy of SCI. Although many recent strategies have focused on the molecular mechanisms of SCI, motor dysfunction remains a devastating outcome for patients and may be exacerbated by persistent neuronal necrosis. Our previous studies have shown that overproduction of reactive oxygen species (ROS) is associated with the development and progression of neuronal survival in the acute phase of SCI [4]. Moreover, we have demonstrated that NLRP3 inflammasome plays an important role in neuron death after SCI [5]. In addition, we found that the activation of neuronal autophagy is one of the pathophysiological processes of spinal cord injury [6]. Activation of the known NLRP3 inflammasome can amplify the accumulation of inflammatory cytokines at the injury and in turn, inflammatory cytokines induced by trauma can promote the NLRP3 inflammasome in SCI [7, 8]. In the animal SCI contusion model, IL-1 β overexpression suggests that NLRP3 inflammasome and related signaling pathways are the main mechanisms of the progression of SCI [9].
Mammalian sirtuin (SIRT) 3 is relevant to cell cycle, aging, cancer inhibition, mitochondrial function [10, 11], and metabolism [12]. Current evidence has proved the beneficial effects of SIRT3 under the natural situation, which is mitochondrial NAD-dependent deacetylase [11]. Moreover, SIRT3 can inhibit IL-1β release and ROS production [13], and promote autophagy upregulation [14]. Therefore, SIRT3 has a neuroprotective effect on the central nervous system (CNS) via targeting mitochondrial ROS and NLRP3 inflammasome [15]. Additionally, autophagy plays an integral role in cell steady state. [16] Autophagy has a protective effect at the initiation of damage and can downregulate inflammatory cytokines, especially IL-1β production [17]. Moreover, SIRT3 participates in the mTOR nutrient-sensing pathway and relies on LC3B expression to regulate autophagy activity [18]. Current results have supported that autophagy inhibits the activation of NLRP3 inflammasome in immune-mediated diseases, including SCI [19].
Zinc (Zn) has been proved to involve in growth and development, immune ability, and vitamin functions [20]. Zinc gluconate (ZnG) is an organic zinc supplement that has little stimulation to the gastric mucosa and is easily absorbed by the human body in vivo with a high absorption rate and good solubility [21]. The functional recovery in SCI is largely mediated by neuronal survival, and Zn can effectively modulate neuronal autophagy and neuroinflammation. However, the specific molecular mechanism of ZnG on SCI has not yet been determined. Previous studies have shown the therapeutic effects of ZnG in a contusive SCI model and the mechanism of action of this compound, including up-regulation of SIRT3-mediated autophagy and down-regulation of ROS-mediated NLRP3 inflammasome. These results suggest that ZnG can be a novel drug for the treatment of acute SCI.
Materials and Methods
Mouse Contused SCI Model
Male 8-week-old C57BL/6 mice were placed with standard rodent chow and water in a controlled location. Animals were kept at 22 ± 1 °C with a 12 h light, 12 h dark cycle. Mice spinal cord contusion model was conducted by Allen’s method. Mice were anesthetized by the injection of uratan (30 mg/kg, i.p.) and placed in a prone position. Then, a T8/9 spinal cord laminectomy was used. An impact device with a mass of 10 g and a diameter of 2 mm was dropped on the spinal cord from 25 mm height. The spinal cord was rinsed with 0.9% saline and sutured the incisions after surgery. The bladder was massaged twice a day until bladder function regained normally. Sham group only performed laminectomy. These mice were divided into three groups and administered daily either ZnG (various concentrations) or vehicle (saline) by intraperitoneal injection. The study was permitted by the Jinzhou Medical University Review Board for the care of animals.
Immunochemical Staining
Spinal cord tissues were soaked in 4% paraformaldehyde for 3 days, added to 30% sucrose in 4% paraformaldehyde, and embedded in paraffin for hematoxylin and eosin staining, Nissl staining, and TUNEL staining. IL-1β (ab9722, Abcam) and LC3B (ab48394, Abcam) were used in spinal cord sections, followed by incubation with biotinylated secondary antibodies (Sigma-Aldrich).
