Abstract
Further understanding the mechanism for microglia activation is necessary for developing novel anti-inflammatory strategies. Our previous study found that the activation of sigma-1 receptor can effectively inhibit the neuroinflammation, independent of the canonical mechanisms, such as NF-κB, JNK and ERK inflammatory pathways. Thus, it is reasonable that an un-identified, non-canonical pathway contributes to the activation of microglia. In the present study, we found that a sigma-1 receptor agonist of 2-morpholin-4-ylethyl 1-phenylcyclohexane-1-carboxylate (PRE-084) suppressed lipopolysaccharide (LPS) elevated nitric oxide (NO) content in BV-2 microglia culture supernatant and LPS-raised mRNA levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS) in BV-2 microglia. Moreover, PRE-084 alleviated LPS-increased Ser 9 de-phosphorylation of glycogen synthase kinase-3 beta (GSK-3β), LPS-elevated catalytic activity of calcineurin, and LPS-raised percent and frequency of Ca2+ oscillatory BV-2 cells. We further found that the inhibitory effect of PRE-084 was reversed by a calcineurin activator of chlorogenic acid and a GSK-3β activator of pyrvinium. Moreover, an IP3 receptor inhibitor of 2-aminoethoxydiphenyl borate mimicked the anti-inflammatory activity of PRE-084. Thus, we identified a noncanonical pro-neuroinflammary pathway of Ca2+ oscillation/Calcineurin/GSK-3β and the inhibition of this pathway is necessary for the anti-inflammatory activity of sigma-1 receptor activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation is a common feature of various neurological diseases, such as ischemic stroke, epilepsy, Alzheimer’s disease and Parkinson’s disease [1,2,3]. Excessive activated microglia is the main initiator that elicits the reaction of neuroinflammation via producing pro-inflammatory mediators that cause tissue damage (e.g. TNF-α, IL-1β, iNOS) [4]. Inhibiting the excessive activation of microglia is a main strategy for alleviating neuroinflammation.
Sigma-1 receptor (S1R) is a dual acting protein of both receptor and chaperone. The resting S1R mainly resides at the interface between endoplasmic reticulum and mitochondria [5, 6]. When activated by exogenic and endogenic agonists, such as PRE-084 and (+)-pentazocine, it translocates into other organelles and interacts with diverse kinds of protein (e.g. G-protein coupled receptors, ion channels) and modulates their function in an allosteric manner as a kind of chaperone [6]. It has been reported that the activation of S1R attenuates the process of neuroinflammation [7]. For instance, the selective S1R agonist of PRE-084 reduces nitrosative and oxidative stress and lessens microglia activation in the mice endured traumatic brain injury [8]. We also reported that a S1R allosteric modulator, SKF83959, reduced the expression of TNF-α, IL-1β and iNOS, and suppressed the release of nitric oxide (NO) and the generation of reactive oxygen species in BV-2 microglial cells [9].
Despite of these findings, the exact mechanism for anti-neuroinflammatory action of S1R agonists is still obscure. Zhao and coworkers proposed that inhibition of MAPK/ERK pathway may be involved in the anti-inflammatory action of (+)-pentazocine in in the lipopolysaccharide (LPS)-activated retinal microglia [10]. But, Rosen and coworkers found that the deficiency of S1R selectively enhance activity of IRE1, without influencing the NF-κB, JNK and ERK inflammatory pathways [11]. Our previous study also reported that the ani-neuroinflammatory effect of SKF83959 didn’t depend on the MAPK/ERK and IKK/IκB pathways [9]. Thus, the exact molecular mechanism needs further investigation.
It has reported that glycogen synthase kinase-3 beta (GSK-3β) modulates inflammatory cytokine production via regulating the transcription of inflammatory cytokines [12]. For instance, dexmedetomidine inhibits the GSK-3β/Nrf2 pathways and ameliorates LPS-induced acute kidney injury in rats [13]. Our published study reported that the S1R agonists inhibited the activity of GSK-3β in astrocytes [14]. Thus, we proposed that the anti-inflammatory action of S1R agonists may be mediated by the inhibition of GSK-3β activity.
