Abstract
Chemotherapy-induced neuropathic pain is a major clinical problem with limited treatment options. Here, we show that metformin relieves bortezomib (BTZ)-evoked induction and maintenance of neuropathic pain by preventing the reduction in the expression of Beclin-1, an autophagy marker, in the spinal dorsal horn. Application of rapamycin or 3-methyladenine, autophagy inducer and inhibitor, respectively, affected the mechanical allodynia differently. Co-application of 3-methyladenine and metformin partially inhibited the effect of metformin in recovering Beclin-1 expression and in reducing the pain behavior in rats subjected to BTZ treatment. BTZ treatment also reduced the expression of AMPKa2 in the dorsal horn, which was recovered by metformin treatment. Overexpression of AMPKa2 attenuated the BTZ-evoked reduction in Beclin-1 expression and mechanical allodynia, whereas intrathecal injection of AMPKa2 siRNA decreased the Beclin-1 expression and induced mechanical allodynia in naive rats. Moreover, BTZ treatment increased the GATA3 expression in the dorsal horn, and GATA3 siRNA attenuated the AMPKa2 downregulation and mechanical allodynia induced by BTZ. Chromatin immunoprecipitation further showed that BTZ induced an increased recruitment of GATA3 to multiple sites in the AMPKa2 promoter region. Furthermore, decreased acetylation and increased methylation of histone H3 in the AMPKa2 promoter in the spinal dorsal horn was detected after BTZ treatment. Our findings suggest that metformin may regulate AMPKa2-mediated autophagy in the dorsal horn and alleviate the behavioral hypersensitivity induced by BTZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bortezomib (BTZ), a 20S proteasome complex inhibitor, is a first-line chemotherapeutic agent for treatment of multiple myeloma [1]. Neuropathic pain is a significant dose-dependent toxicity of BTZ, which often leads to dose reduction or discontinuation of chemotherapy in patients [2]. The mechanism underlying BTZ-induced neuropathic pain has been proposed to mainly involve peripheral nerve inflammation [3, 4], oxidative stress [5] or axonal degeneration [6]. Pharmacological management is essential for treating neuropathic pain. Tricyclic antidepressants, dual reuptake inhibitors of serotonin and norepinephrine, calcium channel alpha(2)-delta ligands, topical lidocaine and glycine transporter inhibitors were recommended as first-line treatment options[7, 8]. However, there are still plenty of negative results in clinical trials. Further understanding of its pathogenesis is essential to treat neuropathic pain and optimize the use of BTZ or other chemotherapeutics in cancer patients.
Metformin, one of the most widely used hypoglycemic drugs for type 2 diabetes, reportedly ameliorates various neurodegenerative disorders including diabetes-associated brain neurodegeneration [9, 10], ethanol-induced neuronal apoptosis [11], and experimental stroke [12]. Notably, application of metformin prevents cisplatin and paclitaxel-induced neuropathic pain [13]. Metformin is as an activator of adenosine monophosphate-activated kinase (AMPK, encoded by Prkaa). According to the difference in its catalytic subunit, AMPK is divided into AMPKa1 and AMPKa2 [14]. It regulates a variety of cellular processes, including protein translation, activity of other kinases, and mitochondrial metabolism [15]. It is not clear whether metformin can regulate the activity of specific AMPK isoforms and prevent and treat BTZ-induced behavioral hypersensitivity in a BTZ-induced neuropathic pain setting.
Autophagy is a physiological process through which cells remove superfluous and dysfunctional components [16]. In the nervous system, autophagy is a fundamental machinery for maintaining functional homeostasis and plays an important role in several neurological diseases, such as Alzheimer’s disease [17], Parkinson’s disease [18], and chronic pain. For example, abnormal cleavage of phosphatidylinositol-binding clathrin assembly protein, PICALM, causes a reduction in autophagy flux in the brain to mediate Alzheimer’s disease [19]. Autophagy also works as an anti-inflammatory mechanism to stop the damage caused by various endogenous or exogenous pernicious stimuli [20]. Increasing evidence indicates that autophagy is intensively involved in the regulation of immune inflammation and production of oxidative molecules, which remodel the neuronal function [18]. Although BTZ-induced pain is closely related to inflammatory response, it is unclear whether autophagy is involved in this process.