Western Blot Analysis
Proteins were extracted from spinal cord tissues and cultured cells and used antibodies against mouse NLRP3 (15101S, Cell Signaling Technology), Caspase-1 (3866S, Cell Signaling Technology), IL-1β (ab9722, Abcam), Nrf2 (ab31163, Abcam), Atg5 (ab108327, Abcam), LC3B (ab48394, Abcam), SIRT3 (2627S, Cell Signaling Technology), and Goat per-oxidase (HRP)-conjugated IgG antibodies (SA0000-2, Proteintech). β-Tubulin (10094–1-1AP, Proteintech) was done as a control protein. The membranes were developed using ChemiDoc-ItTMTS2Imager (UVP, LLC, Upland, CA, USA), and relative optical density was analyzed by ImageJ2x software(National Institute of Health, Bethesda, MD, USA).
Quantitative Real-Time PCR Analysis
A 0.5 cm length of spinal cord tissue after the mice were sacrificed by the overdose of anesthetic, and primary astrocytes were taken from the injured point for an experiment of quantitative real-time PCR (qRT-PCR), as described [22]. Total RNA extracts were obtained using TRIzol Reagent (Ambion, Foster City, CA, USA), and 5 μg of total RNA was used to synthesize cDNA (Promega, Fitchburg, WI, USA). Samples were analyzed by real-time polymerase chain reaction (RT-PCR) and SYBR Green Supermix used primers in parallel for analysis. The primers for NLRP3 and RPS18 are given in Table 1.
MDA and SOD Activity Assay
To weight the spinal cord tissue including the injury point according to the manufacturer's instructions, add the extract, homogenize it in the ice bath, centrifuge 8000 g at 4 °C for 10 min, and take the supernatant. The levels of SOD and MDA in the supernatant were measured using a commercial kit. The absorbance at 560 nm (SOD) and 532 nm (MDA) was measured with a microplate reader. The concentration was calculated according to the manufacturer's instructions and standardized as the total protein concentration.
Cell Injury Models
The murine neurons PC12 cells line was cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and 200 μL penicillin–streptomycin solution (Gibco) at 37 °C in a 5% CO2 incubator. Briefly, cells were incubated with lipopolysaccharides (LPS, 1 μg/mL) and adenosine triphosphate (ATP, 5 mmol/L) for 6 h to serve as the priming signal of the NLRP3 inflammasome, and then treated with ZnG (100 μM) for 24 h. These cells were used to measure mitochondrial ROS activity.
Stable Expression of SIRT3 shRNA
Lentivirus transduction particles carrying shSIRT3 in PC12 cells were constructed according to the manufacturer's instructions as described.[23] The cells were then infected with lentivirus-bearing specific shRNAs of SIRT3 to select stably infected cells for further experiments.
Data Analysis
Data were expressed as mean ± SD and analyzed by SPSS 19.0. Student’s t-test and one-way ANOVA determined the data of two groups and more groups. In addition, the BMS tests were analyzed using the Mann–Whitney U-test. Differences were considered statistically significant with a value of p < 0.05.
Results
Appropriate Concentration of ZnG to Promote Functional Recovery After SCI
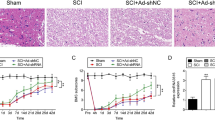
The administrations of ZnG were injected at various concentrations (10, 30, 50, 100 mg/kg) after SCI, and the mice were sacrificed four weeks post-injury. Based on the results of BMS scores, this concentration (30 mg/kg) of ZnG was beneficial to mice for a longer period of four weeks post-injury (Fig. 1A, 1B). After four weeks following the treatment of ZnG, the lesion size of hit points in the injured spinal cord decreased significantly compared to the SCI group (Fig. 1C). Moreover, the staining results showed a decreased area of spared white matter in the injured lesion for the SCI group, substantially increased in the ZnG group (Fig. 1D). Four weeks after the treatment, the ventral neurons were significantly reduced in the SCI group compared to the Sham group, the group treated with ZnG highly induced neurons survival in the ventral horn (Fig. 1E). The assessment of neurons number revealed substantial improvement by the administration of ZnG (Fig. 1F). The images of TUNEL staining revealed a significantly decreased apoptosis level after the treatment of ZnG (Fig. 1G, 1H). Based on these results, the proper concentrations of ZnG (at 30 mg/kg) were beneficial to SCI mice in vivo experiments.