Recent studies reveal that the activity of GSK-3β is modulated by calcineurin in neuroblast cells and T-cell acute lymphoblastic leukemia cells [15, 16]. Calcineurin is a well-known signal molecule in the activating process of immune cells. When directly interact with GSK-3β, calcineurin leads to the Ser 9 dephosphorylation and consequently increases GSK-3β kinase activity. The predominant activator of calcineurin is the intracellular Ca2+. Coordinating with calcineurin, Ca2+ binds with the subunit B of calcineurin, leading to activation of calcineurin and subsequent dephosphorylation of target proteins. Ca2+ oscillation is a process of intracellular Ca2+ periodic fluctuation. As a highly efficient signal, Ca2+ oscillation can lead to the activation of calcineurin and subsequent dephosphorylation of target proteins, while avoiding intracellular Ca2+ overload. Meanwhile, the high affinity and slow interaction between calcineurin and Ca2+ allow calcineurin to respond gradually and consistently to the fast changing intracellular Ca2+. In present study, we identified a noncanonical pro-neuroinflammatory pathway of Ca2+ oscillation/calcineurin/GSK-3β and the inhibition of this noncanonical pathway contributes to the anti-neuroinflammatory effect of S1R activation.
Materials and Methods
Drugs
LPS and PRE-084 was purchased from Sigma-Aldrich Co. (St.Louis, MO, USA) directly dissolved in PBS. Chlorogenic acid (CGA), an activator of calcineurin, was purchased from Aladdin Biochemical Technology (Shanghai, China), and was dissolved in water. 2-aminoethoxydiphenyl (2-APB), an antagonist of IP3R, was purchased from GlpBio Technology (Montclair, CA, USA). Tacrolimus (FK-506), an inhibitor of calcineurin was purchased from ChemeGen (Los Angeles, CA, USA). 2-APB and FK-506 were initially dissolved in dimethyl sulfoxide (DMSO) for stock solutions and diluted with cell medium (DMSO ≤ 0.2%). Fluo-4 AM and Hanks’ balanced salt solution were purchased from Beyotime Biotechnology (Shanghai, China). 1 mM HEPES solution (PH 7.2) was purchased from Coolaber Science & Technology (Beijing, China). Anti-TNF-α and anti-iNOS antibodies were purchased from Proteintech Campany (Cat. No. 17590-1-AP, 18985-1-AP, Wuhan, Hubei Province, China). Anti-IL-1β antibodies were purchase from Bioworld Company (Cat. No. BS6067, Nanjing, Jiangsu Province, China). Antibodies against phospho-GSK-3β (Ser9) (Cat. No.9323T), total GSK-3β (Cat. No. 12456T), were obtained from Cell Signaling Technology Corporation (Danvers, MA, USA). Antibodies against phospho-AKT (Cat. No. ab192623) and total AKT (Cat. No. ab179463) were purchased from Abcam (Cambridge, MA, USA).
Cell Culture
The murine microglial BV-2 cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum and penicillin/streptomycin (100 units and 100 μg/ml). Cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C.
The procedure of primary microglia was the same as that in our published paper [17]. Mixed cells were shaken at 220 rpm for 1 h. After the primary microglia was collected and plated for 24 h, they were used for the imaging of intracellular calcium.