GATA transcription factors are a superfamily of zinc finger proteins characterized by their ability to bind to a specific DNA sequence. The GATA superfamily consists of six members, GATA1 through GATA6. Generally, GATA1, GATA2, and GATA3 play primarily roles in the hematopoietic system [21], whereas GATA4, GATA5, and GATA6 are expressed in nonhematopoietic tissues and exhibit diverse developmental roles in other systems [22]. GATA3 has conventionally been regarded as a transcription factor that mediates the activation and repression of transcription. For example, it increases the expression of many Th2 cell genes through positive transcriptional regulation [23]. Importantly, it was recently reported that GATA3 is involved in BTZ-induced chronic pain via enhancing the CCL21 transcription [24]. However, whether GATA3 negatively regulates the expression of other proteins, such as AMPK, at the transcriptional level in BTZ-induced neuropathic pain is unclear.
In this study, we aimed at investigating whether metformin can prevent and treat BTZ-induced behavioral hypersensitivity in a BTZ-induced neuropathic pain setting using a rodent model, and the mechanism underlying any such effect.
Results
Metformin Relieves BTZ-Induced Neuropathic Pain via Enhanced Autophagy Flux in the Spinal Dorsal Horn
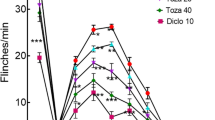
First, we found that intraperitoneal injection of metformin significantly prevented the induction and maintenance of mechanical allodynia following BTZ treatment (Fig. 1a, b). Results of western blot analysis showed that Beclin-1, an autophagy marker, was significantly downregulated in the spinal dorsal horn on days 4 (the initiation of neuropathic pain) and 12 (the maintenance of neuropathic pain), following BTZ treatment (Fig. 1c). Furthermore, we found that consecutive intrathecal injection of an autophagy inducer, rapamycin (2 μg/10 μL for 10 days), attenuated mechanical allodynia (Fig. 1d) induced by BTZ treatment, and the application of 3-methyladenine (3-MA), an autophagy inhibitor, induced mechanical allodynia (Fig. 1e) in naïve rats. These data indicate that, in the spinal cord, autophagy is involved in the development of BTZ-induced chronic pain. Importantly, we found that metformin inhibited the downregulation of Beclin-1 expression by BTZ (12 days after treatment) (Fig. 1f). Notably, co-application of 3-MA (i.t.) partially inhibited the effect of metformin to mitigate Beclin-1 downregulation (Fig. 1g) and mechanical allodynia following BTZ treatment (Fig. 1h–i).
Metformin relieves bortezomib (BTZ)-induced neuropathic pain via enhanced autophagy flux in the spinal dorsal horn. a The induction of mechanical allodynia induced by BTZ was significant ameliorated by metformin administration (n = 12; two-way ANOVA repeated measures). The arrows indicate the time points of metformin injection. b Metformin could relieve the maintenance of mechanical allodynia after BTZ injection (n = 12; two-way ANOVA repeated measures). The arrows indicate the time points of metformin injection. c After BTZ injection, Beclin-1 expression was decreased on days 4 and 12 (n = 4). d Rapamycin injection alleviates BTZ-induced mechanical allodynia (n = 8; two-way ANOVA repeated measures). e Intrathecal injection of 3-MA in naive rats leads to a downregulation of mechanical thresholds (n = 6; two-way ANOVA repeated measures). f Metformin inhibits the reduction in Beclin-1 expression induced by BTZ in the spinal dorsal horn (n = 4). g Co-administration of metformin and 3-MA partially prevented the Beclin-1 upregulation induced by metformin treatment (n = 3). h, i Intrathecal injection of 3-MA reversed the pain relief effect of metformin (n = 6; two-way ANOVA repeated measures). The arrows indicate the time points of metformin injection. Data are plotted as the mean ± SEM. **P < 0.01, ## P < 0.01
AMPKa2/Autophagy Signaling Pathway Plays an Important Role in the Alleviation of BTZ-Induced Neuropathic Pain by Metformin
As metformin is a known AMPK activator, we speculated the AMPK pathway might be the key molecular mechanism underlying metformin-regulated autophagy in the spinal dorsal horn. Results of western blot analysis showed that AMPKa2, but not AMPKa1, was downregulated on days 4 and 12 after BTZ treatment (Fig. 2a), which was significantly inhibited upon metformin application (Fig. 2b). Immunofluorescence staining also confirmed the downregulation of AMPKa2 in the dorsal horn and the effect of metformin in rodents subjected to BTZ treatment (Fig. 2c). Furthermore, overexpression of AMPKa2 by intraspinal AAV (adenovirus-associated virus) injection (Fig. 2d) upregulated the Beclin-1 expression (Fig. 2e) and alleviated BTZ-induced mechanical allodynia (Fig. 2f). Moreover, intrathecal injection of a specific AMPKa2 siRNA (Fig. 2g) induced downregulation of Beclin-1 expression and mechanical hypersensitivity in naive rats (Fig. 2h–i). These results suggest that metformin relieves BTZ-induced neuropathic pain by inhibiting the AMPKa2/autophagy downregulation.
