Abstract
Hyperpolarization-activated cyclic nucleotide-gated channels and purinergic P2X receptors play critical roles in the nerve injury-induced pain hypersensitivity. Both HCN channels and P2XR are expressed in dorsal root ganglia sensory neurons. However, it is not clear whether the expression and function of P2X2 and P2X3 receptors can be modulated by HCN channel activity. For this reason, in rats with chronic constriction injury of sciatic nerve, we evaluated the effect of intrathecal administration of HCN channel blocker ZD7288 on nociceptive behavior and the expression of P2X2 and P2X3 in rat DRG. The mechanical withdrawal threshold was measured to evaluate pain behavior in rats. The protein expression of P2X2 and P2X3 receptor in rat DRG was observed by using Western Blot. The level of cAMP in rat DRG was measured by ELISA. As a result, decreased MWT was observed in CCI rats on 1 d after surgery, and the allodynia was sustained throughout the experimental period. In addition, CCI rats presented increased expression of P2X2 and P2X3 receptor in the ipsilateral DRG at 7 d and 14 d after CCI operation. Intrathecal injection of ZD7288 significantly reversed CCI-induced mechanical hyperalgesia, and attenuated the increased expression of P2X2 and P2X3 receptor in rat DRG, which open up the possibility that the expression of P2X2 and P2X3 receptor in DRG is down-regulated by HCN channel blocker ZD7288 in CCI rats. Furthermore, the level of cAMP in rat DRG significantly increased after nerve injury. Intrathecal administration of ZD7288 attenuated the increase of cAMP in DRG caused by nerve injury. Subsequently, effects of HCN channel activity on ATP-induced current (IATP) in rat DRG neurons were explored by using whole-cell patch-clamp techniques. ATP (100 μM) elicited three types of currents (fast, slow and mixed IATP) in cultured DRG neurons. Pretreatment with ZD7288 concentration-dependently inhibited three types of ATP-activated currents. On the other hand, pretreatment with 8-Br-cAMP (a cell-permeable cAMP analog, also known as an activator of PKA) significantly increased the amplitude of fast, slow and mixed IATP in DRG neurons. The enhanced effect of 8-Br-cAMP on ATP-activated currents could be reversed by ZD7288. In a summary, our observations suggest that the opening of HCN channels could enhance the expression and function of P2X2 and P2X3 receptor via the cAMP-PKA signaling pathway. This may be important for pathophysiological events occurring within the DRG, for where it is implicated in nerve injury-induced pain hypersensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain is commonly caused by injuries in the peripheral or central nervous system, which manifests as allodynia, hyperalgesia and spontaneous pain [1]. It is clear that peripheral sensitization is involved in the development of chronic pain induced by peripheral nerve injury. Hyper-excitability of dorsal root ganglia (DRG) sensory neurons, as a result of afferent ectopic discharges from the site of nerve injury, is necessary to kindle the sensitization of spinal nociceptive pathway. Some different types of ion channels in DRG sensory neurons play an important role in nociceptive information transmission from the peripheral to the central nervous system. Recently, numerous studies have shown that hyperpolarization-activated cyclic nucleotide-gated (HCN) channels which mediate responses to noxious stimuli are expressed in DRG. HCN channels comprise four members (HCN1-HCN4). HCN1 and HCN2 are the isoforms most strongly expressed in primary somatosensory neurons in the DRG. The increased expression of HCN1 and HCN2 in DRG were observed in rodent models of inflammatory and neuropathic pain. Moreover, intraperitoneal or intrathecal injection with ZD7288 (4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyrimidinium chloride, a HCN channel inhibitor) suppressed pain hypersensitivity, and attenuated the increased expression of HCN1 and HCN2 in DRG neurons of these pain models [2,3,4,5,6,7]. In addition, HCN1 or HCN2-deficient mice exhibited reduced experimental pain sensitivity than wild-type littermate, which indicate an important role for HCN1 and HCN2 in pain perception [7,8,9,10]. It looks likely that inhibition of the function of HCN1 and HCN2 are effective at reducing the spontaneous pain behavior in animal models of neuropathic pain, which means that HCN channels appear to be a promising analgesic drug target.
It is well known that extracellular adenosine triphosphate (ATP) can serve as an important chemical neurotransmitter that can modulate pain perception through the activation of P2 purinoceptors-mediated nociceptive afferent pathway. P2 receptor can be divided into two main groups: the ATP-gated ionotropic P2X family and the G protein-coupled metabotropic P2Y receptors. Immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR) demonstrate that P2 receptors, including P2X1-6, P2Y1 and P2Y4, are expressed in DRG neurons [11, 12]. Previous studies have shown that P2X3 receptor play a crucial role in facilitating the transmission of pain in neuropathic pain states [13, 14]. In situ hybridization and whole-cell patch-clamp recording revealed that capsaicin-sensitive, small-sized DRG neurons mainly expressed homomeric P2X3 subunit, and capsaicin-insensitive, medium-sized DRG neurons expressed heteromeric receptor with P2X2 and P2X3 [15, 16]. A growing body of research confirms that the increased expression of P2X2 and P2X3 receptors in DRG contribute to chronic inflammatory and neuropathic pain, and selective blockade of P2X2 and P2X3 receptor activation can inhibit mechanical and thermal hyperalgesia [13, 17, 18]. For example, Wang et al. [16] report that ATP-induced current mediated by P2X3 receptors in DRG neurons is significantly enhanced after spared nerve injury (SNI). In addition, in complete Freund's adjuvant (CFA)-induced inflammation pain rats, ATP-induced both fast and slow currents (the responsible receptors are homomeric P2X3 and heteromeric P2X2/3, respectively) were significantly enhanced in DRG neurons [19]. Furthermore, Cockayne et al. [20] found that DRG neurons from P2X2-/- mice only presented fast current responded to ATP and reduced pain-related behavior in response to intra plantar injection of formalin, which suggests that an important contribution of heteromeric P2X2/3 receptors to nociceptive responses. These experimental results suggest that overactivation of P2X2 and P2X3 receptors in DRG sensory neurons is responsible for the neuropathic and inflammatory pain behaviors.
As a selective blocker of HCN currents, ZD7288 has been widely used as a tool for studying the role of HCN channels in neuronal functions. A growing body of research has shown that ZD7288 can affect the function of other ion channels and receptors of neurons. For example, ZD7288 inhibited Na+ currents in DRG neurons and HEK293 cells transfected with Na(v)1.4 plasmids, respectively [21]. In addition, ZD7288 attenuated the amplitude of both AMPA and NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) [22, 23], and blocked high-frequency tetanic stimulation induced long-term potentiation (LTP) at the mossy fiber-CA3 synapses [22] and perforant path-CA3 synapses [23, 24], which suggests that HCN channels may affect glutamate receptor function and thus contribute to regulation of plasticity in the hippocampus.
Some recent studies have shown that there is an interaction between HCN channels and P2 purinoceptors. It was reported that P2Y1 receptors activation lead to facilitation of the current mediated by HCN channels (Ih) in mesencephalic trigeminal neurons [25]. But Khakh et al. found that activation of P2X receptor channels caused an apparent inhibition of Ih in neurons of rat trigeminal mesencephalic nucleus [26]. It is not clear whether there is an interaction between HCN channels and P2X receptors in DRG neurons. For this reason, in the current study, we examined whether the expression and function of P2X2 and P2X3 receptors were affected by HCN channel blocker ZD7288.