NO Measurement
The level of NO was determined by measuring the amount of nitrite released into BV-2 cells culture supernatants using the Griess reagents (Beyotime Biotechnology, Shanghai, China). 50 µl of cell culture supernatant was collected to mix with an equal volume of Griess reagent I and Griess reagent II into a new 96-well plate. Then, the absorbance was read at 540 nm on a Microplate Reader (Multiskan MK3, Thermo Scientific, USA). The NO concentration was calculated based on the NO standard curve, which was obtained from a gradient of concentration of sodium nitrite.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (Q-PCR)
Total RNA was extracted from BV-2 cells using Trizol reagent (Invitrogen, San Diego, CA), and further purified by phenol/chloroform extraction method. cDNA was reversely transcribed from 1 μg total RNA using the PrimeScript RT Reagent Kit (TakaRa, Japan). Real-time quantitative PCR was performed using TB Green premix Ex Taq II (TakaRa, Japan) according to the manufacturer's instructions in a LightCycler 480 system (Roche Applied Science, Mannheim, Germany). For relative comparison of each gene, real-time quantitative data were evaluated using the equation 2–△Ct test/2–△Ct control. The primer sequences are showed as follows:
-
GAPDH, forward 5′-TGTGTCCGTCGTGGATCTGA-3′, reverse 5′-TTGCTGTTGAAGTC GCAGGAG-3′;
-
iNOS, 5′-TAGGCAGAGATTGGAGGCCTTG-3′, reverse, 5′-GGGTTGTTGCTGAACTTCCAGTC-3′;
-
TNF-α, forward 5′-CAGG AGGGAGAACAGAAACTCCA-3′, reverse, 5′-CCTGGTTGGCTGCTTGCTT-3′;
-
IL-1β, forward 5′-TCCAGGATGAGGACATGAGCAC-3′, reverse, 5′-GAACGTCACACACCAGCAGGTTA-3′;
-
IL-10, forward, 5′-GCTCTTACTGACTGGCATGAG-3′, reverse, 5′-CGCAGCTCTAGGAGCATGTG-3′;
-
TGF-β, forward 5′-TCCTTGCCCTCTACAACCAACACA-3′, reverse, 5′-TTGCAGGAGCGCACAATCATGT-3′.
Western Blotting
BV-2 cells were collected and lysed using RIPA buffer (Beyotime Biotechnology, Shanghai, China), phenylmethylsulfonylfluoride (PMSF) (KeyGEN, Nanjing, China) and an inhibitor of protease phosphatase (KeyGEN, Nanjing, China) at 4 °C for at least 30 min and then centrifuged at 4 °C at 12000 g for 15 min. The supernatant was collected for western blotting. The supernatant protein concentration was measured using the BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). After that, the supernatant with loading buffer were denatured by heating at 100 °C for 10 min. The protein samples were separated by sodium dodecyl sulfate/poly-acrylamide gel electrophoresis (10%), and transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were blocked using 5% skim milk for 1 h at room temperature, then, incubated overnight at 4 °C with primary antibodies including anti-TNF-α (1:1000), anti-IL-1β (1:1000), anti-iNOS (1:1000), anti-GSK-3β (1:1000), anti-p-GSK-3β (1:800), anti-AKT (1:1000) and anti-p-AKT (1:1000). Then, the membranes were incubated with the corresponding secondary antibodies (1:10000; Odyssey, USA) and visualized with Odyssey Infrared Imaging System (Odyssey Sa, USA). The signal densities were analyzed by Image J software (National Institutes of Health). Each experiment was replicated at least three times.
Ca2+ Imaging
The intracellular calcium oscillation of microglia was assessed using a sensitive Ca2+ indicator Fluo-4 AM. Briefly, BV-2 cells were seeded in a 12 well culture plate at a density of 3 × 105 per well. After 12 h, cells were loaded with 2 μM Fluo-4 AM in Hank’s balanced salt solution (138 mM NaCl, 4.2 mM NaHCO3, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 5.3 mM KCl, 0.4 mM MgSO4, 1.26 mM CaCl2). Then, they were washed with Hank’s solution 3 times and maintained in Ca2+-free HEPES solution (145 mM NaCl, 4.5 mM KCl, 10 mM Glucose, and 5 mM HEPES, pH 7.0–7.2), and put on the stage of a fluorescence microscope (Olympus Corporation, Tokyo, Japan). Fluo-4 AM-incubated cells were excited by a laser source (488 nm) and the emission fluorescence signal (520 nm) was collected by a long-term exposure charge coupled device camera. Images were taken every 1 s and each cell was observed for 3 min. The fluorescence intensity of cells was quantitatively analyzed and the intensity-time curves were obtained by Fiji ImageJ (National Institutes of Health, Version 2). The percent of oscillating cells and the frequency of Ca2+ oscillation was calculated using the intensity-time curves.