AMPKa2/autophagy signaling pathway plays an important role in the alleviation of BTZ-induced chronic pain by metformin. a After BTZ injection, AMPKa2 expression was decreased on days 4 and 12 (n = 3). b Administration of metformin activated AMPKa2 but not AMPKa1 (n = 3). c Immunofluorescence staining showed the upregulation of AMPKa2 after metformin treatment (scale bar: 100 μm). d qPCR results demonstrate the efficiency of overexpression of AMPKa2 (n = 4; one-way ANOVA). e Overexpression of AMPKa2 in BTZ-treated rats reversed the decline in Beclin-1 expression (n = 4). f Overexpression of AMPKa2 in BTZ-treated rats alleviates mechanical allodynia (n = 6; two-way ANOVA repeated measures). g Intrathecal injection of AMPKa2 siRNA in naive rats leads to a decrease in AMPKa2 expression (n = 4). h Intrathecal injection of AMPKa2 siRNA in naive rats leads to the increase in Beclin-1 expression (n = 4). i Knockdown of AMPKa2 in naive rats leads to a reduction in the mechanical threshold (n = 5; two-way ANOVA repeated measures). Data are plotted as the mean ± SEM. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01
Transcription Factor GATA3 Negatively Regulates the Expression of AMPKa2
To understand the specific molecular mechanism through which BTZ regulates AMPKa2, we examined the expression of AMPKa2 mRNA in the dorsal horn in the model and control rats. The results showed that BTZ application significant downregulated the expression of AMPKa2 mRNA (Fig. 3a). To elucidate the specific role of GATA family members in regulating AMPKa2 transcription, we performed qPCR with primers targeting GATA1 through GATA6 mRNAs, and found that only GATA3 mRNA was significantly upregulated in the spinal dorsal horn on days 4 and 12 after BTZ treatment (Fig. 3b). Results of western blot analysis also revealed the upregulation of GATA3 protein on days 4 and 12 following BTZ treatment (Fig. 3c). Furthermore, double immunofluorescence staining showed that AMPKa2 was co-expressed in GATA3-positive cells in the spinal dorsal horn (Fig. 3d). In addition, knock down of GATA3 by intrathecal injection of an siRNA significantly reversed the downregulation of AMPKa2 mRNA and protein after BTZ treatment (Fig. 3e, f) and alleviated the mechanical allodynia induced by BTZ (Fig. 3g). These results suggest that upregulation of GATA3 negatively regulates the expression of AMPKa2 at the transcriptional level and mediates mechanical allodynia induced by BTZ.