Representative BMS scores of mice from various concentration of ZnG groups at different time points (A) and 4 weeks post-injury (B) (n = 10–14/group). H&E staining of the spinal cord of three groups (C) and semiquantitative analysis (D) (n = 6 /group). Scale bar = 25 μm. Nissl staining of the spinal cord of three groups (E) and semiquantitative analysis (F) (n = 6 /group). Scale bar = 100 μm. TUNEL staining of the spinal cord of three groups (G) and semiquantitative analysis (H) (n = 6 /group). Scale bar = 100 μm. *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data presented as mean ± SD
Administration of ZnG to Decrease the Expressions of Proinflammatory Cytokines in Injured Spinal Cord Tissue
The pathological process of SCI involves the progression of inflammation. The main change during acute SCI is upregulation in the expression of inflammation-related cytokines. We assessed cytokine expression by assessing protein chips using injured local spinal cord tissue from the SCI model treated with ZnG or vehicle (Figs. 2A). Moreover, we found that many pro-inflammation cytokines (including IL-1β, TNF-α, IL-6) in the ZnG group were decreased on the first day compared to the SCI group, and anti-inflammatory cytokines (including IL-10, G-CSF) upregulated in one-day post-injury mice treated with ZnG (Fig. 2B). After being injected with ZnG for three days, anti-inflammatory cytokines display more significantly increased levels compared to the SCI group (Fig. 2C). According to previous studies [3], most of the changed profiles of cytokines were related to the activation or initiation of the NLRP3 inflammasome. Collectively, these results indicated that ZnG may be involved in the process of NLRP3 inflammasome in acute SCI.
Administration of ZnG to Inhibit NLRP3 Inflammasome in Injured Spinal Cord
To examine whether ZnG specifically regulates the activity of NLRP3 inflammasome in 3-days post-injury, we stained the expression of IL-1β in injured spinal cord lesions. As shown in Fig. 3A, the immunohistochemical staining of IL-1β displayed significant production in the SCI group compared to the Sham group and could decrease the expression of IL-1β (Fig. 3A). Besides, the protein levels of inflammation-related factors via western blot (Fig. 3B), statistics showed that NLRP3 (Fig. 3C), Caspase-1 (Fig. 3D), and IL-1β (Fig. 3E) were significantly reduced in the treatment group compared with SCI mice, although the former showed greatly increased protein levels compared to Sham mice. Moreover, the treatment of ZnG exhibited significantly downregulated mRNA levels of these genes including NLRP3 (Fig. 3F), Caspase-1 (Fig. 3G), and IL-1β (Fig. 3H) compared with SCI mice. These results showed that ZnG therapy inhibits the activation of NLRP3 inflammasome after SCI for 3 days.
Immunohistochemically staining of mice in three groups (A). Scale bar = 100 μm. Western blot of the spinal cord in three groups (B) and semiquantitative analysis of NLRP3 (C), Caspase-1 (D) and IL-1β (E). Representative RT-qPCR analysis of NLRP3 (F), Caspase-1 (G) and IL-1β (H) in three groups *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001.Data presented as mean ± SD. (n = 6 /group)
Treatment of ZnG to Promote Autophagy in Injured Spinal Cord
Autophagy reduces NLRP3 inflammasome activation, whereas activated SIRT3 promotes autophagy. As shown in Fig. 4A, the levels of LC3B were significantly induced after treatment with ZnG. These results indicated that the expression of Atg5 (Fig. 4B), LC3B (Fig. 4C) proteins were gradually upregulated until the third day after treatment with ZnG (Fig. 4D). Next, we assessed whether SIRT3 could be modulated by ZnG. As shown in Fig. 4E, the results showed that ZnG treatment significantly increased SIRT3 levels compared with SCI mice, and the statistical results were shown in Fig. 4F. In addition, the mRNA levels of SIRT3 confirmed the same results (Fig. 4G). These results suggest that downregulation of the NLRP3 inflammasome and activation of autophagy are temporally synchronized following treatment with ZnG.