Measurement of Intracellular Ca2+
BV-2 cells were loaded with Fluo-4 AM (5 μM) 30 min at 37 °C in the dark in Hanks’ balanced salt solution, subsequently washed twice with Hanks’ balanced salt solution. After incubation in Ca2+-free HEPE’S buffer solution (145 mM NaCl, 4.5 mM KCl, 10 mM Glucose, 5 mM HEPES, pH 7.2) 30 min at 37 °C in the dark, fluorescence signals were obtained with excitation at 488 and emission at 520 nm for 30 min on a Microplate Reader.
The test of store-operated Ca2+ entry (SOCE) was referred to the published study [18]. The basal intracellular Ca2+ fluorescence was obtained once per 30 s for 6 min. Then, LPS (1000 ng), PRE-084 (1 μM) and thapsigargin (1 μM) were added. 10 min later, 2.0 mM CaCl2 was added into the cells. The intracellular Ca2+ concentration was calculated using the following equation: \(\left[{\mathrm{Ca}}^{2+}\right]\mathrm{i}={K}_{d}\frac{\left(F-{F}_{min}\right)}{{F}_{max}-F}\), where kd = 345 nM. F was the measured fluorescence, and Fmax was determined after addition of 0.1% Triton X-100 in HBS with 10 mM Ca2+, and Fmin was determined when the cells were incubated with HBS 10 mM EGTA. Amplitudes of SOCE (Δ[Ca2+]i) were defined as the difference between the peak [Ca2+]i after the addition of CaCl2 and the trough [Ca2+]i. before the addition of extracellular CaCl2.
The Activity Test of Calcineurin
The calcineurin activity in BV-2 cells was assayed using a colorimetric assay kit according to the manufacturer’s instruction (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In this assay, nitrophenylphosphate was used as the substance of calcineurin, releasing the free phosphate when being catalyzed by calcineurin. The free phosphate release was detected by the classic malachite green assay, in which free phosphate formed the green complex with Malachite Green, molybdate, and the absorbance measured at 620 nm on a plate reader. The calcineurin activity was defined in the term of the amount of free phosphate released by calcineurin in 1 mg protein per hour.
Statistical Analysis
Ca2+ oscillation frequencies were expressed as medians, with interquartile ranges. Other data were expressed as mean ± SEM. Ca2+ oscillation frequencies were evaluated for difference using Kruskal–Wallis test, followed by Dunnett's multiple-comparison test. The difference of number of oscillating cells between groups was tested using x2 test. Other data were assessed for difference using one-way ANOVA plus Dunnett’s multiple-comparison. In some experiments (specifically marked in Results section), the two-way ANOVA followed by Bonferroni’s post hoc test were used. Dose–response cures were fitted using the following equation: \(Response=Bottom +\frac{Top-Bottom}{1+\left(\frac{Dose}{IC50}\right)}\). p < 0.05 was considered statistically significant. Data were analyzed using the GraphPad Prism software Version 7.0 (GraphPad Software, Inc. La Jolla, CA, USA).