GATA3 expression negatively regulates the expression of AMPKa2 following BTZ treatment. a The AMPKa2 mRNA was significantly downregulated after BTZ treatment (n = 5; two-sample t-tests). b The qPCR results showed the expression of GATA 1 through 6 mRNAs in the spinal dorsal horn of rats after BTZ treatment (n = 3; one-way ANOVA). c The GATA3 levels in the spinal dorsal horn upregulated on days 4 and 12 after administration of BTZ to rats (n = 3; one-way ANOVA). d Immunofluorescence staining images showing colocalization of GATA3 with AMPKa2 (scale bar: 100 μm). e The scatter plots showing the expression of AMPKa2 mRNA after GATA3 siRNA injection (n = 5; one-way ANOVA). f The blots showing the upregulation of AMPKa2 protein after knock down of GATA3 relative to that in the BTZ group (n = 3). g Application of GATA3 siRNA significantly ameliorated the mechanical allodynia induced by BTZ (n = 12; two-way ANOVA repeated measures). Data are plotted as the mean ± SEM. *P < 0.05, **P < 0.01, ##P < 0.01
Mechanism of Transcriptional Repression of AMPKa2 by GATA3
To elucidate the molecular mechanism through which GATA3 negatively regulates the transcription of AMPKa2, we first analyzed the possible binding sites of GATA3 in the AMPKa2 promoter using Jaspar. Next, we performed ChIP-PCR to identify the specific binding sites of GATA3 in the AMPKa2 promoter among the five potential binding sites with high scores. The binding of GATA3 at sites 1, 2, and 4 was increased in the BTZ group compared with that in the vehicle group (Fig. 4a). Several studies have shown that specific histone modification, including acetylation and methylation, are closely related to gene expression [25]. Indeed, we found hypoacetylated histone H3 on sites 1 and 4 of the AMPKa2 promoter in BTZ-treated rats relative to that in the vehicle group (Fig. 4b), whereas no change in histone H4 acetylation was detected on these sites in the model rats (Fig. 4c). Furthermore, we also assessed the methylation level of histone H3 on sites 1 and 4 of the AMPKa2 promoter, and found that the methylation level of histone H3 (k9) on sites 1 and 4 was significantly enhanced in the BTZ group (Fig. 4d). Together, these results suggest that GATA3 potentially modifies the acetylation and methylation levels of histone H3 on sites 1 and 4, through which it contributes to BTZ-induced AMPKa2 downregulation in the dorsal horn.
GATA3 upregulation contributes to changes in acetylation and methylation levels of histone H3 in the AMPKa2 promoter. a ChIP-PCR reveals the binding of GATA3 at different sites in the AMPKa2 promoter (n = 4; two-sample t-tests). b Levels of histone H3 acetylation (acH3) on sites 1 and 4 were significantly decreased in the BTZ groups (n = 6; two-sample t-tests). c Levels of histone H4 acetylation (acH4) on sites 1, 2, and 4 were explored in the vehicle and BTZ groups (n = 5; two-sample t-tests). d Methylation levels of histone H3 (H3K9me2) on sites 1 and 4 were significantly increased following BTZ treatment (n = 5; two-sample t-tests). Data are plotted as the mean ± SEM. **P < 0.01
Discussion
This study demonstrates that metformin treatment can attenuate the induction and maintenance of BTZ-induced neuropathic pain. We found that metformin potentially recovered the impaired autophagy as it mitigated the downregulation of a key autophagy marker, Beclin-1, in the dorsal horn induced by BTZ. Consistent with the previous studies indicating the involvement of metformin or autophagy in neuropathic pain [26, 27], the present study provides the first evidence that metformin may regulate the autophagy function and improve the chemotherapeutic drug-induced neuropathic pain. We also found that application of the autophagy inducer, rapamycin, or its inhibitor, 3-MA, changes the mechanical allodynia differently, and co-application of 3-MA partially inhibited the effect of metformin to recover Beclin-1 expression and reduce mechanical hypersensitivity in rats subjected to BTZ treatment. Rapamycin and 3-MA are the agonist and inhibitor of mTOR and PI3K signaling pathways, respectively [28, 29]. Here, we found that BTZ treatment consistently reduced the AMPKa2 expression in the spinal dorsal horn, which was recovered by intraperitoneal injection of metformin. Importantly, overexpression of AMPKa2 rescued the Beclin-1 expression and attenuated mechanical allodynia induced by BTZ. In naive rats, knockdown of AMPKa2 by intrathecal injection of an siRNA also decreased the Beclin-1 expression and induced mechanical allodynia. These results suggest that AMPKa2 may serve as an important signaling pathway to mediate the analgesic effect of metformin in rodents subjected to BTZ treatment. This is consistent with previous findings that the decreases in AMPKa expression in the spinal dorsal horn is involved in the acute pain induced by the chemotherapeutic drug oxaliplatin [30]. AMPK, as a metabolic stress-sensing protein, can regulate several pain-related signaling proteins, such as SIRT1 and TRPA1, which may be involved in the neuropathic pain induced by nerve injury [31, 32]. Taken together, the present study demonstrates that metformin may regulate AMPKa2-mediated signaling pathways and relieve chronic pain induced by BTZ.