Immunohistochemically staining of mice in three groups (A). Scale bar = 100 μm. Western blot of the spinal cord of groups in various times (D) and semiquantitative analysis of Atg5 (B), and LC3B (C). Western blot of the spinal cord in three groups (E) and semiquantitative analysis of SIRT3 (F). Representative RT-qPCR analysis of SIRT3 (G) in three groups *p < 0.1, ** p < 0.01, ***p < 0.001, ****p < 0.0001.Data presented as mean ± SD. (n = 6 /group)
Injection of ZnG to Suppress ROS Production in Injured Spinal Cord
The production of mitochondrial ROS plays an essential role in the activation of the NLRP3 inflammasome. Both SCI mice from SCI and ZnG groups had significantly higher ROS levels compared to control and Sham mice, but this activation of ROS was significantly inhibited in the mice treated with ZnG (Fig. 5A, B). As shown in Fig. 3C, the levels of Nrf2, as an antioxidant-specific protein, were significantly increased in both ZnG and SCI mice compared with control and sham mice, but this effect was induced in ZnG mice (Fig. 5C, D). In contrast, MDA was greatly reduced in ZnG mice compared with SCI mice (Fig. 5E). Furthermore, a greatly increased SOD was observed in ZnG mice compared with SCI mice (Fig. 5F). These results suggest that ZnG downregulates ROS activity, which is the main initiating signal of the NLRP3 inflammasome.
ROS staining of mice in four groups (A) and semiquantitative analysis (B). Scale bar = 100 μm. Western blot of the spinal cord in four groups (C) and semiquantitative analysis of Nrf2 (D). Representative MDA (E) and SOD (F) analysis of the spinal cord in four groups *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001.Data presented as mean ± SD. (n = 6/group)
Administration of ZnG to Induce Autophagy-Mediated Inhibition of NLRP3 Inflammasome in Cultured Neurons
Cell viability was tested by MTT assay, which showed 100 μmol/L ZnG was the optimal dose for PC12 cells (Fig. 6A). It has been reported that activation of the NLRP3 inflammasome requires both priming signals such as LPS and activation signals such as ATP to produce mature IL-1β [9]. Next, to test whether ZnG could regulate neuron activity after treated LPS + ATP, we found that LDH release was significantly increased after culturing with LPS + ATP, compared to the control group, ZnG downregulated LDH release (Fig. 6B) and reversed cell death induced by LPS + ATP stimulation (Fig. 6C).
Cell viability of ZnG to PC12 cells (A). LDH release of three groups to PC12 cells (B). BrdU percentage of three groups to PC12 cells (C). Quantitative analysis of IL-1β of five groups in ELISA (D). Immunohistochemically staining of LC3B in five groups (E). Scale bar = 100 μm. Western blot of cells in five groups (F) and semiquantitative analysis of Atg5 (G) and LC3B (H). IP of IL-1β of cells in four groups. ZnG induced autophagy-mediated inhibition of NLRP3 inflammasome in PC12 cells (I). *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data presented as mean ± SD (n = 6/group)
Autophagy can inhibit priming and activation signals of the NLRP3 inflammasome [23], so we examined whether ZnG can suppress the activation of the NLRP3 inflammasome in PC12 neurons. Rapamycin stimulates autophagy in mammals [24, 25]. As shown in Fig. 6D, IL-1β production was reduced in cells treated with rapamycin or ZnG compared to LPS + ATP-induced cells. The production of LC3B (Fig. 6E–H and S3) and Atg5 (Fig. 6F, G) was increased after incubation with rapamycin or ZnG compared to LPS + ATP-induced cells, but these effects were suppressed by 3 -MA reversal, an autophagy inhibitor. Furthermore, rapamycin or ZnG significantly downregulated the expression of mature IL-1β by immunoprecipitation (Fig. 6I). Furthermore, ZnG inhibited the production of NLRP3 and mature IL-1β based on the western blot analysis, but this effect was altered by the addition of 3-MA (Figure S2A-2C). The results suggest that ZnG exerts its anti-inflammatory effect by promoting autophagy activation and subsequently inhibiting NLRP3 inflammasome activity.
