Abstract
Hyssopus officinalis L. is one of the most important medicinal plants in traditional medicine used to treat seizures. In this study, we assessed the effects of H. officinalis hydroalcoholic extract against pentylenetetrazol (PTZ)-induced seizures in rat. The anti-seizure activity of the extract was assessed in three doses of 25, 50, and 100 mg/kg. Kindling was induced by intraperitoneal injection of PTZ (35 mg/kg) every 48 h, and H. officinalis extract was administered daily and behavioral tests performed. The possible involvement of GABA receptors in the extract activity was investigated using flumazenil. Tonic seizure threshold and mortality rate were measured following intraperitoneal injection of 60 mg/kg PTZ on the 14th day, following 14 days administration of H. officinalis hydroalcoholic extract. Blood and hippocampus samples were prepared to measure brain and serum antioxidant capacity, malondialdehyde (MDA), and nitric oxide (NO). Finally, the expression of GABA receptor gene in brain tissue was investigated. H. officinalis extract increased tonic seizure threshold and decreased mortality due to PTZ. Flumazenil, as a GABA receptor antagonist, reduced the tonic seizure threshold. Extract treatment significantly improved memory and learning, increased brain antioxidant capacity, decreased brain MDA and NO in kindled rats. It also increased GABA receptor gene expression in pre-treated groups compared to the negative control group. H. officinalis extract probably exerts potential antiepileptic effects through the GABAergic system. Also, H. officinalis extract has a supportive effect against hippocampal neuronal damage and improves memory and learning in kindled rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seizure is a temporary dysfunction of the brain caused by an excessive and abnormal discharge of cortical neurons. Epilepsy is a central nervous system disorder characterized by repetitive seizures that usually occur without provocation due to a genetic predisposition or an underlying chronic pathological condition. Epileptic seizures are related to a change in ion transport mechanisms in neuronal networks and directly cause the imbalance of the excitation–inhibition and indirectly cause oxidative stress and neuroinflammation [1,2,3]. Stress is one of the most important stimuli of seizure that can exacerbate it [4].
Among the various neurotransmitters and their receptors, induced changes in the GABAergic-glutamatergic systems and NMDA receptors are important in increasing the excitability of pentylenetetrazole (PTZ)-kindled neocortex [5]. Various studies have shown that the GABAergic system plays an important role in the coordination of local neural networks, the communication of brain areas and their function [6, 7]. PTZ kindling is a model for chronic epilepsy that primarily affects the cortex [8]. The structure of PTZ action in the development of epileptic seizures involves competitive inhibition of GABA receptors and inactivation of chlorine channels, and is widely used to induce epilepsy in laboratory animals [9, 10].
Hyssopus officinalis L. belongs to the family Lamiaceae [11]. The main constituents of H. officinalis extract include various polyphenolic compounds, such as flavonoids, apigenin, quercetin, diosmin, luteolin, and caffeic acids [12]. In traditional medicine, H. officinalis is used to treat respiratory, urinary, and gastrointestinal infections [13]. It has been reported to exert antioxidant, anti-inflammatory, anti-convulsive, and anti-depressant effects [14,15,16]. The healing properties of H. officinalis against convulsive seizures have not yet been investigated; therefore, the current study was aimed to investigate the therapeutic effects of H. officinalis hydroalcoholic extract against PTZ-induced convulsive seizures in the rat.
Materials and Methods
Plant Material and Extraction
After preparation of the aerial parts of the plant from a reputable store and authentication of its scientific name by a botanist, H. officinalis was registered with herbarium number 177 in the Medicinal Plants Research Center at Shahrekord University of Medical Sciences. Extraction was performed by maceration. The powdered sample of the aerial parts of the plant was mixed with 70% alcohol; after 72 h, the extract was filtered and concentrated in a rotary apparatus. To conduct final drying, the concentrated extract was incubated at 37 °C [17].
Determination of Antioxidant Capacity of the Extract and Measuring Total Phenolic and Flavonoid Content
After the stock of extract and DPPH was prepared, the sample was incubated in the dark for 15 min and the absorbance of the samples was read by spectrophotometer at 517 nm wavelength. Methanol was used as blank and methanol plus DPPH was considered a control. After calculating the inhibition rate of free radicals by the extract, the antioxidant activity was calculated as IC50 [18]. The total phenol of H. officinalis was measured by Folin-Ciocalteau reagent and aluminum chloride colorimetric method. The experiments were conducted in triplicate and the total phenol and flavonoid contents of the extract were measured and reported in mg/g dried extract [19, 20].
Animal Grouping and Study Design
Adult male Wistar rats weighing 200–250 g were kept under standard laboratory conditions (12-hour periods of darkness and light, at 22 ± 2 °C, and free access to water and food). Animals were divided into the following groups (n = 10): Negative control group received normal saline daily and 35 mg/kg PTZ every 48 h; positive control group received 2 mg/kg diazepam (daily) 30 min before receiving 35 mg/kg PTZ (every 48 h); the animals in sham group were given only normal saline daily; H. officinalis extract-treated groups received the extract with doses of 25, 50, 100 mg/kg (daily) 30 min before receiving 35 mg/kg PTZ (every 48 h). It should be noted that due to the high rate of mortality following injection of high dose of PTZ on 14th day (60 mg/kg), this group was used for behavior and biochemical evaluations, not for seizure experiment.