The GATA molecular family is involved in mediating various physiological processes, such as inflammatory responses and cell growth and development [21, 22, 33]. We examined the altered expression levels of GATA 1 through 6 mRNAs in the spinal dorsal horn of rats and found that only GATA3 was consistently upregulated on days 4 and 12 after BTZ treatment. In addition, GATA3 was expressed in AMPKa2-positive cells, and its knockdown significantly reversed the downregulation of AMPKa2 and relieved BTZ-induced neuropathic pain. Whereas previous studies showed that GATA3 plays a role in BTZ-induced pain models by upregulating the expression of inflammatory cytokine CCL21 [24], the present study indicates that GATA3 can also participate in the BTZ-induced neuropathic pain by inhibiting AMPKa2 expression, which suggests that GATA3 differentially regulates the expression of various pain-related molecules in BTZ-induced neuropathic pain model. Several studies have shown that changes in acetylation or methylation of histone are closely related to gene expression [25]. In the ChIP analysis performed to explore the mechanism through which GATA3 regulates AMPKa2 transcription, we found that the interaction between GATA3 and sites 1, 2, and 4 of the AMPKa2 promoter significantly increased following BTZ application. Moreover, the decreased acetylation level of histone H3, as well as the hypermethylation of H3, on sites 1 and 4 in the AMPKa2 promoter potentially contributed to the downregulation of AMPKa2 following BTZ treatment.
In summary, these results show that the GATA3-mediated epigenetic mechanism is involved in the suppression of AMPKa2 transcription, which potentially impairs autophagy and contributes to mechanical allodynia induced by BTZ. Metformin prevents the downregulation of AMPKa2 and restores the autophagy function in the dorsal horn, thereby, relieving BTZ-induced neuropathic pain.
Methods
Animals
Male Sprague Dawley rats weighting 200–250 g were obtained from the Institute of Experimental Animals at Sun Yat-Sen University. All animals were housed separately at 24 ± 1 °C and 50–60% relative humidity on a 12 h/12-h light/dark cycle and given access to food and water ad libitum. All the experimental protocols were approved by the Institutional Animal Care Committee and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the suffering and the number of rats used. All animals were randomly assigned to different experimental or control groups in this study.
Lumbar Subarachnoid Catheterization
For intrathecal injection, after animals were anesthetized with 2% isoflurane, polyethylene intrathecal catheters (PE-10, USA) were implanted into rats. In brief, the catheter was inserted into the L5/L6 intervertebral subarachnoid space with the tip of the catheter located near the L5 spinal segmental level. Following intrathecal implantation of catheters, animals were allowed 5 days to recover from surgery prior to subsequent drug injection, and any animal with hind limb paresis or paralysis was excluded from the present study. Ten microliter of 2% lidocaine was injected to confirm that the catheter was correctly placed, as indicated by transient bilateral hind limb paralysis.
Drug and Adenovirus-Associated Virus Administration
Bortezomib (Topscience, Shanghai, China) was intraperitoneally injected at 0.4 mg/kg once per day for 5 consecutive days, as described previously [34]. Control animals received an equivalent volume of the vehicle, saline. The cholesterol-conjugated siRNAs were commercially obtained from Ribo Bio. GATA3 siRNA (1 nmol/10 μL), non-targeting control siRNA (1 nmol/10 μL), AMPKa2 siRNA (1 nmol/10 μL), 3-MA (30 μg/10 μL), rapamycin (2 μg/10 μL) or vehicle saline (10 μL) was intrathecally administrated prior to BTZ treatment. Metformin was intraperitoneally injected at doses of 50 mg/kg/day, which were based on previous reports [34].
For intraspinal injection of the adenovirus-associated virus (AAV), the L4–L5 vertebrae were exposed, and the vertebral column was mounted in a stereotaxic frame. A slight laminotomy was performed, and the dura was incised to expose the spinal cord. AAV was injected into both the sides of the spinal dorsal horn at four injection sites (150 nL of AAV was injected at each site). The micropipette was withdrawn 10 min after virus injection, and the incision was closed with stitches.