Results
Activation of S1R Suppresses M1 Microglia Polarization and LPS-Induced Neuroinflammation
First of all, we tested the effect of PRE-084 on the viability of BV-2 cells, and found that no significant cytotoxic activity in the range of 0.01–10 μM (Supplemental Material. 1a). Thus, in the following experiments, the concentrations of PRE-084 were selected in this tested range. The level of NO in culture supernatant was higher than in the LPS-stimulated group than in control group (Fig. 1a F(4, 10) = 56.77, p < 0.0001). The mRNA levels of TNF-α, IL-1β and iNOS displayed the similar tendency (Fig. 1c–e F(4, 10) = 78.34, F(4, 10) = 21.33, F(4, 10) = 24.15, respectively; p < 0.0001 for each cytokine). By contrast, the levels of NO and the mRNA of TNF-α, IL-1β and iNOS were significantly lower than in the PRE-084 1 μM + LPS treated groups than the single LPS treated group (Fig. 1a, c–e p < 0.05 for every comparison). Moreover, the value of IC50 was 0.28 μM (95% Confidential interval: 0.08–1.36 μM) for the inhibition of PRE-084 on NO levels in culture supernatant in dose-dependent manner (Fig. 1b). Then, we examined the protein levels of TNF-α, IL-1β and iNOS in the above group. The protein levels of TNF-α, IL-1β and iNOS in the LPS-treated group were higher than the control group, and PRE-084 decreased the levels of TNF-α, IL-1β and iNOS Fig. 1f–i. F(4, 15) = 5.557, F (4, 15) = 5.436, F (4, 10) = 12.90, respectively; p < 0.01 for each cytokine]. But for the mRNA expression of anti-inflammatory cytokines of TGF-β and IL-10, no significant differences were found between all of the PRE-084 + LPS treated groups and the single LPS treated group (Supplementary Material 1b, c. p > 0.05). This data demonstrated that PRE-084 suppresses M1 microglia polarization without changing the tendency of M2 polarization.
Effect of PRE-084 on proinflammatory cytokine and anti-inflammatory cytokines from BV-2 microglia induced by lipopolysaccharide (LPS). a Nitric oxide (NO) levels in culture supernatants 24 h after the stimulation of LPS. b The dose–response curve for NO. c–e Proinflammatory cytokines mRNA level in LPS-activated BV-2 cells (c TNF-α, d IL-1β, e iNOS mRNA). f, g Anti-inflammatory mRNA levels of IL-10 and TGF-β in activated BV-2 microglia (f IL-10; g TGF-β). f The presentative WB images of TNF-α, IL-1β and iNOS. g–i The statistical graph of TNF-α pretein (g), IL-1β (h) and iNOS (i). BV-2 microglia were pretreated with 0.01–10 μM PRE-084 for 30 min, and then treated with LPS (200 ng/mL). NO levels in culture supernatants were measured using the Griess reaction. The mRNA expressing levels of TNF-α, IL-1β, iNOs were quantified by Q-PCR. The protein level was examined using western blot. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests. *p < 0.05, compared with the control group (without LPS and PRE-084 treatment); #p < 0.05, compared with the single LPS-treated group n = 3 independent experiments
Next, we tested if the anti-inflammatory activity of PRE-084 depended on the activation of S1R. When BV-2 microglia were pretreated with a sigma-1 receptor antagonist of BD1047 (10 μM), the mRNA levels of TNF-α, IL-1β and iNOS were significantly higher than those in the single PRE-084 treated group (Fig. 2a–c, F(1, 8) = 40.02, p < 0.001, F (1, 8) = 17.74, p < 0.01, F (1, 8) = 20.84, p < 0.01). The protein levels of TNF-α, IL-1β, and iNOS showed the similar tendency (Fig. 2d–f, F(1, 8) = 14.87, p < 0.05, F (1, 8) = 5.25, p < 0.05, F (1, 8) = 6.35, p < 0.05). These results indicated that the activation of S1R is involved in the anti-inflammatory action of PRE-084 in BV-2 microglia.