To evaluated the probable involvement of benzodiazepine receptors in the anti-seizure action of the extract we then used flumazenil as a benzodiazepine antagonist and 50 mg/kg extract as the best effective dose. This group received 50 mg/kg extract 30 min before receiving 0.5 mg/kg flumazenil, and 60 mg/kg PTZ 30 min later. This group was called flumazenil group and was compared with the group which received the same drugs without flumazenil.
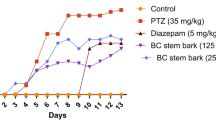
All injections were performed intraperitoneally. PTZ was freshly prepared daily and was administered to the studied groups at 35 mg/kg every 48 h for 14 days. On the last day, tonic seizure threshold and mortality percent was recorded during 30 min by the camera and observed accurately after intraperitoneal injection of 60 mg/kg PTZ in all groups [21]. Treatment with H. officinalis extract was performed daily (Fig. 1). Finally, rats underwent deep anesthesia with interaperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg) [22, 23], and the blood samples were taken from the hearts. Then, hippocampus was separated on the ice and analyzed biochemically. The hippocampus was placed in formaldehyde 10% to conduct histopathological investigations.
Behavior Tests
Behavior tests, including Morris Water Maze test and Shuttle box were performed on 13th day of the experiment (after training as follows) to evaluate spatial memory and passive avoidance memory, respectively.
Morris Water maze test
In this test, each rat was trained in a maze for 4 days and 4 times a day. In each experiment, the rat was randomly placed to one side of the maze. Each rat was given an opportunity of 60 s to find the platform. The rats were given 30 s rest between the two trials to examine the surroundings. Rats were removed from the water for approximately 10 min between the blocks and rested in cages. On the day 5 that was probe day only once performed without the platform [24].
Shuttle box test
This test was performed for 4 days. On days 1 and 2, each mouse was placed in the instrument and placed for 5 min to adapt to it. An acquisition test was performed on day 3. The mice were placed individually in a lighted room. After a period of adaptation (2 min), the guillotine door was opened and after the rat entered the dark room, the door was closed and an electric shock was applied to the rat to the extent that it only paddled (1 mA, 1 s, 1 time). In this test, the primary latency (t1) to enter the dark room was recorded on day 3 and secondary latency (t2) was recorded on day 4 [25].
Measuring the Brain and Serum Antioxidant Capacity
The antioxidant power of the serum and brain was determined by measuring its ability to reduce ferric-tripiridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+) using FRAP method and a complex formed between Fe3+ and TPTZ that created a blue color. Its absorbance was calculated at 593 nm [26].
Measuring the Brain and Serum MDA Levels
Serum/homogeneous brain tissue (200 µl) was mixed with 1.5 ml of acetic acid 20%, 1.5 ml of thiobarbituric acid (0.8%), and 200 µl of SDS 8.1%, and boiled in a bain-marie of boiling water for 60 min. Then, the samples were cooled and 1 ml of distilled water and 5 ml of n-butanol-pyridine mixture were added and the tubes were shaken. The mixture was centrifuged at 4000 rpm for 10 min and the optical absorbance of the supernatant was recorded at 523 nm wavelength [26].
Measuring the Brain and Serum NO Levels
Brain and serum sample (100 µl) was poured in triplicate into a 96-well plate and then 100 µl of sulfanilamide solution (1 g of sulfanilamide in 100 ml of phosphoric acid 5%) was added to it. The plate was incubated for 5–10 min in the dark at ambient temperature. N-1-naphthylnethylenediamine (NEDD) solution (50 µl) was added to the wells and incubated for 30 min at ambient temperature, and then the optical absorbance was read at 540 nm wavelength by ELISA. The amount of nitrite in the samples was determined using the standard curve [26].
Histopathological Investigations
For histological investigations, the rat was anesthetized, the brain was removed from the skull and placed in 10% fixative solution. After a few days, the samples were subjected to dewatering, clarification, paraffin immersion, and finally dominance. After dominance, 5 μm thick sections were prepared using microtome and stained with hematoxylin eosin (H&E). Then, these sections were examined and photographed using an optical microscope.
Gamma-aminobutyric acid type A Receptor alpha4 Subunit gene Expression
Total RNA was extracted from hippocampus tissue using the RNeasy Lipid Tissue Mini Kit (cat. no. 74,804; Qiagen) according to the manufacturer’s protocol. RNA purity was tested by Thermo Scientific™ NanoDrop 2000. One µg of total RNA was used in a reverse-transcription reaction utilizing random hexamer primers according to the manufacturer’s protocol (TaKaRa, Japan). Next, 1 µL of cDNA Synthesis Kit (Oligo Co) was amplified in the Step One plus Real-Time PCR thermal cycler (Rotor Gene 3000) in a total volume of 10 µL containing 5 µL of SYBR Green Master Mix (TaKaRa, Japan) along with gene-specific primers. Transcript levels of target genes were normalized to the expression level of beta actin. Differential expression was analyzed according to the 2-ΔΔCt method. Primer pairs (Table 1) were designed by the oligo (Version 7) and Perl-primer softwares and synthesized by oligo (Germany).
Data Analysis
Statistical analysis of the data was performed using Prism 5 software. Data were presented as Means ± SEM and analyzed by one-way ANOVA and Tukey’s post hoc tests. The level of significance was considered at P < 0.05.
Results
Antioxidant Potency by DPPH Method and Total Phenol and Flavonoid Content
Scavenging the capacity of radicals and antioxidant activity of hydroalcoholic H. officinalis (IC50) was derived about 268.51 ± 0.03 µg/ml. The total phenol and flavonoid contents of H. officinalis extract were derived 26.41 ± 0.01 mg GAE/g dry extract and 18.24 ± 0.01 mg RE/g dry extract.