Behavioral Assessment
For each rat, mechanical allodynia was assessed by the hind paw mechanical withdrawal threshold. All rats were placed in a plastic box on a metal mesh and were allowed to acclimate for 3 consecutive days (30 min/day) before testing. Von Frey filaments of different bending forces were applied alternately to the midplantar surface of the hind paw. A nociceptive response was defined as a brisk paw withdrawal or paw flinching following Von Frey filament application. In the absence of a paw withdrawal response, a stronger stimulus was presented; when paw withdrawal occurred, the next weaker stimulus was chosen. An optimal threshold calculation using this method required five responses in the immediate vicinity of the 50% threshold. The 50% paw withdrawal threshold was calculated following a previously validated up–down procedure [35]. The behavioral tests were conducted by an experimenter who was blinded to all treatments.
Immunofluorescence Staining
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and cardiac perfusion were performed using 0.9% physiological saline, followed by 4% paraformaldehyde in PBS. Next, L4–L6 spinal cord tissues were removed and post-fixed in the same fixative overnight and then dehydrated with 30% sucrose. Cryostat sections (25 μm thick) were cut and processed for immunofluorescent staining with primary antibodies for GATA3 (1:50, Santa Cruz, sc-268) and AMPKa2 (1:100, Proteintech, 18167-1-AP) at 4 °C overnight; the sections were then incubated with Cy3-conjugated or Alexa 488-conjugated secondary antibodies for 1 h at 26 °C. The stained sections were then examined with a Leica (Nikon, Japan) fluorescence microscope equipped with a digital camera, and images were captured.
RNA Extraction and Quantitative (Real-Time) PCR (qPCR)
Total RNA was extracted from the dorsal horn (L4–L6) tissues by TRIzol reagent (Invitrogen, USA). Evo M-MLV RT Premix (AG, China) and SYBR Green Pro Tap Premix (AG, China) were used to quantify the amounts of mRNAs, according to a protocol based on the manufacturer’s instructions. The relative expression ratio of mRNA was quantified using the 2−△△CT method [36]. The sequences of specific primers used are listed in Table 1.
Western Blot Analysis
The L4–L6 spinal dorsal horn tissues of rats were removed and homogenized in a lysis buffer (Beyotime, China) after the animals were anesthetized with 50 mg/kg sodium pentobarbital (i.p.). Next, the lysates of the L4–L6 dorsal horn tissues were prepared, separated by gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride membrane. They were then preincubated for 1 h at room temperature in the block buffer (EpiZyme, China). After incubating overnight with diluted primary antibodies against GATA3 (1:100, Santa Cruz, sc-268), AMPKa2 (1:1000, Proteintech, 18167-1-AP), Beclin1 (1:1000, CST, 3738), β-actin (1:1000, CST, 3700 or 4967), or GAPDH (1:1000, Abcam, ab8245; 1:1000, CST, 2118) at 4 °C, the membranes were incubated in horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Finally, the bands on the membranes were visualized by enhanced chemiluminescence (ECL, Millipore), as per the manufacturer’s instructions. The bands were quantified with a computer-assisted imaging analysis system (ImageJ, NIH, USA).
ChIP Assays
ChIP assays were performed using the ChIP Assay Kit (Cell Signaling Technology, cat #9005). The L4 and L5 spinal cord segments of animals were removed quickly and placed in 1% formaldehyde for 10 min at room temperature to crosslink transcription factors with chromatin. Formaldehyde was subsequently inactivated by addition of glycine. Micrococcal nuclease was used to digest DNA to approximately 150–900 bp length, and the DNA fragments were immunoprecipitated overnight using antibodies targeting GATA3 (5 μg, Thermo Fisher Scientific, PA5-20892), ac-H3 (5 μg, Abcam, ab32129), ac-H4 (5 μg, Sigma, 06-598), H3K9me2 (5 μg, Sigma, D5567), or IgG at 4 °C. The next day, ChIP-grade protein G magnetic beads were added and incubated for 2 h at 4 °C with rotation. Thereafter, the chromatin-protein-antibody-bead complexes were eluted, and the bound DNA was extracted. The precipitated DNA was purified using spin columns, and qPCR was performed as described above. Finally, the ratio of ChIP/input in the spinal dorsal horn was calculated.