Methods
Animals
Sprague Dawley (SD) rats were used in this study. The protocol was prepared from SD rats in accordance with the National Institutes of Health guide-lines in a manner that minimized animal suffering and animal numbers. All experiments were carried out in accordance with China animal welfare legislation, and were approved by the Zunyi Medical University Committee on Ethics in the Care and Use of Laboratory Animals. 2 to 3-week-old SD rats were used in the electrophysiology study. Adult SD rats (6–8 weeks, 180–220 g) were used in behavioral experiments [27, 28]. The rats were housed in separated cages with free access to water and food in a standard 12-h light/dark cycle [21, 29].
Implantation of Intrathecal Catheter
Lumbosacral intrathecal catheters were implanted as described by Storkson et al. [30]. Under anesthesia with pentobarbital sodium (40 mg/kg, i.p.), rats were fixed and 2 cm longitudinal incision was made above vertebrae L5–L6. A PE-10 polyethylene catheter was implanted intrathecally at the level of the L5-L6 until a sudden movement of the tail or the hind limb was observed [29], and then passed gently 2 cm upward to reach the level of the lumbar enlargement. The tip of the catheter was immobilized onto the neck under the skin. The correct intrathecal catheter position was confirmed through paralysis of bilateral hind limbs after injection of 2% lidocaine (10 μl). Rats exhibiting postoperative neurologic deficits were excluded. The rats were housed individually after implantation of an intrathecal catheter, and allowed to recover 5 d before the chronic constriction injury (CCI) operation [31].
Intrathecal administration of drug is via a microsyringe connected to the intrathecal catheter. ZD7288 (Sigma) was made with 0.9% sodium chloride. The drug was injected once a day for 14 consecutive days after CCI [29].
The Chronic Constriction Injury of Sciatic Nerve (CCI) Model
The rat CCI model was established with reference to the method described by Bennett et al. [32]. All surgical procedures were performed under strict sterile conditions. Under anesthesia with pentobarbital sodium (40 mg/kg, i.p.), the left sciatic nerve proximal to the sciatic trifurcation was exposed by blunt dissection of the biceps femoris muscle. Four loose ligatures were tied in the isolated sciatic nerve with sterile 4.0 chromic guts, 1 mm apart. The intensity of the ligation was determined by the slight twitch of the calf muscle [32, 33]. Muscle and skin were closed in layers. The sciatic nerve of sham operated rats was exposed without ligation. Rats exhibiting neurologic impairments or infection were not included in this study. After CCI surgery, rats were housed in separate cages to avoid scratching each other.
Measurement of Mechanical Allodynia
The paw mechanical withdrawal threshold (MWT) was measured using an electronic von Frey plantar aesthesiometer (IITC, Wood Dale, IL, USA) [31]. A rigid filament was applied perpendicularly to the medial surface of the left hind paw with increasing force until a paw withdrawal occurred. The paw withdrawal was considered as a positive response and the pressure value recorded by the device was MWT. The test was repeated three times at 5-min intervals and the average value was recorded as MWT for the rat [34,35,36].
Western Blot (WB)
The rats were terminally anesthetized with pentobarbital sodium (40 mg/kg, i.p.). The ipsilateral L4-L6 DRG of CCI operation was rapidly dissected and rinsed in cold phosphate-buffered saline, then homogenized in 1 ml ice-cold radioimmunoprecipitation (RIPA) lysis buffer. Protein concentration was determined via Bicinchoninic Acid (BCA) methods [32]. Total proteins were diluted in 4 × loading buffer and incubated at 100 °C for 5 min for denaturation. Samples (80 μg) were loaded onto a 10% Tris–HCl SDS-PAGE gel (BioRad, Hercules, CA) for electrophoresis separation and transferred onto polyvinylidene fiuoride (PVDF) membranes [19, 37]. The PVDF membranes were blocked for 1 h at room temperature in nonfat dried milk solution [10% in 0.1% Tween-Tris-buffered saline (TTBS)] and then were incubated with goat anti-P2X2 (1:500, ab229151, Abcam) [38], goat anti-P2X3 (1:500, ab90905, Abcam) and goat anti-GAPDH polyclonal antibody (1:5000, ab8245, Abcam, Cambridge, UK) overnight at 4 °C [39]. The PVDF membranes were washed three times with TBST and then incubated with HRP-tagged secondary antibodies at room temperature for 1 h [40]. The immunoreactive proteins were visualized using enhanced chemiluminescence (ECL) reagent Beyo ECL plus (Beyotime Institute of Biotechnol-ogy) [19]. Images of the blots were captured using a ChemiDoc XRS system (Bio-Rad Laboratories, Inc.). The image was scanned, and band intensity was semi-quantified using Quantity One software v4.52 (Bio-Rad Laboratories, Inc.).
Cell Culture
DRG neurons were obtained from 2 to 3-week-old SD rats. Briefly, rat was anesthetized and L4-L6 DRGs were dissected surgically under sterile condition. The dissected DRG were desheathed, cut and incubated in 4 ml Dulbecco,s Modified Eagle,s Medium with F-12 supplement (DMEM/F-12, GIBCO, USA) containing 0.3% collagenase (Class I, Sigma, St Louis, MO, USA) for 60 min at 37 °C. After wash three times, the tissue was then incubated in 4 ml DMEM/F-12 containing 0.125% trypsin (Sigma) at 37 °C for 20 min. The action of trypsin was stopped by DMEM/F12 containing 10% fetal bovine serum. The tissue was then dissociated into single neurons by gentle trituration using fire polished glass pipets. The cells were collected by centrifugation at 1000 rpm for 5 min. The supernatant was discarded and the cells were suspended in growth medium comprising of DMEM/F-12 supplemented with 10% bovine serum, 50 ng/ml nerve growth factor, 200 IU/ml penicillin and 200 IU/ml streptomycin. DRG cells were plated onto sterile glass coverslips pre-coated with 10 μg/ml poly-d-lysine (Sigma) followed by 10 μg/ml Laminin-I (Sigma) and maintained in a 95% air and 5% CO2 humidified incubator at 37 °C, and used within 48 h.
Whole-Cell Clamp Recording
Recordings were performed at room temperature (22 ~ 25 °C) [35, 41]. Cells were transferred to a submersion-type recording chamber and perfused (1–2 ml/min) with extracellular solution (in mM): 135 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgCl2, 10 glucose, and 10 HEPES, pH 7.2 [27, 41]. Recording electrodes (resistance 5–10 MΩ) were filled with internal solution, which contained (in mM): 145 K-gluconate, 2 MgCl2, 5 K2 ATP, 0.5 EGTA (ethylene glycol tetra acetic acid), 5HEPES, pH 7.2–7.4 [42]. Membrane currents were recorded using an Axopatch 200B amplifier, digitized with a Digidata 3200A interface (Axon Instruments, Foster City, CA.), and acquired at a frequency of 2 kHz using pClamp 9 [43]. Under voltage-clamp conditions, the whole-cell patch recording technique was used for current recordings. Membrane potential was held at −50/−60 mV [27]. After giga-ohm seal formation and patch rupture, series resistance was compensated at 60–80% and continually monitored throughout the experiment [35]. Neurons were given at least 5 min to stabilize before data were collected [35]. ATP (100 μM) was applied rapidly for 2 s at 5-min intervals through a manifold comprised of 8 capillaries, with 200 µm internal diameter [37].