The Threshold of Tonic Seizure
The threshold of tonic seizure was significantly higher in the positive control group than in the negative control group (P < 0.001). The threshold of tonic seizure was significantly higher in the group receiving 50 and 100 mg/kg doses of the extract plus PTZ than the negative control group (P < 0.001 and P < 0.01, respectively). The threshold of tonic seizure was significantly lower in the group receiving 25 mg/kg extract plus PTZ than the positive control group (P < 0.05). There was no significant difference between the groups receiving the extract and PTZ. The threshold of tonic seizure was lower in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg extract plus PTZ (P < 0.01) (Fig. 2).
The effect of various doses of Hyssopus officinalis extract on seizure threshold in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the rat group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ. Flu + PE50: the group receiving flumazenil (benzodiazepine receptor antagonist) and 50 mg/kg extract with PTZ. **P < 0.01 and ***P < 0.001: compared to the Neg Cont group, $P < 0.05: compared to pos Cont group. ##P < 0.01: compared to the group receiving 50 mg/kg extract with PTZ.
Mortality Percent
The Chi-square results showed that there was a significant difference between different groups (P < 0.001). Tukey’s post test showed that the mortality rate was 0% in the positive control group and the groups receiving 25, 50, and 100 mg/kg extract plus PTZ, with a significant difference compared to the PTZ group (P < 0.001). The mortality rate was significantly higher in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg extract plus PTZ (P < 0.05) (Fig. 3).
Mortality percent in various groups of rats receiving pentylenetetrazol (PTZ). Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam and PTZ; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ; Flu + PE50 group: the group receiving flumazenil, 50 mg/kg extract, and PTZ. ***P < 0.001: compared to the Neg Cont group, #P < 0.05: compared to the PE50 group
Spatial Learning in the Morris Water Maze Test
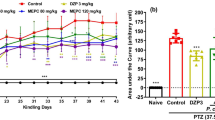
The one-way ANOVA results on day 1 showed that there was no significant difference between different groups (P > 0.05). The results on day 2 showed that the latency of finding the platform was significantly lower in the sham group and the group receiving 50 mg/kg extract than the negative control group (P < 0.001 and P < 0.05, respectively). The latency of finding the platform on day 3 was significantly lower in the positive control group than the negative control group (P < 0.01). Also, the latency of finding the platform was significantly lower in the sham group and the groups receiving the extract at doses 25, 50, and 100 mg/kg plus PTZ than the negative control group (P < 0.001).
On day 4, there was a significant difference between different groups (P < 0.001). The latency of finding the platform was significantly lower in the positive control group and the groups receiving 25 and 100 mg/kg extract (P < 0.01) and also in the sham group and the group receiving 50 mg/kg extract than the negative control group (P < 0.001). The latency of finding the platform was significantly lower in the group receiving 50 mg/kg extract plus PTZ than the group receiving 100 mg/kg extract plus PTZ (P < 0.05). There was no significant difference between the groups receiving the extract plus PTZ and the positive control group on all days under study (P > 0.05) (Fig. 4).
The effect of various doses of Hyssopus officinalis extract on spatial learning (Morris water maze test), in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group; the group receiving 1 ml/kg normal saline but not PTZ; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ. **P < 0.01 and ***P < 0.001; compared to the Neg Cont group. †P < 0.05; comparison between the PE50 and PE100 groups
Spatial Learning on Probe day in Morris Water maze test
The swimming time in the target quarter was significantly lower in the negative control group than the other groups (P < 0.001). There was no significant difference between the groups receiving the extract plus PTZ and the positive control group (P > 0.05) (Fig. 5).
The effect of various doses of Hyssopus officinalis extract on spatial learning of the prob day (Morris water maze test), in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group: the group receiving 1 ml/kg normal saline but not PTZ; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ. ***P < 0.001; compared to the Neg Cont group
Passive Avoidance Memory in Shuttle Box Test
The primary latency was significantly higher in the negative control group than in the other groups (P < 0.001). There was no significant difference between the groups receiving the extract plus PTZ and the positive control group (P > 0.05). Secondary latency was significantly higher in the positive control group, sham group and the groups receiving 50 and 100 mg/kg extract plus PTZ than the negative control group (P < 0.001). This parameter was significantly higher in the group receiving 25 mg/kg extract and PTZ than in the negative control group (P < 0.01). The secondary latency was significantly lower in the groups receiving 25 and 100 mg/kg extract plus PTZ than the positive control group (P < 0.05 and P < 0.01, respectively) (Fig. 6).
The effect of various doses of Hyssopus officinalis extract on passive avoidance memory (shuttle box test) in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group: the group receiving 1 ml/kg normal saline but not PTZ; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ. ***P < 0.001; compared to initial latency in the Neg Cont group, ++P < 0.01, +++P < 0.001; compared to secondary latency in the Neg Cont group, $$P < 0.01; compared to secondary latency in the Pos Cont group, †P < 0.05; compared to secondary latency in the PE25 and PE50 groups
The Brain and Serum Antioxidant Capacity
The brain antioxidant capacity was significantly higher in the positive control group and the groups receiving 25, 50, and 100 mg/kg extract plus PTZ and the sham group than the negative control group (P < 0.001, P < 0.01, P < 0.001, P < 0.001, P < 0.05, respectively). The brain antioxidant capacity was significantly lower in the group receiving 25 mg/kg extract plus PTZ than the positive control group (P < 0.05). This parameter was lower in other groups receiving the extract than the positive control group, but this decrease was not significant (P > 0.05). The brain antioxidant capacity was significantly lower in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg extract plus PTZ (P < 0.001).