Application of ZnG to Downregulate IL-1β Production Through Enhancing SIRT3-Mediated Autophagy in Cultured Neurons
SIRT3 has been reported to enhance autophagy [26]. As shown in Fig. 7A, the treatment with ZnG or rapamycin significantly increased the protein expression of SIRT3, and that effect was reversed by 3-MA (Fig. 7B). To clarify whether SIRT3 is involved in autophagy activation in cells treated with ZnG. As shown in Fig. 7C, ZnG treatment significantly increased Atg5 expression in PC12 cells (Fig. 7D), but this effect was abolished in cells lacking SIRT3 (Fig. 7E). Furthermore, although ZnG decreased IL-1β secretion, this effect was reversed by the administration of shSIRT3 (Fig. S3). Taken together, the results suggest that ZnG blocks the activation of the NLRP3 inflammasome through SIRT3-mediated induction of autophagy, thereby promoting neuronal survival.
Discussion
In this learning process, we found the neuroprotective effect of ZnG in SCI mice and the molecular mechanism for suppression of ROS-mediated NLRP3 inflammasome and activation of SIRT3-mediated NLRP3 inflammasome downregulation. The previous study has put examples because of the over-producing of ROS activates the NLRP3 inflammasome involved in the progress of the mouse SCI model and cultured neurons [4]. SIRT3 has been suggested to suppress the mitochondrial ROS producing and induce autophagy in CNS disorders [11]. Our finding of the molecular effects of ZnG on SIRT3 expression suggests that changes in these proteins are consistent with activation of autophagy. These observations are associated with the downregulation of the NLRP3 inflammasome protein in the injured spinal cord and neurons after culture. Treated with ZnG process.
To assess the role of ZnG in upstream conduction of the NLRP3 inflammasome, we evaluated the effect of this pure compound on SCI mice and LPS + ATP-treated cultured neurons. We showed ZnG treatment-induced autophagy in neurons within 1–3 days. Furthermore, these results indicated that ZnG-treated mice or neurons induced decreased expression of initiation and activation signals of the NLRP3 inflammasome, but induction of the NLRP3 inflammasome was again elevated after treatment with 3-MA. Moreover, the results showed that ZnG had a significant neuroprotective effect on SCI mice in vivo, and functional recovery was improved after ZnG injection. Consistent with the in vivo and in vitro experiments, we found that the expression of autophagy-related proteins increased substantially as early as the first day after SCI and lasted for three days. Using saline-treated mice as a control, the effect of ZnG on autophagy induction in SCI mice and its potential therapeutic effect on SCI were confirmed.
Previous evidence has demonstrated that ATP was an important component involving the NLRP3 inflammasome-mediated mature IL-1β release [23]. In this way, the priming signal and formation of NLRP3 inflammasome might need both ATP and LPS and trigger neuroinflammation in SCI. The death of spinal cord neurons is the main sign of SCI. In our previous study, we have indicated that ROS production and activation of the NLRP3 inflammasome triggered by LPS + ATP are major secondary lesions in the progression of SCI. However, whether other types of nerve cells, such as astrocytes, are present in the injured spinal cord, and whether their role in the mechanism warrants further investigation.