ELISA
The level of cAMP in the DRG of rats was measured by ELISA. The ipsilateral L4-L6 DRG of CCI operation was rapidly separated, ground, and homogenized with an ultrasonic tissue homogenizer. Samples were centrifuged and the supernatants were collected for cAMP analysis. The content of cAMP was evaluated using ELISA kit (Hushang Biological Technology Co., Ltd., Shanghai, People’s Republic of China). The optical density (OD) value at 450 nm was recorded using a microplate reader. The average level of cAMP was calculated in term of the standard curve as directed by manufacturer’s instructions for the kit.
Statistical Analysis
In vivo data were presented as the mean ± standard deviation (SD). SPSS18.0 (SPSS Inc., Chicago, IL, USA) was used to analyze data. Normality of distribution of data for each test index was verified using the Kolmogorov–Smirnov test. Given the normal distribution of data, statistical analysis was conducted with one-way analysis of variance (ANOVA) followed by application of Dunnett’s multiple comparison test or Student’s t-test. P < 0.05 was considered statistically significant. In vitro data were presented as the mean ± standard error (Sem). In this study, ATP-induced current was compared before and after the administration of a drug in the same cell. Statistical analysis was performed with Paired sample t-test. P < 0.05 was considered statistically significant.
Results
Analgesic Effect of ZD7288 on Hyperalgesia in CCI Rats
ZD7288 was used to determine the involvement of HCN channels in CCI-induced neuropathic pain. Previous studies have reported that ZD7288 produced a concentration-dependent reversal of mechanical nociceptive threshold, with maximal reversal observed at 2 h after ZD7288 injection in spinal nerve ligation-induced neuropathic pain rats and diabetic neuropathic pain rats [44]. In the present study, three concentrations of ZD7288 (10 µg, 30 µg, 50 µg/10 µl) were intrathecally administered. We found that application of ZD7288 at 10 µg, 30 µg and 50 µg/10 µl all showed increased MWT in comparison to CCI rats (P < 0.05). The analgesic effect of ZD7288 reached its peak 2 h after intrathecal injection, and gradually decreased thereafter (Fig. 1A). Among them, ZD7288 at 10 µg/10 µl showed slight but statistically significant analgesic effect only 2 h after injection (17.33 ± 1.86 vs. 13.96 ± 1.75 g. P < 0.05; n = 8; Fig. 1A), while ZD7288 at concentrations of 30 and 50 µg/10 µl had more significant analgesic effect (30.3 ± 3.39 and 31.38 ± 3.79 g vs. 13.96 ± 1.75 g. P < 0.01; n = 8; Fig. 1A). Some of the rats injected with 50 μg/10ul ZD7288 showed sluggish in action, while the rats injected with 30 μg/10ul ZD7288 did not show this side effect. Then, ZD7288 at concentration of 30 µg/10 µl was selected in the following experiments.
Mechanical hypersensitivity in different groups was determined by measuring the mechanical withdrawal threshold (MWT). All values represent mean ± SD. A Effect of ZD7288 (10 μg, 30 μg and 50 μg/10 μl) on the MWT in CCI rats at 1 h, 2 h, 4 h and 8 h after the administration of ZD72888. Statistically significant differences were observed at 1 h, 2 h, 4 h and 8 h after the administration of ZD7288 (10 μg: *P < 0.05; 30 μg and 50 μg: **P < 0.01, ++P < 0.01). Intrathecal injection of 10 μg/10 μl ZD7288 had an analgesic effect only 2 h after administration, and other detection time was not statistically significant. Intrathecal injection of ZD7288 of 30 μg and 50 μg/10 μl had significant analgesic effect during the detection period. B The MWT in CCI rats (at 1, 3, 5, 7, 10 and 14 d after CCI operation) was significantly lower than in the sham group (**P < 0.01). The MWT in ZD7288-treated CCI rats were significantly higher than CCI rats (++P < 0.01)
On the other hand, we noticed that decreased MWT were exhibited on 1 d after nerve injury compared to sham rats (25.13 ± 3.47 vs. 36.32 ± 3.81 g. P < 0.01; n = 8; Fig. 1B). The allodynia lasted until day 14 after CCI operation (12.78 ± 1.42 vs. 36.72 ± 3.5 g. P < 0.01; n = 8; Fig. 1B). Rats of the sham group failed to exhibit mechanical allodynia. In addition, intrathecal injection of ZD7288 (30 µg/10 µl) significantly reversed mechanical hyperalgesia, and had analgesic effect 14 d after CCI operation (29.07 ± 2.89 vs. 12.78 ± 1.42 g. P < 0.01; n = 8; Fig. 1B). These data confirmed that HCN channels are involved in the development of CCI-induced neuralgia.
Blocking the Function of HCN Channels can Inhibit the Expression of P2X2 and P2X3 Receptor in DRG of CCI Rats
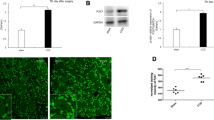
The P2X receptors in DRG have been found to greatly contribute to pain transmission sensitization [13, 14]. In this study, the protein expression of P2X2 and P2X3 receptor in DRG significantly increased after nerve injury compared to sham rats (P < 0.05, Fig. 2). To explore whether HCN channels regulate the expression of P2X receptor in DRG, we detected the P2X2 and P2X3 receptors expression after the administration of ZD7288. Western blot analysis demonstrated that intrathecal injection of ZD7288 significantly attenuated the increased expression of P2X2 and P2X3 receptor in CCI rats (P < 0.05, Fig. 2). These data confirmed that HCN channels activation after CCI operation can promote the expression of P2X2 and P2X3 receptors in DRG.
Effect of HCN channel blockers ZD7288 on the protein expression of P2X2 and P2X3 receptor in DRG in CCI rats (n = 4). A Intrathecal administration of ZD7288 significantly decreased the P2X2 receptor expression in the DRG of CCI rats (P < 0.05); B Intrathecal administration of ZD7288 significantly decreased the P2X3 receptor expression in the DRG of CCI rats (P < 0.05)
Blockage of HCN Channels can Reduce the Increase of cAMP in DRG of CCI Rats
Previous studies have shown that enhanced cAMP signaling promotes neuropathic and inflammatory pain [45,46,47]. The cAMP signaling is also involved in the activation of HCN channels [48, 49]. In this experiment, the level of cAMP in DRG significantly increased at 7d and 14d after nerve injury (29.17 ± 1.88 and 36.57 ± 1.27 pmol/mg vs. 21.55 ± 1.25 pmol/mg. P < 0.05; n = 6; Fig. 3). Intrathecal administration of HCN channels antagonist ZD7288 attenuated the increase of cAMP in DRG caused by nerve injury (7d: 24.54 ± 2.51 pmol/mg vs. 29.17 ± 1.88 pmol/mg; 14d: 25.80 ± 1.24 pmol/mg vs. 36.57 ± 1.27 pmol/mg. P < 0.01; n = 6; Fig. 3). These results suggest that the elevation of cAMP level in DRG caused by nerve injury may promote the activation of HCN channel, and then promote the generation of neuropathic pain. The analgesic effect of blocking HCN channels may be related to the decrease of cAMP level in DRG of rats with nerve injury.