The serum antioxidant capacity was significantly higher in the positive control group and the group receiving 25, 50, and 100 mg/kg extract plus PTZ than the negative control group (P < 0.001, P < 0.05, P < 0.001, P < 0.05, respectively). The serum antioxidant was significantly higher in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg extract plus PTZ (P < 0.05) (Fig. 7).
The effect of various doses of Hyssopus officinalis extract on the brain and serum antioxidant capacities in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group; the group receiving 1 ml/kg normal saline but not PTZ; PE 25, 50, and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ; Flu + PE50: the group receiving flumazenil and 50 mg/kg extract, and PTZ. *P < 0.05, **P < 0.01 and ***P < 0.001; compared to the Neg Cont group; $$$P < 0.001; compared to the Pos Cont group, ###P < 0.001; compared to the Flu + PE50 group and the PE50 group
The Brain and Serum MDA
The brain MDA was significantly lower in the positive control, sham group, and the groups receiving 25, 50 and 100 mg/kg extract plus PTZ than the negative control group (P < 0.001). The brain MDA was significantly higher in the groups receiving 25, 50, and 100 mg/kg extract plus PTZ than the positive control group (P < 0.001). The brain MDA was significantly higher in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg extract plus PTZ (P < 0.05).
The serum MDA was significantly lower in the positive control, sham group, and the groups receiving 25, 50, and 100 mg/kg extract plus PTZ than the negative control group (P < 0.001). The serum MDA was significantly higher in the group receiving flumazenil with 50 mg/kg extract and PTZ than the group receiving 50 mg/kg plus PTZ (P < 0.05) (Fig. 8).
The effect of various doses of Hyssopus officinalis extract on the brain and serum malondialdehyde (MDA) in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group: the group receiving 1 ml/kg normal saline, PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ. Flu + PE50: the group receiving flumazenil and 50 mg/kg extract with PTZ. ***P < 0.001, compared to the Neg Cont group; $$$P < 0.001, compared to the Pos Cont group; ##P < 0.01, #P < 0.05: comparison of the Flu + PE50 group and PE50 group
The Brain and Serum Nitric Oxide
The brain nitric oxide was significantly lower in the sham group than the negative control group (P < 0.05). The brain nitric oxide was lower in other groups than the negative control group, but this decrease was not significant (P > 0.05). Significant difference was not observed between the groups receiving the extract plus PTZ and the positive control group (P > 0.05).
Results showed that the serum nitric oxide was significantly lower in the sham group than the negative control group (P < 0.01). The serum nitric oxide was lower in other groups than the negative control group but this decrease was not significant (P > 0.05). Significant difference was not observed between the groups receiving the extract plus PTZ and the positive control group (P > 0.05) (Fig. 9).
The effect of various doses of Hyssopus officinalis extract on the brain and serum nitric oxide in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group: the group receiving 1 ml/kg normal saline; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ; Flu + PE50: the group receiving flumazenil and 50 mg/kg extract with PTZ. *P < 0.05, **P < 0.01; compared to the Neg Cont group
Histological Changes
The results of histological pathology in all groups are shown in Fig. 10 In microscopic observations, the hippocampal tissue in the sham rats was intact. In hippocampal tissue samples in the negative control group, the number of pyramidal cells decreased and, in some areas, no pyramidal cells were observed. In hippocampal tissue samples in the positive control group, a number of granular cells in the dentate gyrus were destroyed. The cells in the molecular region, especially small cells in the CA1 and CA2, decreased and gliosis increased. In hippocampal tissue samples in the group receiving 25 mg/kg extract plus PTZ, a number of small pyramidal cells in the C1 and C2 were destroyed. Cell thickness decreased and scattering was seen in the granular layer.
In hippocampal tissue samples in the group receiving 50 mg/kg extract plus PTZ, granular layer was almost intact. The cells in some areas were scattered. Pyramidal cells were scattered in C1, C2, C3, and C4. The molecular layer of some glial cells was seen and some neurons increased in volume. In hippocampal tissue samples in the group receiving the extract at 100 mg/kg plus PTZ, cases of apoptosis and scattering in the granular layer were seen. Also, large and small pyramidal cells died and their number decreased and gliosis was seen. In hippocampal tissue samples in the group receiving flumazenil with 50 mg/kg extract and PTZ, granular cells in the dentate gyrus layer were dispersed and the number of pyramidal cells decreased. The thickness of the granular layer in the CA1, CA2, and CA3 decreased. Also, cases of gliosis were observed.
The effect of various doses of Hyssopus officinalis extract on hippocampal structure in pentylenetetrazol (PTZ)-induced seizures. A: Hippocampal structure in the sham group; layers and cells are normal; a: Granular layer, b: Molecular layer. B: Hippocampal structure in negative control group; a: Reduction of granular layer cells, b: Scattering of granular cells, c: Reduction of molecular layer cells, d: Cell vacuolization. C: Hippocampal structure in positive control group; the layers and cells are almost normal; a: Decreased granular layer cells in some areas. D: Structure of the hippocampus in the group receiving 25 mg/kg extract; a: Reduction and scattering of cells in the pyramidal region. E: Hippocampal structure in the group receiving 50 mg/kg extract; a: Gliosis in the molecular area, b: Cell enlargement in molecular area. F: Hippocampal structure in the group receiving 100 mg/kg extract; a: Apoptosis, b: Gliosis. G: Hippocampal structure in the group receiving flumazenil and extract; a: Severe cell scattering in the dentate gyrus, b: Reduction and scattering of cells in the C4, c: Reduction of cells in the C3
Expression of GABAA Receptor Subunit 4α Gene
The gene expression of GABAA receptor subunit 4α gene significantly increased in the positive control group and also in the group receiving 50 mg/kg extract plus PTZ compared to the control group (P < 0.01 and P < 0.05, respectively). Although, the expression of this gene in other groups increased but this increase was not significant (P > 0.05) (Fig. 11).