Effect of BD1047 on the anti-inflammatory activity of RPE-084. a TNF-α mRNA level in LPS-activated BV-2 microglia; b IL-β mRNA level; c iNOS mRNA level. d The presentative WB images of TNF-α, IL-1β and iNOS. e–g The statistical graph of TNF-α pretein (e), IL-1β (f) and iNOS (g). BV-2 microglia were pretreated with 1 μM PRE-084, 10 μM BD1047 or the combination of 1 μM PRE-084 and 10 μM BD1047 for 30 min, and then activated by 200 ng/ml LPS. The mRNA expressing levels of TNF-α, IL-1β and iNOS were quantified by Q-PCR. The protein levels were examined using western blot. Data were expressed as mean ± SEM and analyzed using two-way ANOVA followed by Bonferroni’s post hoc test. *p < 0.05, compared with the control group (without LPS and PRE-084 treatment); #p < 0.05, compared with the sole LPS-treated group n = 3 independent experiments
Decreased GSK-3β Activity is Involved in PRE-084’s Anti-Inflammatory Action
GSK-3β is an important signal molecule mediating the activation of microglia [19]. Our previous study has also demonstrated that the activation of S1R promotes the serine-9 phosphorylation of GSK-3β and consequently inhibits the activation of GSK-3β. Thus, we next examined the activity of GSK-3β in PRE-084 treated BV-2 cells. After 3 h of LPS stimulation, the level of S9 phosphorylated GSK-3β was significantly lower in the LPS treated group than that in the control (non-LPS stimulated) group (Fig. 3a, b. F (2, 6) = 15.67, p < 0.05). While the cell was co-treated with PRE-084 and LPS, the level of p-GSK-3β were higher than that in the LPS treated group (Fig. 3a, b. p < 0.05).
Increased p-GSK-3β participated in anti-inflammatory activity of PRE-084. a, b PRE-084 reversed the inhibition of GSK-3β phosphorylation induced by LPS. a Representative Western blot of p-GSK-3β, total GSK-3β and GAPDH. b Quantitative summary of results from three sets of independent experiments. BV-2 microglia were pretreated with PRE-084 10 μM for 30 min, and then activated by 200 ng/ml LPS. p-GSK-3β, total GSK-3β and GAPDH was examined using Western blot 3 h after the treatment of LPS. Data was expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests n = 3 independent experiments. c, d The GSK-3β activator pyrvinium decreased the level of p-GSK-3β. c Presentative western blot. d Quantitative summary of results from three sets of independent experiments. BV-2 microglia were pretreated with pyrvinium (0–0.5 μM) for 30 min, and then activated by 200 ng/ml LPS. p-GSK-3β, total GSK-3β and GAPDH was examined using Western blot 3 h after the treatment of LPS. Data were expressed mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests. n = 3 independent experiments. *p < 0.05, compared with the pyrvinium 0 μM group. e, f Pyrvinium reversed the inhibition of TNF-α. e IL-1β (f) and iNOS. g BV-2 microglia were pretreated with PRE-084, pyrvinium, or their combination for 30 min, and then activated by 200 ng/ml LPS. The mRNA expressing levels of TNF-α, IL-1β and iNOS were quantified by Q-PCR 6 h after the stimulation of LPS. Data were expressed as mean ± SEM and analyzed using two-way ANOVA followed by Bonferroni’s post hoc test n = 3 independent experiments
Then, we utilized a recently discovered GSK-3β activator, pyrvinium, to confirm the role of GSK-3β in PRE-084’s anti-inflammatory activity. As shown in Fig. 3c, d, pyrvinium reduced the level of p-GSK-3β in the BV-2 cells in a dose-dependent manner (Fig. 3c, d, F (3, 8) = 15.64, p < 0.01). The TNF-α mRNA level was higher in the PRE-084 + pyrvinium treated group than the sole PRE-084 treated group (Fig. 3e, two-way ANOVA, F(1, 8) = 17.17, p < 0.05), despite that pyrvinium itself didn’t change the expression of TNF-α mRNA. Similar tendency was found regarding to IL-1β and iNOS (Fig. 3f, g, two-way ANOVA; F(1, 8) = 17.63, p < 0.05, F(1, 8) = 6.493, p < 0.05, respectively). Collecting these results, we proposed that the enhancement of GSK-3β S9 phosphorylation contributes to the anti-inflammatory effect of S1R.