Intrathecal administration of HCN channel blockers ZD7288 inhibited the increase of cAMP content in DRG of CCI rats (n = 6). The content of cAMP was increased in DRG of CCI rats compared with the control group (P < 0.05). Intrathecal injection of ZD7288 attenuated the increase of cAMP content in DRG of CCI rats (P < 0.05)
HCN Channel Blocker ZD7288 Inhibit ATP-Induced Currents (IATP) in DRG Neurons
Currents Evoked by ATP in DRG Neurons
Whole-cell currents were recorded from small and medium-sized (20- to 35-mm in diameter) DRG neurons using whole-cell patch-clamp recording. ATP (100 μM, applied for 2 s) elicited inward currents in 220 of 253 (87%) cells. The responses of cells to ATP exhibited diverse kinetics and could be subdivided into three types: fast, slow and mixed kinetics responses. It is well known that the responsible receptor is homomeric P2X3 (fast IATP) and heteromeric P2X2/3 (slow IATP) subtypes, and the mixed current is attributable to the presence of homomeric P2X3 and heteromeric P2X2/3 receptors in the same cell [50].
In our experiments, among the cultured DRG neurons (n = 253), approximately 26.5% cells (n = 67) displayed fast currents, 27.3% cells (n = 69) displayed slow currents, and 33.2% cells (n = 84) displayed mixed current. There are also some cells (13.0%, n = 33) that did not respond to ATP.
ZD7288 Inhibited ATP-Induced Currents in DRG Neurons
To characterize the effects of HCN channels on P2X receptor function, we recorded IATP in DRG neurons pre-incubated with ZD7288. We noticed that pre-incubation of ZD7288 (100 μM, 10 min) caused a significant decrease in the peak amplitude of three types of IATP (rapid, slow and mixed IATP). In our experiments, ZD7288 inhibited rapid, slow and mixed IATP by 65.2% (n = 23), 62.5% (n = 32), and 82.8% (n = 32), respectively. The inhibitory effect of ZD7288 on IATP in DRG neurons was dose-dependent. As shown in Fig. 4, ZD7288 inhibited ATP-induced fast currents to 82.4 ± 3.8%, 41.8 ± 1.6% and 21.3 ± 1.9% of control (2829.50 ± 158.26 pA, 1434.23 ± 66.81 pA and 731.88 ± 54.65 pA vs. 3433.54 ± 73.10 pA, respectively. P < 0.05; n = 8; Fig. 4A and D) at 0.01 M, 0.1 M and 1 M, respectively. ZD7288 inhibited ATP-induced slow currents to 86.0 ± 2.0%, 60.1 ± 2.5% and 27.1 ± 2.1% of control (2898.66 ± 160.37 pA, 2026.1 ± 77.30 pA and 914.49 ± 46.15 pA vs. 3371.36 ± 109.12 pA, respectively. P < 0.05; n = 8; Fig. 4B and E) at 0.01 M, 0.1 M and 1 M. In addition, ZD7288 inhibited ATP-induced mixed currents. The inhibition of ZD7288 on the fast and slow components of mixed IATP were similar to that of ZD7288 on rapid IATP and slow IATP, respectively. ZD7288 inhibited the fast component of mixed IATP to 55.6 ± 4.1%, 31.2 ± 5.5% and 9.8 ± 8.2% of control (880.8 ± 83.94 pA, 493.53 ± 85.07 pA and 154.87 ± 16.42 pA vs. 1583.5 ± 130.86 pA, respectively. P < 0.05; n = 9; Fig. 4C and F) at 0.01 M, 0.1 M and 1 M. ZD7288 inhibited the slow component of mixed IATP to 61.2 ± 8.9%, 31.8 ± 12.5% and 11.0 ± 11.2% of control (483.97 ± 48.23 pA, 251.48 ± 53.54 pA and 86.64 ± 11.08 pA vs. 790.21 ± 86.27 pA, respectively. P < 0.05; n = 9; Fig. 4C and G) at 0.01 M, 0.1 M and 1 M. The inhibitory effect of ZD7288 on rapid, slow and mixed IATP was reversible after 10 min washouts (Fig. 5). In conclusion, HCN channels blocker ZD7288 can significantly inhibit ATP-induced rapid, slow and mixed currents mediated by homomeric P2X3 and heteromeric P2X2/3, which means that HCN channels activation can enhance P2X2,3 receptor function in DRG neurons.
Concentration-dependent inhibitory effects of ZD7288 on the fast, slow and mixed current evoked by ATP (100 μM) in DRG neurons. The inhibitory effect of ZD7288 on ATP-evoked fast currents in DRG neurons was concentration-dependent, and fast IATP currents were inhibited to 82.4 ± 3.8%, 41.8 ± 1.6% and 21.3 ± 1.9% of control at 0.01 M, 0.1 M and 1 M, respectively (A and D). The inhibitory effect of ZD7288 on ATP-evoked slow currents in DRG neurons was concentration-dependent, and slow IATP currents were inhibited to 86.0 ± 2.0%, 60.1 ± 2.5% and 27.1 ± 2.1% of control at 0.01 M, 0.1 M and 1 M. (B and E). The inhibitory effect of ZD7288 on ATP-evoked mixed currents in DRG neurons was concentration-dependent. In addition, the inhibition of ZD7288 on the fast current and slow current components of mixed IATP was similar to that of ZD7288 on rapid IATP and slow IATP. ZD7288 inhibited the fast component to 55.6 ± 4.1%, 31.2 ± 5.5% and 9.8 ± 8.2% of control at 0.01 M, 0.1 M, and 1 M, and inhibited the slow component to 61.2 ± 8.9%, 31.8 ± 12.5% and 11.0 ± 11.2% of control at 0.01 M, 0.1 M and 1 M, respectively (C, F and G). The error bars represent the difference of the amplitude of ATP-induced currents (fast, slow and mixed IATP) in multiple DRG neurons. Responses to ATP in the presence of ZD7288 were normalized with respect to the control current in the same neuron. **P < 0.01, versus control
Effects of 100 μM ZD7288 on the amplitude of the currents evoked by ATP (100 μM) in DRG neurons. Representative traces show the inhibition by ZD7288 (100 μM, 10 min) on the amplitude of fast, slow and mixed currents. ZD7288 inhibited ATP-induced fast currents to 43.4 ± 10.3% of control. The inhibitory effect of ZD7288 on rapid IATP was reversible after 10 min washouts (n = 10, A and D). ZD7288 inhibited ATP-induced slow currents to 61.7 ± 8.6% of control. The inhibitory effect of ZD7288 on slow IATP was reversible after 10 min washouts (n = 14, B and E). Similarlly, ZD7288 inhibited the fast component of mixed currents to 44.2 ± 11.4% of control, and inhibited the slow component of mixed currents to 36.2 ± 12.7% of control (n = 13, C, F and G).*P < 0.05, compared with ATP; +P < 0.05, compared with ZD7288 + ATP
Involvement of cAMP-PKA Signaling in the Inhibitory Effect of ZD7288 on ATP-Activated Currents
Previous research shows that the intracellular carboxyl terminus of P2X receptor contains several consensus phosphorylation sites for cAMP-dependent PKA, suggesting that the function of the P2X purinoceptor could be regulated by cAMP-PKA signaling [51, 52]. The cAMP-PKA signaling is also a significant modulator of HCN channels activation. Cytoplasmic cAMP can facilitate the opening of HCN channels by affecting the structure of the C-linker and cyclic nucleotide-binding domain [48, 49]. In the present study, 8-Br-cAMP (a cell-permeable cAMP analog), an activator of PKA, significantly increased the amplitude of three types of IATP (fast, slow and mixed IATP) in DRG neurons. 8-Br-cAMP enhanced the amplitude of the fast currents evoked by ATP to 143.8 ± 5.4% of control (3244.68 ± 416.13 pA vs.2257.13 ± 305.23 pA, P < 0.05; n = 9; Fig. 6D), and enhanced the amplitude of the slow currents evoked by ATP to 142.5 ± 8.9% of control (3116.42 ± 577.13 pA vs.2186.48 ± 410.18 pA, P < 0.05; n = 9; Fig. 6E). Similarly, 8-Br-cAMP enhanced the fast component of mixed currents to 155.3 ± 8.7% of control (1657.69 ± 74.87 pA vs.1067.63 ± 87.63 pA, P < 0.05; n = 9; Fig. 6F), and enhanced the slow component of mixed currents to 166.2 ± 7.0% of control (994.03 ± 70.91 pA vs.598.2 ± 60.83 pA, P < 0.05; n = 9; Fig. 6G). In addition, HCN channels blocker ZD7288 significantly inhibited the effect of 8-Br-cAMP on ATP-induced currents. In this study, pre-incubation of ZD7288 (100 μM, 10 min) can inhibit the effect of 8-Br-cAMP on ATP induced fast, slow and mixed currents in DRG neurons. ZD7288 inhibited the fast currents to 113.5 ± 3.2% of control (2562.1 ± 338.17 pA vs.2257.13 ± 305.23 pA, P < 0.05; n = 9; Fig. 6D), and inhibited the slow currents to 116.2 ± 6.6% of control (2541.49 ± 529.80 pA vs.2186.48 ± 410.18 pA, P < 0.05; n = 9; Fig. 6E), respectively. Similarly, pre-incubation of ZD7288 inhibited the effect of 8-Br-cAMP on the fast component of mixed currents to 125.0 ± 6.8% of control (1334.37 ± 97.72 pA vs.1067.63 ± 87.63 pA, P < 0.05; n = 9; Fig. 6F), and inhibited the effect of 8-Br-cAMP on the slow component of mixed currents to 114.2 ± 5.0% of control (682.97 ± 75.61 pA vs.598.2 ± 60.83 pA, P < 0.05; n = 9; Fig. 6G), respectively. These results suggest that cAMP-PKA signaling is involved in the regulation of HCN channels to P2X receptor function.