The effect of various doses of Hyssopus officinalis extract on the expression of GABAA receptor subunit α4 gene in pentylenetetrazol (PTZ)-induced seizures. Neg Cont: the group receiving normal saline with PTZ; Pos Cont: the group receiving 2 mg/kg diazepam with PTZ; sham group: the group receiving 1 ml/kg normal saline; PE 25, 50 and 100: the groups receiving the extract at doses 25, 50, and 100 mg/kg with PTZ; Flu + PE50: the group receiving flumazenil and 50 mg/kg extract with PTZ. **P < 0.01, *P < 0.05: compared to the Neg Cont group
Discussion
Because PTZ at 60 mg/kg leads to death by inducing tonic contractions and generalized tonic-clonic seizures [27], we used this dose to induce seizures. In this study, hydroalcoholic H. officinalis extract could increase the threshold of tonic seizures and reduce the mortality rate in a PTZ-induced kindling rat model. Diazepam was used to compare the anticonvulsant capability of the plant extract. Diazepam, the same as other benzodiazepines, acts by effect on GABA receptors and eventually chlorine ions entering neurons [28]. Our results showed that diazepam at 2 mg/kg dose produced a stronger effect than the extract in inhibiting seizure. Therefore, it may be concluded that this dose of the extract is not sufficiently strong compared to the diazepam. Also, Flumazenil (a specific GABAA receptor antagonist) was used to investigate the mechanism action of H. officinalis extract on PTZ-induced kindling. The results showed that this substance reduced the antiepileptic effect of the plant extract in rats. Therefore, one of the main mechanisms involved in its anticonvulsant effect may be stimulation of the GABA system and benzodiazepine receptors. One of the basic mechanisms of anticonvulsant effect is the activation of the inhibitory GABA system, and benzodiazepines play an essential role in stabilizing the nervous system [29]. In the study of Fatahizad et al. (2011), apigenin 7-O-β-D-glucuronide was extracted as one of the most important flavonoids in H. officinalis extract [13].
Some H. officinalis flavonoids exhibit benzodiazepine-like activity, one of which is apigenin. However, in one study, the affinity of apigenin for benzodiazepine receptors was low and its effect was not blocked by a specific benzodiazepine receptor antagonist [30, 31]. Because in our study, flumazenil injection reduced the effect of the hydroalcoholic plant extract on seizure, and the expression of GABA receptor gene increased in the group receiving 50 mg/kg extract, it is highly likely that one of the mechanisms of H. officinalis for reduction of seizure is related to the effect of the extract’s compounds on activating the brain’s benzodiazepine-GABA system.
In the study of Hashemi et al. (2019), it was observed that epigenin had significant anticonvulsant activity, improved memory, and increased the number of living nerve cells in the hilus [32]. Also, it has been shown that epigenin inhibits glutamate secretion in the rat hippocampus [33]. Therefore, balancing between inhibitory and excitatory neurotransmitters in the hippocampus can be another possible mechanism to explain the anticonvulsant activity of apigenin.
In one study, the effect of aqueous H. officinalis extract on PTZ-induced acute seizure and hippocampal iNOS gene expression was investigated. The results showed that 100 mg/kg extract could delay the onset of stages 5 and 6 of seizure, which coincide with tonic-clonic attacks. Furthermore, a significant increase in iNOS gene expression was observed at this dose, but at a dose of 200 mg/kg of the extract, delay and duration of seizure stages changed and were more similar to the results in the PTZ group [16]. Based on the results of the present study and the other studies, it seems that the doses of H. officinalis extract used in this study have antiepileptic effect. Seizure attacks lead to impaired memory and learning in the affected person [34]. The direct role of flavonoids in the acquisition, memory has already been described in a study involving neural signaling-induced activation and gene expression in the brain [35].
The results of behavioral tests in this study showed that seizure in male Wistar rats reduced spatial memory and passive avoidance memory, while pretreatment with H. officinalis extract could improve spatial memory and passive avoidance memory following seizure in rats. In addition, the latency to find the platform in the target quarter in Morris water maze test was higher in all H. officinalis-treated groups than the PTZ (negative control) group.
Pentylenetetrazol injection has been shown to damage the CA1, CA3, and dentate regions of hippocampus, as well as some neurons of amygdala and entorhinal cortex, which are involved in memory and learning [34] explaining the possible reason for memory and learning deficits in epilepsy. Furthermore, dramatical oxidative stress enhancement in these regions of the brain, especially in hippocampus, following seizures increase the possibility of damage in these regions [36]. Therefore, the reduction of spatial learning and memory in the water maze test and increase of the latency time in shuttle box test in the present study can be resulted from these damages. Furthermore, antioxidants have been shown to increase the cell membrane stability and enhance neuron resistance against oxidative stress [37]. Learning and memory enhancement by H. officinalis, which had high level of antioxidant compounds and reduced seizure attacks may confirm the opinion of involving the pathogenesis of epilepsy and brain damage, especially the regions involved in memory and learning such as hippocampus [38]. In this regard, autophagy has also been shown to increase in some regions of the brain in epilepsy, and antioxidant agents can reduce autophagy and neuronal damage [39]. Therefore, at least in part, the beneficial effect of H. officinalis extract might be due to its neuroprotective effects in reducing damage to hippocampal neurons following PTZ injection.