Statistical Analysis
SPSS 25.0 was used to analyze the data, and the results are shown as the mean ± SEM. The data from behavioral tests were analyzed using two-way ANOVA with repeated measures. The qPCR and western blot data were analyzed using the two independent samples t-test or one-way ANOVA followed by Dunnett’s T3 or Tukey’s post hoc test. When tests of normality were not satisfied, the nonparametric tests were substituted. The criterion of statistical significance was 0.05. All measurements were taken from distinct samples.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rule S (2018) Bortezomib-based chemotherapy in mantle cell lymphoma. Lancet Oncol 19:1419–1421
Stockstill K, Doyle TM, Yan X, Chen Z, Janes K, Little JW, Braden K, Lauro F, Giancotti LA, Harada CM, Yadav R, Xiao WH, Lionberger JM, Neumann WL, Bennett GJ, Weng HR, Spiegel S, Salvemini D (2018) Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J Exp Med 215:1301–1313
Moschetti G, Amodeo G, Maftei D, Lattanzi R, Procacci P, Sartori P, Balboni G, Onnis V, Conte V, Panerai A, Sacerdote P, Franchi S (2019) Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy. J Neuroinflamm 16:89
Liu C, Luan S, OuYang H, Huang Z, Wu S, Ma C, Wei J, Xin W (2016) Upregulation of CCL2 via ATF3/c-Jun interaction mediated the Bortezomib-induced peripheral neuropathy. Brain Behav Immun 53:96–104
Duggett NA, Flatters SJL (2017) Characterization of a rat model of bortezomib-induced painful neuropathy. Br J Pharmacol 174:4812–4825
Geisler S, Doan RA, Cheng GC, Cetinkaya-Fisgin A, Huang SX, Hoke A, Milbrandt J, DiAntonio A (2019) Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program. JCI 4:e129920
Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, Kent JL, Krane EJ, Lebel AA, Levy RM, Mackey SC, Mayer J, Miaskowski C, Raja SN, Rice AS, Schmader KE, Stacey B, Stanos S, Treede RD, Turk DC, Walco GA, Wells CD (2010) Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 85:S3-14
Al-Khrasani M, Mohammadzadeh A, Balogh M, Kiraly K, Barsi S, Hajnal B, Koles L, Zadori ZS, Harsing LG Jr (2019) Glycine transporter inhibitors: a new avenue for managing neuropathic pain. Brain Res Bull 152:143–158
Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, Monteiro P, Seica R, Oliveira CR, Moreira PI (2008) Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem 4:358–364
El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci 34:77–87
Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13:11
Li J, Benashski SE, Venna VR, McCullough LD (2010) Effects of metformin in experimental stroke. Stroke 41:2645–2652
Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ (2014) The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS ONE 9:e100701
Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716
Maixner DW, Yan X, Hooks SB, Weng HR (2016) AMPKalpha1 knockout enhances nociceptive behaviors and spinal glutamatergic synaptic activities via production of reactive oxygen species in the spinal dorsal horn. Neuroscience 326:158–169
Mizushima N, Levine B (2020) Autophagy in human diseases. N Engl J Med 383:1564–1576
Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16:345–357
Ghosh A, Tyson T, George S, Hildebrandt EN, Steiner JA, Madaj Z, Schulz E, Machiela E, McDonald WG, Escobar Galvis ML, Kordower JH, Van Raamsdonk JM, Colca JR, Brundin P (2016) Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci Transl Med 8:368ra174
Moreau K, Fleming A, Imarisio S, Lopez Ramirez A, Mercer JL, Jimenez-Sanchez M, Bento CF, Puri C, Zavodszky E, Siddiqi F, Lavau CP, Betton M, O’Kane CJ, Wechsler DS, Rubinsztein DC (2014) PICALM modulates autophagy activity and tau accumulation. Nat Commun 5:4998
Deretic V (2021) Autophagy in inflammation, infection, and immunometabolism. Immunity 54:437–453
van Bergen M, Marneth AE, Hoogendijk AJ, van Alphen F, van den Akker E, Laros-van Gorkom B, Hoeks M, Simons A, de Munnik S, Janssen JJ, Martens J, Jansen JH, Meijer AB, Van der Reijden BA (2021) Specific proteome changes in platelets from individuals with GATA1-, GFI1B- and RUNX1-linked bleeding disorders. Blood 138:86