The Inhibition of Calcineurin Promotes the S9 Phosphorylation of GSK-3β and is Involved in the Anti-Neuroinflammatory Activity of PRE-084
The S9 state of GSK-3β is in the equilibrium between phosphorylation and de-phosphorylation. AKT is a key kinase that promotes the phosphorylation in BV-2 cells. But we didn’t find that the activity of AKT changed in LPS or PRE-084 treated BV-2 cells (Supplemental Material. 1d). The raised GSK-3β S9 phosphorylation of GSK-3β may be due to the inhibition of GSK-3β de-phosphorylation. It has been reported that calcineurin catalyzed the de-phosphorylation of GSK-3β in neuroblast-derived cells [15]. Thus, we firstly examined the relation between calcineurin and GSK-3β in microglia using a calcineurin of competitive activator of chlorogenic acid (CGA) and an antagonist of FK-506. The p-S9 GSK-3β level was significantly lower in the CGA-treated groups than the control group (CGA 0 μM) (Fig. 4a, b, F(3, 12) = 30.42, p < 0.0001) and significantly higher in the FK-506-treated groups than the control group (Fig. 4c, d F(3, 12) = 50.78, p < 0.0001). This finding validated that calcineurin modulates the S9 phosphorylation of GSK-3β in BV-2 cells.
Effect of calcineurin activator chlorogenic acid and inhibitor FK-506 on the activity of GSK-3β in BV-2 microglia. a Representative western blot of p-GSK-3β, total GSK-3β and GAPDH after the treatment of chlorogenic acid. b Quantitative summary of results from three sets of independent experiments illustrated in a. c Representative western blot of p-GSK-3β, total GSK-3β and GAPDH after the treatment of FK-506. d Quantitative summary of results from three sets of independent experiments illustrated in c. BV-2 microglia were treated with different doses of chlorogenic acid and FK-506 for 30 min. p-GSK-3β, total GSK-3β and GAPDH was examined using Western blot. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests. n = 4 independent experiments. *p < 0.05, compared with the chlorogenic acid 0 μM group (Subplot b) or FK-506 0 μM treated group (Subplot d)
Then, we examined the activity of calcineurin in BV-2 cells after the LPS stimulation and the treatment of drugs. The relative catalytic activity was significantly higher in the LPS treated group than the negative control group, while PRE-084 inhibited the LPS-raised calcineurin activity in a dose-dependent manner (Fig. 5a F(4, 10) = 12.58, p < 0.001; LPS vs control: p < 0.05, PRE-084 1 μM + LPS or PRE-084 10 μM + LPS vs LPS: p < 0.05). As to the NO level in the supernatant, the maximal inhibiting value of PRE-084 in combination with 50 μM CGA was twice as much as that without CGA (Fig. 5b 13.26 ± 1.163 vs 5.96 ± 1.375 μM, student’s t-test, p < 0.05). Similarly, the TNF-α mRNA level was higher in the PRE-084 + CGA treated group than the PRE-084 treated group (Fig. 5c. two-way ANOVA, F(1, 8) = 6.399, p < 0.05), despite that GCA itself didn’t change the expression of TNF-α mRNA. Analogous changing moods were found concerning IL-1β and iNOS (Fig. 5d, e two-way ANOVA, F(1, 8) = 8.298, p = 0.0205, F(1, 8) = 9.601, p < 0.05, respectively). Collectively, these results indicated that inhibition of calcineurin and the following dephosphorylation of GSK-3β may be a major mechanism for PRE-084’s anti-inflammatory action.