The influence of ZD7288 on the effect of 8-Br-cAMP on enhancing the currents evoked by ATP (100 μM) in DRG neurons. 8-Br-cAMP enhanced the amplitude of the fast currents evoked by ATP to 143.8 ± 5.4% of control. Pre-incubation of ZD7288 inhibited the effect of 8-Br-cAMP on enhancing the fast currents to 113.5 ± 3.2% of control (P < 0.05; n = 9, A and D). 8-Br-cAMP enhanced the amplitude of the slow currents evoked by ATP to 142.5 ± 8.9% of control. Pre-incubation of ZD7288 inhibited the effect of 8-Br-cAMP on enhancing the slow currents to 116.2 ± 6.6% of control (P < 0.05; n = 9; B and E). Similarly, 8-Br-cAMP enhanced the fast and slow component of mixed currents to 155.3 ± 8.7% and 166.2 ± 7.0% of control, respectively. Pre-incubation of ZD7288 inhibited the effect of 8-Br-cAMP on enhancing the fast and slow component to 125.0 ± 6.8% and 114.2 ± 5.0% of control, respectively (P < 0.05, n = 9, C, F and G). *P < 0.05, compared with ATP; +P < 0.05, compared with 8-Br-cAMP + ATP (n = 9)
In addition, it should be noted that the difference in the amplitude of the same type of ATP-induced currents in different figures was caused by recording in different cells.
Discussion
HCN channels are widely distributed in the sensory neurons of the nervous system. There's a lot of evidence that HCN channels in DRG participate in the development of inflammatory [53,54,55,56] and neuropathic pain [3, 57, 58]. In the present study, intrathecal injection of ZD7288, a HCN channels specific inhibitor, produced a concentration-dependent analgesic effect on CCI rats, which also suggest that the hyperactivation of HCN channels in DRG promote CCI-induced neuropathic pain. Previous studies have shown that increased expression of HCN1 [4, 59] and HCN2 [3,4,5, 60] in DRG sensory neurons plays a key role in promoting the occurrence and development of pathological pain.
Numerous studies show that P2X1–6 receptors are widely expressed in DRG neurons [12]. Among them, P2X3 is the predominant purinergic receptor subtype in small- and medium-sized neurons (nociceptive C-fibers and Aδ-fibers) of DRG, and has been demonstrated to play an important role in mediating nociceptive information transmission [61, 62]. It has been reported that the expression of P2X2 and P2X3 receptors in DRG neurons increased in inflammatory, neuropathic and cancer pain [63, 64]. A-317491 (antagonist selective for P2X3 and P2X2/3 subunit-containing channels) can effectively relieve pathological pain [65,66,67]. In this study, intrathecal injection of ZD7288 not only produced analgesic effects, but also inhibited the expression of P2X2 and P2X3 receptors in DRG of CCI rats. These results suggest that the activation of HCN channel can promote the expression of P2X2 and P2X3 receptors, and further promote the occurrence of pathological pain after peripheral nerve injury. On the other hand, these results imply that the analgesic effect of ZD7288 is not only related to the blocking of HCN channels, but also to the indirect inhibition of P2X receptor expression in DRG.
In the present study, ATP-induced currents were recorded from small and medium-sized DRG neurons, which transmit peripheral nociceptive information to the spinal cord. Whole cell patch clamp recording showed that the responses of cells to ATP exhibited fast, slow and mixed kinetics responses. It is well known that ATP-induced fast and slow currents are mediated by homomeric P2X3 and heteromeric P2X2/3 receptors, respectively, and the mixed current is attributable to the presence of homomeric P2X3 and heteromeric P2X2/3 receptors in the same cell [50]. In our experiment, pre-incubation of ZD7288 inhibited the fast, slow and mixed currents induced by ATP in cultured DRG neurons. These results show that activation of HCN channels can promote the opening of homomeric P2X3 and heteromeric P2X2/3 receptors, and increase the excitability of primary sensory neurons.
It is reported that overactivation of cAMP-PKA signaling promotes the occurrence of pathological pain [68]. The cAMP-PKA signaling is also a significant modulator of HCN channels activation. As a positive HCN channels modulator, cAMP can facilitate the opening of HCN channels by affecting the structure of the C-linker and cyclic nucleotide-binding domain. The response of HCN subtypes to cAMP varies greatly (HCN4 > HCN2 > HCN3 > HCN1) [69]. In addition, recent studies have shown that activation of PKA can also promote the opening of HCN channels. For example, Cheng et al. [70] found that PKA inhibitor KT5720 inhibited the current mediated by HCN channels (Ih) in DRG neurons. On the other hand, recent studies have shown that HCN channels can in turn affect cAMP content and PKA activity. For example, Du et al. [41] found that local infusion of HCN channels blocker ZD7288 attenuated CCI-induced up-regulation of cAMP content in ventral-lateral periaqueductal gray. In this experiment, we also observed that intrathecal injection of ZD7288 reduced the elevation of cAMP in DRG induced by CCI operation.