According to these results, ZnG therapy improved behavior and pathological recovery after SCI, possibly subsequently increasing the autophagic response and reducing the production of inflammation. While the exact mechanism of SCI warrants further investigation, the emergence of complex interactions during autophagy induction and the NLRP3 inflammasome remains unclear. Activation and response of the NLRP3 inflammasome occur in SCI mice. Our hypothesis is supported by the following results of this study. Application of an autophagy inhibitor (3-MA) restored IL-1β cytokine release, indicating that ZnG induced autophagy in LPS + ATP-induced cultured neurons. Although enhanced induction of autophagy was observed in SCI mice, autophagy activation may be a protective mechanism during acute SCI. In the present study, ZnG mediated SIRT3-related autophagy induction and autophagy-related NLPR3 inflammasome downregulation.
Conclusions
In conclusion, ZnG effectively enhanced the recovery of SCI. This beneficial effect may include inactivation of the mitochondrial ROS-initiating NLRP3 inflammasome and differential regulation of the autophagy/NLRP3 inflammasome axis by mediating SIRT3. Therefore, it is necessary to further develop ZnG therapy as a candidate for SCI recovery. Combining all the experimental results, we believe that zinc ions have anti-inflammatory and neuroprotective effects, which can shed light on the clinical treatment of spinal cord injury.
Data Availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- SCI:
-
Spinal cord injury
- CNS:
-
Central nervous system
- ROS:
-
Reactive oxygen species
- SIRT:
-
Sirtuin
- qRT-PCR:
-
Quantitative real-time PCR
- RPS18:
-
Protein S18
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- DMEM:
-
Dulbecco’s modified eagle medium
- LPS:
-
Lipopolysaccharides
- ATP:
-
Adneosine triphosphate
References
Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, Fehlings MG (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018. https://doi.org/10.1038/nrdp.2017.18
McDonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet 359:417–425. https://doi.org/10.1016/S0140-6736(02)07603-1
Bracken MB (2012) Steroids for acute spinal cord injury. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001046.pub2
Li D, Tian H, Li X, Mao L, Zhao X, Lin J, Lin S, Xu C, Liu Y, Guo Y, Mei X (2020) Zinc promotes functional recovery after spinal cord injury by activating Nrf2/HO-1 defense pathway and inhibiting inflammation of NLRP3 in nerve cells. Life Sci 245:117351. https://doi.org/10.1016/j.lfs.2020.117351
Lin JQ, Tian H, Zhao XG, Lin S, Li DY, Liu YY, Xu C, Mei XF (2021) Zinc provides neuroprotection by regulating NLRP3 inflammasome through autophagy and ubiquitination in a spinal contusion injury model. CNS Neurosci Ther 27:413–425. https://doi.org/10.1111/cns.13460
Lin S, Tian H, Lin J, Xu C, Yuan Y, Gao S, Song C, Lv P, Mei X (2020) Zinc promotes autophagy and inhibits apoptosis through AMPK/mTOR signaling pathway after spinal cord injury. Neurosci Lett 736:135263. https://doi.org/10.1016/j.neulet.2020.135263
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. https://doi.org/10.1038/s41577-019-0165-0
Mortezaee K, Khanlarkhani N, Beyer C, Zendedel A (2018) Inflammasome: its role in traumatic brain and spinal cord injury. J Cell Physiol 233:5160–5169. https://doi.org/10.1002/jcp.26287
Zendedel A, Mönnink F, Hassanzadeh G, Zaminy A, Ansar MM, Habib P, Slowik A, Kipp M, Beyer C (2018) Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol 55:1364–1375. https://doi.org/10.1007/s12035-017-0400-2
Chen J, Wang A, Chen Q (2017) SirT3 and p53 deacetylation in aging and cancer. J Cell Physiol 232:2308–2311. https://doi.org/10.1002/jcp.25669