Fan Y, Gu X, Zhang J, Sinn K, Klepetko W, Wu N, Foris V, Solymosi P, Kwapiszewska G, Kuebler WM (2020) TWIST1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of GATA-6 and BMPR2. Am J Respir Crit Care Med 202:1283–1296
Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A (2012) The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37:634–648
Zheng Y, Sun Y, Yang Y, Zhang S, Xu T, Xin W, Wu S, Zhang X (2019) GATA3-dependent epigenetic upregulation of CCL21 is involved in the development of neuropathic pain induced by bortezomib. Mol Pain 15:1744806919863292
Sadakierska-Chudy A, Filip M (2015) A comprehensive view of the epigenetic landscape. Part II: histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotox Res 27:172–197
Li J, Tian M, Hua T, Wang H, Yang M, Li W, Zhang X, Yuan H (2021) Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 17:4062
Baeza-Flores GDC, Guzman-Priego CG, Parra-Flores LI, Murbartian J, Torres-Lopez JE, Granados-Soto V (2020) Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol 11:558474
Benjamin D, Colombi M, Moroni C, Hall MN (2011) Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 10:868–880
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM (2010) Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285:10850–10861
Ling YZ, Li ZY, Ou-Yang HD, Ma C, Wu SL, Wei JY, Ding HH, Zhang XL, Liu M, Liu CC, Huang ZZ, Xin WJ (2017) The inhibition of spinal synaptic plasticity mediated by activation of AMP-activated protein kinase signaling alleviates the acute pain induced by oxaliplatin. Exp Neurol 288:85–93
Li C, Sun W, Gu C, Yang Z, Quan N, Yang J, Shi Z, Yu L, Ma H (2018) Targeting ALDH2 for therapeutic interventions in chronic pain-related myocardial ischemic susceptibility. Theranostics 8:1027–1041
Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y (2018) Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes 67:98–109
Iyyanki T, Zhang B, Wang Q, Hou Y, Jin Q, Xu J, Yang H, Liu T, Wang X, Song F, Luan Y, Yamashita H, Chien R, Lyu H, Zhang L, Wang L, Warrick J, Raman JD, Meeks JJ, DeGraff DJ, Yue F (2021) Subtype-associated epigenomic landscape and 3D genome structure in bladder cancer. Genome Biol 22:105
Chen K, Fan J, Luo ZF, Yang Y, Xin WJ, Liu CC (2018) Reduction of SIRT1 epigenetically upregulates NALP1 expression and contributes to neuropathic pain induced by chemotherapeutic drug bortezomib. J Neuroinflamm 15:292
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinform Biomath 3:71–85
Acknowledgements
This study was funded by National Natural Science Foundation of China (31970936, 81801103 and 81971048), Natural Science Foundation of Guangdong (2019A1515010871 and 2019A1515110716), Shanghai Pujiang Program (2020PJD059) and Youth Cultivation Project for Special Basic Medical Research of the First Affiliated Hospital of Naval Medical University (2021JCQN02).
Funding
This work was supported by National Natural Science Foundation of China (31970936, 81801103 and 81971048), Natural Science Foundation of Guangdong (2019A1515010871 and 2019A1515110716), Shanghai Pujiang Program (2020PJD059) and Youth Cultivation Project for Special Basic Medical Research of the First Affiliated Hospital of Naval Medical University (2021JCQN02).
Author information
Authors and Affiliations
Contributions
ML: Investigation, Writing—Original Draft. YTZ: Investigation, Formal analysis. YYL: Investigation, Formal analysis. TX: Formal analysis. DL: Formal analysis. YCX: Visualization, Supervision. WJX: Visualization, Supervision. SYL: Conceptualization, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
All experimental protocols were approved by the Local Animal Care Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Zhao, YT., Lv, YY. et al. Metformin Relieves Bortezomib-Induced Neuropathic Pain by Regulating AMPKa2-Mediated Autophagy in the Spinal Dorsal Horn. Neurochem Res 47, 1878–1887 (2022). https://doi.org/10.1007/s11064-022-03571-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03571-7