On the other hand, P2X receptors have been shown to be regulated by both PKA and PKC activation, which suggests that they might be substrates for phosphorylation [71]. Chow et al. [51] report that the intracellular carboxyl terminus of P2X2 receptor contains several consensus phosphorylation sites for cAMP-dependent PKA, suggesting that the function of the P2X2 purinoceptor could be regulated by the protein phosphorylation. In addition, Wang et al. [52] found that P2X3 receptor activation induced [Ca2+]i elevation in DRG neurons was blocked by protein kinase A inhibitor H-89. In this study, 8-Br-cAMP (a cell-permeable cAMP analog), an activator of PKA, enhanced both the fast current mediated by homomeric P2X3 receptor and the slow current mediated by heteromeric P2X2/3 receptor, suggesting that the function of P2X2 and P2X3 receptors in DRG neurons are regulated by the cAMP-PKA signaling. That is, the increase of cAMP-PKA activity can promote the opening of P2X2 and P2X3 receptors in DRG neurons. Moreover, pre-incubation of ZD7288 significantly inhibited the enhancement of ATP-induced current by 8-Br-cAMP, which means that HCN channel is involved in the effect of 8-Br-cAMP. We speculate that 8-Br-cAMP first promoted the activation of HCN channel, which in turn promoted the opening of P2X2 and P2X3 receptors in DRG neurons. Meanwhile, it can be seen from Fig. 6 that incubation of ZD7288 did not completely block the enhancement of ATP-induced current by 8-Br-cAMP. We hypothesized that although blocking HCN channel could reduce the level of cAMP in DRG neurons, exogenous 8-Br-cAMP could directly activate the P2X receptor through PKA, leading to its opening. These results suggest that HCN channels can affect the activity of cAMP-PKA, and then regulate the functions of P2X2 and P2X3 receptors in DRG neurons.
How does HCN channel affect the cAMP-PKA signaling? Huang et al. [22] report that the opening of HCN channels can increase [Ca2+]i and further Ca2+/calmodulin-dependent activation of adenylate cyclase, leading to increased intracellular cAMP concentration and PKA activity. As for the reason that HCN channel activation leads to the increase of [Ca2+]i, it may be mainly due to the depolarization of the cell membrane caused by Na+ influx due to HCN channel opening. Then, membrane depolarization leads to the opening of the voltage-dependent calcium channel and the increase in [Ca2+]i [72]. For example, Felix et al. [73] found that the HCN channel antagonist ZD7288 could block the T-type Ca2+ currents in mouse spermatogenic cells and HEK-293 cells. On the other hand, in addition to being permeable to Na+ and K+, it is reported that HCN channels are also weakly permeable to Ca2+. Therefore, HCN channels activation may lead to Ca2+ influx and the increase of [Ca2+]i [74]. In this study, whether HCN channels affect cAMP-PKA activity by altering [Ca2+]i and further Ca2+/calmodulin-dependent activation of adenylate cyclase in DRG neurons remains to be further confirmed.
In summary, this study demonstrates that intrathecal administration of HCN channel antagonist ZD7288 can relieve CCI-induced neuropathic pain. In addition, ZD7288 significantly inhibits the increased expression of P2X2 and P2X3 receptors in DRG in CCI rats, which strongly suggest that HCN channels activation can promote the expression of P2X2 and P2X3 receptors in DRG after peripheral nerve injury. Furthermore, whole cell patch clamp recording shows that HCN channels antagonist ZD7288 can inhibit ATP-induced fast, slow and mixed currents and the effect of 8-Br-cAMP on enhancing ATP-induced currents in cultured DRG neurons. These results suggest that HCN channels activation may enhance the functions of P2X2 and P2X3 receptors by the following possible mechanisms: HCN channels activation enhances the cAMP-PKA activity in DRG neurons, which promotes the opening of purinergic P2X receptor, thereby promoting peripheral sensitization and the occurrence of neuropathic pain after peripheral nerve injury.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Costigan M, Scholz J, Woolf CJ (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32. https://doi.org/10.1146/annurev.neuro.051508.135531
Jansen LR, Forster LA, Smith XL, Rubaharan M, Murphy AZ, Baro DJ (2021) Changes in peripheral HCN2 channels during persistent inflammation. Channels (Austin) 15(1):165–179. https://doi.org/10.1080/19336950.2020.1870086
Smith T, Al Otaibi M, Sathish J, Djouhri L (2015) Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience 295:90–102. https://doi.org/10.1016/j.neuroscience.2015.03.041
Dini L, Del Lungo M, Resta F, Melchiorre M, Spinelli V, Di Cesare Mannelli L, Ghelardini C, Laurino A, Sartiani L, Coppini R, Mannaioni G, Cerbai E, Romanelli MN (2018) Selective blockade of HCN1/HCN2 channels as a potential pharmacological strategy against pain. Front Pharmacol 9:1252. https://doi.org/10.3389/fphar.2018.01252
Weng X, Smith T, Sathish J, Djouhri L (2012) Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors. Pain 153(4):900–914. https://doi.org/10.1016/j.pain.2012.01.019
Djouhri L, Al Otaibi M, Kahlat K, Smith T, Sathish J, Weng X (2015) Persistent hindlimb inflammation induces changes in activation properties of hyperpolarization-activated current (Ih) in rat C-fiber nociceptors in vivo. Neuroscience 301:121–33. https://doi.org/10.1016/j.neuroscience.2015.05.074
Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA (2011) HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 333(6048):1462–1466. https://doi.org/10.1126/science.1206243
Yang YC, Meng QT, Pan X, Xia ZY, Chen XD (2014) Dexmedetomidine produced analgesic effect via inhibition of HCN currents. Eur J Pharmacol 740:560–564. https://doi.org/10.1016/j.ejphar.2014.06.031
Tsantoulas C, Laínez S, Wong S, Mehta I, Vilar B, McNaughton PA (2017) Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med 9(409):6072. https://doi.org/10.1126/scitranslmed.aam6072
Momin A, Cadiou H, Mason A, McNaughton PA (2008) Role of the hyperpolarization-activated current Ih in somatosensory neurons. J Physiol 586(24):5911–29. https://doi.org/10.1113/jphysiol.2008.163154
Liu X, Zeng J, Zhao Y, Xiao Z, Fang C, Ruan H (2010) Inhibition of ATP-induced Ca2+ influx by corticosterone in dorsal root ganglion neurons. Neurochem Res 35(5):804–10. https://doi.org/10.1007/s11064-010-0138-y
Chen L, Liu YW, Yue K, Ru Q, Xiong Q, Ma BM, Tian X, Li CY (2016) Differential expression of ATP-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signal 12(1):79–87. https://doi.org/10.1007/s11302-015-9481-4
Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, Faltynek CR, Jarvis MF, Lynch K (2002) Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain 99(1–2):11–9. https://doi.org/10.1016/s0304-3959(02)00032-5
Gao Y, Liu H, Deng L, Zhu G, Xu C, Li G, Liu S, Xie J, Liu J, Kong F, Wu R, Li G, Liang S (2011) Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull 84(6):406–13. https://doi.org/10.1016/j.brainresbull.2011.01.017