Salvatori I, Valle C, Ferri A, Carrì MT (2017) SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem Int 109:184–192. https://doi.org/10.1016/j.neuint.2017.04.012
Zheng Y, Shi B, Ma M, Wu X, Lin X (2019) The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J Cell Physiol 234:5488–5495. https://doi.org/10.1002/jcp.27329
Zhou TY, Wu YG, Zhang YZ, Bao YW, Zhao Y (2019) SIRT3 retards intervertebral disc degeneration by anti-oxidative stress by activating the SIRT3/FOXO3/SOD2 signaling pathway. Eur Rev Med Pharmacol Sci 23:9180–9188. https://doi.org/10.26355/eurrev_201911_19408
Han D, Jiang L, Gu X, Huang S, Pang J, Wu Y, Yin J, Wang J (2020) SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J Cell Physiol 235:8839–8851. https://doi.org/10.1002/jcp.29727
Tyagi A, Nguyen CU, Chong T, Michel CR, Fritz KS, Reisdorph N, Knaub L, Reusch J, Pugazhenthi S (2018) SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep 8:17547. https://doi.org/10.1038/s41598-018-35890-7
Dikic I, Elazar Z (2018) Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19:349–364. https://doi.org/10.1038/s41580-018-0003-4
Piccioli P, Rubartelli A (2013) The secretion of IL-1β and options for release. Semin Immunol 25:425–429. https://doi.org/10.1016/j.smim.2013.10.007
Ho L, Wang L, Roth TM, Pan Y, Verdin EM, Hsiao EC, Nissenson RA (2017) Sirtuin-3 promotes adipogenesis, osteoclastogenesis, and bone loss in aging male mice. Endocrinology 158:2741–2753. https://doi.org/10.1210/en.2016-1739
Cao Z, Wang Y, Long Z, He G (2019) Interaction between autophagy and the NLRP3 inflammasome. Acta Biochim Biophys Sin (Shanghai) 51:1087–1095. https://doi.org/10.1093/abbs/gmz098
Maywald M, Rink L (2015) Zinc homeostasis and immunosenescence. J Trace Elem Med Biol 29:24–30. https://doi.org/10.1016/j.jtemb.2014.06.003
Cervantes J, Eber AE, Perper M, Nascimento VM, Nouri K, Keri JE (2018) The role of zinc in the treatment of acne: a review of the literature. Dermatol Ther. https://doi.org/10.1111/dth.12576
Li X, Chen S, Mao L, Li D, Xu C, Tian H, Mei X (2019) Zinc improves functional recovery by regulating the secretion of granulocyte colony stimulating factor from microglia/macrophages after spinal cord injury. Front Mol Neurosci 12:18. https://doi.org/10.3389/fnmol.2019.00018
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. https://doi.org/10.1038/s41583-018-0055-7
Wei YM, Li X, Xu M, Abais JM, Chen Y, Riebling CR, Boini KM, Li PL, Zhang Y (2013) Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem 31:925–937. https://doi.org/10.1159/000350111
Cai Z, Yan LJ (2013) Rapamycin, Autophagy, and Alzheimer’s Disease. J Biochem Pharmacol Res 1:84–90
O’Shea TM, Burda JE, Sofroniew MV (2017) Cell biology of spinal cord injury and repair. J Clin Invest 127:3259–3270. https://doi.org/10.1172/JCI90608
Acknowledgements
The authors thank other researchers for the valuable technical assistance in this work.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC) (NO. 81871556 and 82072165) and the Liaoning Revitalization Talents Program (NO. XLYC1902108).
Author information
Authors and Affiliations
Contributions
Designed, performed, and analyzed the data from all experiments, CX and ZZ; methodology, HZ; data curation, PZ; writing—original draft preparation, SL; writing—review and editing, HT; funding acquisition, XM. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional Review Board
The animal study protocol was approved by the Ethics Committee of Jinzhou Medical University (protocol code 2020009 and March 11, 2020).”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Zhou, Z., Zhao, H. et al. Zinc Promotes Spinal Cord Injury Recovery by Blocking the Activation of NLRP3 Inflammasome Through SIRT3-Mediated Autophagy. Neurochem Res 48, 435–446 (2023). https://doi.org/10.1007/s11064-022-03762-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03762-2