Recent studies have shown that oxidative stress and mitochondrial dysfunction can predispose the brain to epileptic seizure. Thus, oxidative stress and the production of free radicals are now recognized as the cause and result of seizure [40, 41]. The results regarding TPC and TFC and antioxidant capacity of H. officinalis in the present study also confirm the high potential of this extract as a natural antioxidant. Moreover, our results showed that the brain antioxidant capacity was significantly decreased in the PTZ group and increased in the extract-treated groups. This result indicates the improvement of brain antioxidant capacity in kindled rats after receiving the extract. The study of antioxidant capacity of the brain in the diazepam group compared to all extract-treated groups led to the hypothesis that diazepam may have antioxidant properties. Serum antioxidant capacity was also decreased in PTZ group and increased in extract-treated groups.
MDA is the product of lipid peroxidation, which is commonly used as a measure of oxidative stress in cells [42]. In the present study, serum and brain MDA levels were higher in PTZ group than in other groups indicating damage due to seizure and lipid peroxidation. In addition, in the diazepam, sham, and extract groups, MDA levels decreased compared to PTZ. In the group receiving flumazenil and the extract (50 mg/kg), an increase was observed in MDA level compared to the 50 mg/kg extract group, strengthening the possibility that the mechanism of action of H. officinalis is realized through the GABAergic system.
Flavonoids have antioxidant activity against free radicals and are known as important agents for inhibiting lipid peroxidation [43]. It seems that the protective effect of this plant on seizures is due to the presence of these compounds. In addition, in the groups treated with H. officinalis, the antioxidant capacity of the brain and serum increased compared to the PTZ group, which indicates the improvement of these variables after administration with the extract. This finding seems to be due to the protective effect of H. officinalis extract components in reducing lipid peroxidation.
Although the role of NO in the pathophysiology of epilepsy remains unclear, observations show that NO causes the destruction of neurons and the proliferation of reactive glia, which may be involved in the pathogenesis of epilepsy [44]. In the present study, serum and brain NO levels increased in the PTZ group compared to other groups and decreased in the diazepam, sham, and extract-treated groups, but this decrease was not significant in any of the groups except for the sham group. Moreover, the level of NO in the flumazenil group increased compared to 50 mg/kg extract group, but this increase was not significant. One of the major biological abnormalities in epileptogenesis and the brain of epileptic patients is neuronal injury and death [45]. Studies have shown that in PTZ (administered in incremental doses)-induced kindling epilepsy, neurons involved in learning and memory in the hippocampus (CA1, CA2 and dentate gyrus) and some amygdala neurons are damaged [34]. It has been reported that seizure may lead to morphological changes such as the production of dark neurons in brain tissue [46].
In the present study, it was found that changes in hippocampal tissue were significantly different in the negative control group with the control group, which was less than the negative control group due to PTZ injection for inducing seizure and changes in the groups receiving the extract.
It is known that some flavonoid compounds can interact with the benzodiazepine site of the GABAA receptor [47]. Although benzodiazepines, like diazepam, are the most suitable drug group for anti-anxiety function, but they mainly have various side effects such as sedation, sleepiness, and dependence [13, 48, 49]. The flavonoid compounds of the H. officinalis plant have selective ligands and relatively mild benzodiazepine receptors with a medicinal profile and have different modulating effects on the benzodiazepine site of the GABAA receptor [50]. There is no report indicating serious side effects for this plant.
According to the positive effects H. officinalis on consecutive seizures, spatial memory, passive avoidance learning, antioxidant capacity, and lipid peroxidation during epilepsy in this study, the use of H. officinalis extract might be beneficia in chronic drug treatment, and after epileptic attacks to improve learning and memory of people who suffer from consecutive seizures. Finally, it is suggested to evaluate H. officinalis plant extract as a supplement in the treatment of epilepsy for people who are willing to participate in clinical trials.
Conclusion
In the present study, PTZ decrease the seizure threshold and impaired spatial memory and passive avoidance in male Wistar rats. Also, it reduced the antioxidant capacity of the brain and serum and increased serum and brain MDA and NO levels. Besides that, intraperitoneal injection of H. officinalis extract for 14 days increased seizure threshold and antioxidant capacity, decreased brain and serum MDA levels, and increased GABA receptor gene expression in the brain. This study showed that H. officinalis exerts have preventive effects in improving seizures. It also is able to improve the memory deficits seen following seizures. Hence, it might be beneficial in these patients.
Data Availability
Data regarding the present study are available at Medical Plants Research Center, Shahrekord University of Medical Science.
Abbreviations
- PTZ:
-
Pentylenetetrazol.
- MDA:
-
Malondialdehyde.
- NO:
-
Nitric oxide.
- TPTZ:
-
tripiridyltriazine.
- NEDD:
-
N-1-naphthylnethylenediamine .