Ueno S, Tsuda M, Iwanaga T, Inoue K (1999) Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 126(2):429–36. https://doi.org/10.1038/sj.bjp.0702319
Chen Y, Li GW, Wang C, Gu Y, Huang LM (2005) Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 119(1–3):38–48. https://doi.org/10.1016/j.pain.2005.09.007
Wang S, Xu H, Zou L, Xie J, Wu H, Wu B, Yi Z, Lv Q, Zhang X, Ying M, Liu S, Li G, Gao Y, Xu C, Zhang C, Xue Y, Liang S (2016) LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal 12(1):139–48. https://doi.org/10.1007/s11302-015-9488-x
Shinoda M, La JH, Bielefeldt K, Gebhart GF (2010) Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J Neurophysiol 104(6):3113–23. https://doi.org/10.1152/jn.00560.2010
Xu GY, Huang LY (2002) Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 22(1):93–102. https://doi.org/10.1523/JNEUROSCI.22-01-00093.2002
Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567(Pt 2):621–39. https://doi.org/10.1113/jphysiol.2005.088435
Wu X, Liao L, Liu X, Luo F, Yang T, Li C (2012) Is ZD7288 a selective blocker of hyperpolarization-activated cyclic nucleotide-gated channel currents? Channels (Austin) 6(6):438–42. https://doi.org/10.4161/chan.22209
Huang CC, Hsu KS (2003) Reexamination of the role of hyperpolarization-activated cation channels in short- and long-term plasticity at hippocampal mossy fiber synapses. Neuropharmacology 44(7):968–81. https://doi.org/10.1016/s0028-3908(03)00098-4
Chen C (2004) ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur J Neurosci 19(3):643–9. https://doi.org/10.1111/j.0953-816x.2003.03174.x
Zhang XX, Min XC, Xu XL, Zheng M, Guo LJ (2016) ZD7288, a selective hyperpolarization-activated cyclic nucleotide-gated channel blocker, inhibits hippocampal synaptic plasticity. Neural Regen Res 11(5):779–86. https://doi.org/10.4103/1673-5374.182705
Huang W, Xiu Y, Yan JA, He WJ, Zhao YD, Hu ZA, Ruan HZ (2010) Facilitation of Ih channels by P2Y1 receptors activation in Mesencephalic trigeminal neurons. Neurosci Lett 482(2):156–9. https://doi.org/10.1016/j.neulet.2010.07.023
Khakh BS, Henderson G (1998) Hyperpolarization-activated cationic currents (Ih) in neurones of the trigeminal mesencephalic nucleus of the rat. J Physiol 510(Pt 3):695–704. https://doi.org/10.1111/j.1469-7793.1998.00695.x
Wang C, Li GW, Huang LY (2007) Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain 3:22. https://doi.org/10.1186/1744-8069-3-22
Zhao J, Gao B, Zhang Y, Zheng B, Liu H, Cao JL (2014) Effects of intrathecal opioids combined with low-dose naloxone on motilin and its receptor in a rat model of postoperative pain. Life Sci 103(2):88–94. https://doi.org/10.1016/j.lfs.2014.03.032
Niu J, Huang D, Zhou R, Yue M, Xu T, Yang J, He L, Tian H, Liu X, Zeng J (2017) Activation of dorsal horn cannabinoid CB2 receptor suppresses the expression of P2Y12 and P2Y13 receptors in neuropathic pain rats. J Neuroinflammation 14(1):185. https://doi.org/10.1186/s12974-017-0960-0
Størkson RV, Kjørsvik A, Tjølsen A, Hole K (1996) Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 65(2):167–72. https://doi.org/10.1016/0165-0270(95)00164-6
Huang D, Yang J, Liu X, He L, Luo X, Tian H, Xu T, Zeng J (2018) P2Y6 receptor activation is involved in the development of neuropathic pain induced by chronic constriction injury of the sciatic nerve in rats. J Clin Neurosci 56:156–162. https://doi.org/10.1016/j.jocn.2018.07.013
Fu M, Meng L, Ren H, Luo F (2019) Pulsed radiofrequency inhibits expression of P2X3 receptors and alleviates neuropathic pain induced by chronic constriction injury in rats. Chin Med J (Engl) 132(14):1706–1712. https://doi.org/10.1097/CM9.0000000000000302
Yang Y, Xia Z, Meng Q, Liu K, Xiao Y, Shi L (2018) Dexmedetomidine relieves neuropathic pain by inhibiting hyperpolarization-activated cyclic nucleotide-gated currents in dorsal root ganglia neurons. Neuroreport 29(12):1001–1006. https://doi.org/10.1097/WNR.0000000000001068
Zhou R, Xu T, Liu X, Chen Y, Kong D, Tian H, Yue M, Huang D, Zeng J (2018) Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain. J Pain Res 11:615–628. https://doi.org/10.2147/JPR.S154437
Gao SH, Shen LL, Wen HZ, Zhao YD, Ruan HZ (2017) Inhibition of metabotropic glutamate receptor subtype 1 alters the excitability of the commissural pyramidal neuron in the rat anterior cingulate cortex after chronic constriction injury to the sciatic nerve. Anesthesiology 127(3):515–533. https://doi.org/10.1097/ALN.0000000000001654
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Tao J, Liu L, Fan Y, Wang M, Li L, Zou L, Yuan H, Shi L, Yang R, Liang S, Liu S (2019) Role of hesperidin in P2X3 receptor-mediated neuropathic pain in the dorsal root ganglia. Int J Neurosci 129(8):784–793. https://doi.org/10.1080/00207454.2019.1567512
Zheng XB, Zhang YL, Li Q, Liu YG, Wang XD, Yang BL, Zhu GC, Zhou CF, Gao Y, Liu ZX (2019) Effects of 1,8-cineole on neuropathic pain mediated by P2X2 receptor in the spinal cord dorsal horn. Sci Rep 9(1):7909. https://doi.org/10.1038/s41598-019-44282-4
Zou L, Yu K, Fan Y, Cao S, Liu S, Shi L, Li L, Yuan H, Yang R, Yi Z, Gao Y, Li G, Greffrath W, Treede RD, Li M, Xu H, Zhang C, Liang S (2019) The Inhibition by Guanfu Base A of Neuropathic Pain Mediated by P2Y12 Receptor in Dorsal Root Ganglia. ACS Chem Neurosci 10(3):1318–1325. https://doi.org/10.1021/acschemneuro.8b00399
Yi Z, Rao S, Ouyang S, Bai Y, Yang J, Ma Y, Han X, Wu B, Zou L, Jia T, Zhao S, Hu X, Lei Q, Gao Y, Liu S, Xu H, Zhang C, Liang S, Li G (2017) A317491 relieved HIV gp120-associated neuropathic pain involved in P2X3 receptor in dorsal root ganglia. Brain Res Bull 130:81–89. https://doi.org/10.1016/j.brainresbull.2017.01.002
Du L, Wang SJ, Cui J, He WJ, Ruan HZ (2013) The role of HCN channels within the periaqueductal gray in neuropathic pain. Brain Res 1500:36–44. https://doi.org/10.1016/j.brainres.2013.01.035
Liu XH, Zeng JW, Zhao YD, Chen PH, Xiao Z, Ruan HZ (2008) Rapid inhibition of ATP-induced currents by corticosterone in rat dorsal root ganglion neurons. Pharmacology 82(2):164–70. https://doi.org/10.1159/000149582
Gerevich Z, Müller C, Illes P (2005) Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol 521(1–3):34–8. https://doi.org/10.1016/j.ejphar.2005.08.001
Ma Y, Chen J, Yu D, Wei B, Jin H, Zeng J, Liu X (2021) cAMP-PKA signaling is involved in regulation of spinal HCN channels function in diabetic neuropathic pain. Neurosci Lett 750:135763. https://doi.org/10.1016/j.neulet.2021.135763
Li ZH, Cui D, Qiu CJ, Song XJ (2019) Cyclic nucleotide signaling in sensory neuron hyperexcitability and chronic pain after nerve injury. Neurobiol Pain 6:100028. https://doi.org/10.1016/j.ynpai.2019.100028
Tao T, Wei MY, Guo XW, Zhang J, Yang LY, Zheng H (2019) Modulating cAMP responsive element binding protein 1 attenuates functional and behavioural deficits in rat model of neuropathic pain. Eur Rev Med Pharmacol Sci 23(6):2602–2611. https://doi.org/10.26355/eurrev_201903_17410.
Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK, Song XJ (2012) Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2. Pain 153(7):1426–1437. https://doi.org/10.1016/j.pain.2012.03.025
Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425(6954):200–5. https://doi.org/10.1038/nature01922
Akimoto M, Zhang Z, Boulton S, Selvaratnam R, VanSchouwen B, Gloyd M, Accili EA, Lange OF, Melacini G (2014) A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP. J Biol Chem 289(32):22205–20. https://doi.org/10.1074/jbc.M114.572164
Shcherbatko A, Foletti D, Poulsen K, Strop P, Zhu G, Hasa-Moreno A, Melton Witt J, Loo C, Krimm S, Pios A, Yu J, Brown C, Lee JK, Stroud R, Rajpal A, Shelton D (2016) Modulation of P2X3 and P2X2/3 receptors by monoclonal antibodies. J Biol Chem 291(23):12254–70. https://doi.org/10.1074/jbc.M116.722330
Chow YW, Wang HL (1998) Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem 70(6):2606–12. https://doi.org/10.1046/j.1471-4159.1998.70062606.x
Wang S, Zhu HY, Jin Y, Zhou Y, Hu S, Liu T, Jiang X, Xu GY (2015) Adrenergic signaling mediates mechanical hyperalgesia through activation of P2X3 receptors in primary sensory neurons of rats with chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 308(8):G710-9. https://doi.org/10.1152/ajpgi.00395.2014
Schnorr S, Eberhardt M, Kistner K, Rajab H, Käer J, Hess A, Reeh P, Ludwig A, Herrmann S (2014) HCN2 channels account for mechanical (but not heat) hyperalgesia during long-standing inflammation. Pain 155(6):1079–1090. https://doi.org/10.1016/j.pain.2014.02.006
Sun W, Yang F, Wang Y, Fu H, Yang Y, Li CL, Wang XL, Lin Q, Chen J (2017) Contribution of large-sized primary sensory neuronal sensitization to mechanical allodynia by upregulation of hyperpolarization-activated cyclic nucleotide gated channels via cyclooxygenase 1 cascade. Neuropharmacology 113(Pt A):217–230. https://doi.org/10.1016/j.neuropharm.2016.10.012
Acosta C, McMullan S, Djouhri L, Gao L, Watkins R, Berry C, Dempsey K, Lawson SN (2012) HCN1 and HCN2 in Rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression. PLoS One 7(12):e50442. https://doi.org/10.1371/journal.pone.0050442
Forster LA, Jansen LR, Rubaharan M, Murphy AZ, Baro DJ (2020) Alterations in SUMOylation of the hyperpolarization-activated cyclic nucleotide-gated ion channel 2 during persistent inflammation. Eur J Pain 24(8):1517–1536. https://doi.org/10.1002/ejp.1606
Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y (2008) Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain 137(3):495–506. https://doi.org/10.1016/j.pain.2007.10.011
Liu Y, Feng Y, Zhang T (2015) Pulsed radiofrequency treatment enhances dorsal root ganglion expression of hyperpolarization-activated cyclic nucleotide-gated channels in a rat model of neuropathic pain. J Mol Neurosci 57(1):97–105. https://doi.org/10.1007/s12031-015-0582-x
Liu DL, Lu N, Han WJ, Chen RG, Cong R, Xie RG, Zhang YF, Kong WW, Hu SJ, Luo C (2015) Upregulation of Ih expressed in IB4-negative Aδ nociceptive DRG neurons contributes to mechanical hypersensitivity associated with cervical radiculopathic pain. Sci Rep 5:16713. https://doi.org/10.1038/srep16713
Richards N, Dilley A (2015) Contribution of hyperpolarization-activated channels to heat hypersensitivity and ongoing activity in the neuritis model. Neuroscience 284:87–98. https://doi.org/10.1016/j.neuroscience.2014.08.058
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304. https://doi.org/10.1016/S0074-7696(04)40002-3
Kobayashi K, Yamanaka H, Noguchi K (2013) Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int 88(1):10–6. https://doi.org/10.1007/s12565-012-0163-9
Sharp CJ, Reeve AJ, Collins SD, Martindale JC, Summerfield SG, Sargent BS, Bate ST, Chessell IP (2006) Investigation into the role of P2X(3)/P2X(2/3) receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. Br J Pharmacol 148(6):845–52. https://doi.org/10.1038/sj.bjp.0706790
Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB (2010) Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 133(9):2549–64. https://doi.org/10.1093/brain/awq194
Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C (2002) A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A 99(26):17179–84. https://doi.org/10.1073/pnas.252537299
McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF (2003) Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol 140(8):1381–8. https://doi.org/10.1038/sj.bjp.0705574
Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, Ilyin VI (2004) A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eur J Pharmacol 504(1–2):45–53. https://doi.org/10.1016/j.ejphar.2004.09.056
Rahn EJ, Guzman-Karlsson MC, David Sweatt J (2013) Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory. Neurobiol Learn Mem 105:133–50. https://doi.org/10.1016/j.nlm.2013.06.008
Alvarez-Baron CP, Klenchin VA, Chanda B (2018) Minimal molecular determinants of isoform-specific differences in efficacy in the HCN channel family. J Gen Physiol 150(8):1203–1213. https://doi.org/10.1085/jgp.201812031
Cheng Q, Zhou Y (2013) Novel role of KT5720 on regulating hyperpolarization-activated cyclic nucleotide-gated channel activity and dorsal root ganglion neuron excitability. DNA Cell Biol 32(6):320–8. https://doi.org/10.1089/dna.2013.2021
Brown DA (1803) Yule DI (2010) Protein kinase A regulation of P2X(4) receptors: requirement for a specific motif in the C-terminus. Biochim Biophys Acta 2:275–87. https://doi.org/10.1016/j.bbamcr.2009.12.002
Siegelbaum SA (2000) Presynaptic facilitation by hyperpolarization-activated pacemaker channels. Nat Neurosci 3(2):101–2. https://doi.org/10.1038/72038
Felix R, Sandoval A, Sánchez D, Gómora JC, De la Vega-Beltrán JL, Treviño CL, Darszon A (2003) ZD7288 inhibits low-threshold Ca(2+) channel activity and regulates sperm function. Biochem Biophys Res Commun 311(1):187–92. https://doi.org/10.1016/j.bbrc.2003.09.197
Michels G, Brandt MC, Zagidullin N, Khan IF, Larbig R, van Aaken S, Wippermann J, Hoppe UC (2008) Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc Res 78(3):466–75. https://doi.org/10.1093/cvr/cvn032
Funding
This study was supported by the National Natural Science Foundation of China (No. 81960161) and the fund of Zunyi Medical University Master's research start (F-978) and the fund of Zunyi Science and Technology (2019) 29.
Author information
Authors and Affiliations
Contributions
Conception and design: XL, YY. Data acquisition, data analysis, and interpretation: XL, XL. Drafting the article or critically revising it for important intellectual content: XL, JZ. Final approval of the version to be published: All authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest in this study.
Ethical Approval
The protocol was prepared from SD rats in accordance with the National Institutes of Health guide-lines in a manner that minimized animal suffering and animal numbers. All experiments were carried out in accordance with China animal welfare legislation and were approved by the Zunyi Medical University Committee on Ethics in the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, X., Zeng, J., Yan, Y. et al. Blockage of HCN Channels Inhibits the Function of P2X Receptors in Rat Dorsal Root Ganglion Neurons. Neurochem Res 47, 1083–1096 (2022). https://doi.org/10.1007/s11064-021-03509-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03509-5