References
Tastemur Y, Gumus E, Ergul M, Ulu M, Akkaya R, Ozturk A, Taskiran AS (2020) Positive effects of angiotensin-converting enzyme (ACE) inhibitor, captopril, on pentylenetetrazole-induced epileptic seizures in mice. Trop J Pharm Res 19:637–643
Devi PU, Manocha A, Vohora D (2008) Seizures, antiepileptics, antioxidants and oxidative stress: an insight for researchers. Expert Opin Pharmaco 9(18):3169–3177
Metcalf CS, Huntsman M, Garcia G, Kochanski AK, Chikinda M, Watanabe E, Underwood T, Vanegas F, Smith MD, Steve White H, Bulaj G (2019) Music-Enhanced Analgesia and Antiseizure Activities in Animal Models of Pain and Epilepsy: Toward Preclinical Studies Supporting Development of Digital Therapeutics and Their Combinations With Pharmaceutical Drugs. Front Neurol 10:277
Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, Hong JSh, Yoneda Y, Kim HCh (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59(2):122–137
Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44(11):S14–S23
Miyazaki H, Matsuura H, Yanagiya C, Mizutani J, Tsuji M, Ishihara C (2003) Inhibitory Effects of Hyssop (Hyssopus officinalis) Extracts on Intestinal ALPHA.-Glucosidase Activity and Postprandial Hyperglycemia. J Nutri Sci Vitaminol 49(5):346–349
Rudolph U, Möhler H (2014) GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol 54:483–507
Singh T, Mishra A, Goel RK (2021) PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: relevance and reliability. Metab Brain Dis 1573–1590
Bazan NG, Tu B, Rodriguez de Turco EB (2002) What synaptic lipid signaling tells us about seizure-induced damage and epileptogenesis. Prog Brain Res 135:175–185
Jesberger JA, Richardson JS (1991) Oxygen free radicals and brain dysfunction. Int J Neurosci 57(1–2):1–17
Angelatou F, Pagonopoulou O, Kostopoulos G (1990) Alterations of A1 adenosine receptors in different mouse brain areas after pentylentetrazol-induced seizures, but not in the epileptic mutant mouse ‘tottering’. Brain Res 534(1):251–256
Tahir M, Khushtar M, Fahad M, Rahman A (2018) Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.). J Appl Pharm Sci 8(07):132–140
Fathiazad F, Hamedeyazdan S (2011) A review on Hyssopus officinalis L.: Composition and biological activities. Acad Journals 5(17):1959–1966
Javadi B, Sahebkar A, Emami S (2017) Medicinal plants for the treatment of asthma: A traditional Persian medicine perspective. Curr Pharm Des 23:1623–1632
Özer H, Şahin F, Kılıç H, Güllüce M (2005) Essential oil composition of Hyssopus officinalis L. subsp. angustifolius (Bieb.) Arcangeli from Turkey. Flavour Fragr J 20:42–44
Gholami M, Jafari F, Baradaran Z, Amri J, Azhdari-Zarmehri H, Sadegh M (2020) Effects of aqueous extract of Hyssopus officinalis on seizures induced by pentylenetetrazole and hippocampus mRNA level of iNOS in rats. Avicenna J Phytomedicine 10(3):213–221
Ullah HA, Zaman S, Juhara F, Akter L, Tareq SM, Masum EH, Bhattacharjee R (2014) Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complem Altern M 14(1):346
Pohle W, Becker A, Grecksch G, Juhre A, Willenberg A (1997) Piracetam prevents pentylenetetrazol kindling-induced neuronal loss and learning deficits. Seizure 6(6):467–474
Sridhar K, Charles AL (2019) In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: estimation methods for EC50 using advanced statistical programs. Food Chem 275:41–49
Derakhshan Z, Ferrante M, Tadi M, Ansari F, Heydari A, Hosseini MS, Conti GO, Sadrabad EKh (2018) Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem Toxicol 114:108–111
Dhir A (2012) Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci 58(1):1–937
Ghazvini H et al (2016) Estrogen and progesterone replacement therapy prevent methamphetamine-induced synaptic plasticity impairment in ovariectomized rats. Addict health 8(3):145
Amiri S et al (2016) NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience 329:122–133
Tripathi Y, Saini N (2019) Total phenolic, total flavonoid content and antioxidant efficacy of leaves of Eupatorium adenophorum. Int J Pharma Bio Sci 10(2):157–166
Peay DN, Saribekyan HM, Parada PA, Hanson EM, Badaruddin BS, Judd JM, Donna ME, Padilla-Garcia D, Conrad CD (2020) Chronic unpredictable intermittent restraint stress disrupts spatial memory in male, but not female rats. Behav Brain Res 383:112519
Khalili M, Roghani M, Ekhlasi M (2010) The effect of aqueous crocus sativus L. extract on intracerebroventricular streptozotocin-induced cognitive deficits in rat: a behavioral analysis. Iran J Pharm Res 185–191
Johnston GA (2005) GABAA receptor channel pharmacology. Curr Pharm Design 11(15):1867–1885
Poucet B (1993) Spatial cognitive maps in animals: new hypotheses on their structure and neural mechanisms. Psychol Rev 100(2):163–182
Duarte FS, Marder M, Hoeller AA, Duzzioni M, Mendes BG, Pizzolatti MG, De Lima TCM (2008) Anticonvulsant and anxiolytic-like effects of compounds isolated from Polygala sabulosa (Polygalaceae) in rodents: in vitro and in vivo interactions with benzodiazepine binding sites. Psychopharmacology 197(3):351–360
Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M (2000) Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol 59(11):1387–1394
Viola H, Wolfman C, Marder M, Goutman J, Bianchin M, Wasowski C, Calvo DJ, Izquierdo I, Paladini AC, Medina JH (2000) 6-Chloro-3′-nitroflavone is a potent ligand for the benzodiazepine binding site of the GABA A receptor devoid of intrinsic activity. Pharmacol Biochem Behav 65(2):313–320
Hashemi P, Babaei JF, Vazifekhah S, Nikbakht F (2019) Evaluation of the neuroprotective, anticonvulsant, and cognition-improvement effects of apigenin in temporal lobe epilepsy: Involvement of the mitochondrial apoptotic pathway. Iran J Basic Med Sci 22(7):752–758
Chang CY, Lin TY, Lu CW, Wang CC, Wang YC, Chou SSP, Wang SJ (2015) Apigenin, a natural flavonoid, inhibits glutamate release in the rat hippocampus. Eur J Pharmacol 762:72–81
Nassiri-Asl M, Mortazavi SR, Samiee-Rad F, Zangivand AA, Safdari F, Saroukhani S, Abbasia E (2010) The effects of rutin on the development of pentylenetetrazole kindling and memory retrieval in rats. Epilepsy Behav 18(1–2):50–53
Spencer JP (2009) Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr 4(4):243–250
Uzüm G, Akgün-Dar K, Aksu U (2010 Nov) The effects of atorvastatin on memory deficit and seizure susceptibility in pentylentetrazole-kindled rats. Epilepsy Behav 19(3):284
Júnior JS, de Almeida AA, Tomé Ada R, Citó AM, Saffi J, de Freitas RM (2011) Evaluation of possible antioxidant and anticonvulsant effects of the ethyl acetate fraction from Platoniainsignis Mart. (Bacuri) on epilepsy models. Epilepsy Behav 22(4):678–684
Costello DJ, Delanty N (2004) Oxidative injury in epilepsy: potential for antioxidant therapy. Expert Rev Neurother 4(3):541–537
Wang J, Zhang YJ, Du S (2012) The protective effect of curcumin on Aβ□ induced aberrant cell cycle reentry on primary cultured rat cortical neurons. Eur Rev Med Pharmacol Sci 16(4):445–454
Yaribeygi H, Panahi Y, Javadi B, Sahebkar A (2018) The Underlying Role of Oxidative Stress in Neurodegeneration: A Mechanistic Review. CNS Neurol Disord Drug Targets 17:207–215
Shekh-Ahmad T, Kovac S, Abramov AY, Walker MC (2019) Reactive oxygen species in status epilepticus. Epilepsy Behav 101:106410
Wolff SP, Dean R (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’in diabetes. Biochem J 245(1):243–250
Rafieian-Kopaei M, Baradaran A, Rafieian M (2013) Oxidative stress and the paradoxical effects of antioxidants. J Res Med Sci 18(7):628
Arhan E, Serdaroghu A, Ozturk B, Ozturk HS, Ozcelik A, Kurt N, Kutsal E, Sevinc N (2011) Effects of epilepsy and antiepileptic drugs on nitric oxide, lipid peroxidant and xanthine oxidase system in children whith idiopathic epilepsy. Seizure 20(2):138–142
Jutila L, Immonen A, Partanen K, Partanen J, Mervaala E, Ylinen A, Alafuzoff I, Paljärvi L, Karkola K, Vapalahti M, Pitkänen A (2002) Neurobiology of epileptogenesis in the temporal lobe.Adv Tech Stand Neurosurg 3–22
Pavlova T, Yakovlev A, Stepanichev MY, Mendzheritskii A, Gulyaeva N (2004) Pentylenetetrazole kindling induces activation of caspase-3 in the rat brain. Neurosci Behav Physiol 34(1):45–47
Huang X, Liu T, Gu J, Luo X, Ji R, Cao Y, Xue H, Wong JTF, Wong BL, Pei G, Jiang H, Chenet K (2001) 3D-QSAR Model of flavonoids binding of benzodiazepine site in GABAA receptors. J Med Chem 4(12):1883–1891
Hanrahan JR, Chebib M, Davucheron NLM, Hall BJ, Johnston GAR (2003) Semisynthetic preparation of amentoflavone: a negative modulator at GABAA receptors. J Bioor Med Chem Lett 13:2281–2284
Wolfson P, Hoffmann DL (2003) An investigation into the efficacy of scutellaria lateriflora in healthy volunteers. J Altern Th 9(2):74–78
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer J (2008) The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 3(3–4):115–126
Acknowledgements
The authors gratefully thank the Research and Technology Deputy of Shahrekord University of Medical Sciences for all supports provided.
Funding
This work was supported by Shahrekord University of Medical Sciences (Grant number 1148).
Author information
Authors and Affiliations
Contributions
NF, ZL, MA, ZR, SK, MRK carried out the experiments, participated in the design of the study, performed the statistical analysis and drafted the manuscript. NF and ZR provided expertise in the behaviors analysis. ZR and ZL participated in the design and coordination of the study. ZR and MRK edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All stages of experimentation were carried out in accordance with the regulations of the University and the Guide for the Care and Use of Laboratory Animals of National Institutes of Health and Guide for the Care and Use of Laboratory Animals. Full efforts were made to diminish the use of animals and to improve their wellbeing.
Consent for Publication
All authors are agreed to publish this manuscript.
Competing Interests
The authors have no conficts of interest to declare regarding the study described in this article and the preparation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fatahinezhad, N., Lorigooini, Z., Arabi, M. et al. Effects of Hyssopus Officinalis Hydroalcoholic Extract on Pentylenetetrazol-Induced Convulsive Seizures in Rat. Neurochem Res 47, 3792–3804 (2022). https://doi.org/10.1007/s11064-022-03759-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03759-x