Effect of LPS, PRE-084 and chlorogenic acid on the calcineurin activity and pro-inflammatory cytokines and mediators. a The calcineurin catalytic activity in the BV-2 microglia. b Supernatant NO concentration. c–e mRNA level of TNF-α (c). IL-1β (d) and iNOS (e). BV-2 microglia were pretreated with PRE-084 1 μM for 30 min, and then activated by 200 ng/ml LPS. The calcineurin activity was assayed using a commercial colorimetric assay kit. NO levels in culture supernatants were measured using the Griess reaction. p-GSK-3β, total GSK-3β and GAPDH was examined using Western blot 3 h after the treatment of LPS. The mRNA expressing levels of TNF-α, IL-1β and iNOS were quantified by Q-PCR. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests. n = 3 independent experiments. CGA cholorogenic acid
The Inhibition of Ca2+ Oscillation Contributes to the Inactivation of Calcineurin
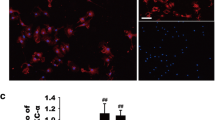
Ca2+ oscillation is a critical and selective event for sustained activation of calcineurin. Then, we examined the role of intracellular oscillation in the anti-inflammatory action of PRE-084. The presentative Ca2+ oscillation was shown in Fig. 6a (also referred to Supplemental Information 2) with diverse shapes (Fig. 6b). In the resting cells (the control group), about 13.3% (8/60) showed the spontaneous Ca2+ oscillation. In the LPS-stimulated group, about 63.3% (38/60) cells displayed Ca2+ oscillation, significantly higher than the control group (Fig. 6c x2-test, p < 0.001). By contrast, only 18.3% cells in the LPS + PRE-084 treated group was found to be oscillating, compared to that in the LPS stimulated group (Fig. 6c x2-test, p < 0.001). The oscillatory frequency was significantly higher in the LPS stimulated group than that in the control group, while the frequency in the PRE-084 treated group was significantly lower than that in the LPS-treated group (Fig. 6d F(2, 54) = 11.97, p < 0.0001).
Ca2+ oscillations and SOCE in BV-2 microglial cells and primary microglia. a A typical series of Fluo-4 AM fluorescence images selected from the stack of images. b The representative time-course of Ca2+ changes after the LPS treatment of LPS and the LPS + PRE-084 treatment in BV-2 cells. c The percent of BV-2 cells showing Ca2+ oscillation. d The frequency changes after the LPS or the LPS + PRE-084 treatment in BV-2 cells. e The percent of primary microglia showing Ca2+ oscillation. f The frequency changes after the LPS or the LPS + PRE-084 treatment in primary microglia. BV-2 microglia or primary microglia were pretreated with PRE-084 for 30 min, and then treated with LPS. 1 min after LPS stimulation, Ca2+ images were captured every second and the time-series of fluorescence intensity was acquired using Image J software. Ca2+ oscillation frequencies were expressed as medians, with interquartile ranges. The difference between three groups was tested using the Kruskal–Wallis test, followed by Dunnett’s post hoc test for multiple comparisons. The difference of cells showing Ca2+ oscillation between groups was tested using x2 test. g Presentative plot of SOCE in BV-2 cells. h The statistical summary of SOCE amplitudes in BV-2 cells. The basal intracellular Ca2+ fluorescence was obtained once per 30 s for 6 min. Then, LPS, PRE-084 and thapsigargin were added. 10 min later, 2.0 mM CaCl2 was added into the cells. The intracellular Ca2+ concentration was obtained and the amplitude of SOCE was calculated. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests. n = 3 independent experiments
To further confirm the role of Ca2+ oscillation in the activation of microglia, we examined the action of Ca2+ oscillation in the primary microglia. The presentative Ca2+ oscillation in activated primary microglia was shown in the video of Supplemental Material 3. Interesting, we found the number of primary microglia displayed the behavior of Ca2+ oscillation than that of BV-2 cells when they were activated by LPS (Fig. 6e, 85 v.s. 63.3%, x2-test, p < 0.01). Pre-084 also decreased the number of Ca2+ oscillation cells and the oscillating frequency (Fig. 6e, f. x2(2) = 73.92, p < 0.001; F(2,73) = 25.72, p < 0.01). Thus, these findings suggested that Ca2+ oscillation is a common phenomenon found in different sources of microglia and inhibition of Ca2+ oscillation maybe a universal anti-inflammatory strategy.
Another mechanism for microglia activation is store-operated Ca2+ entry (SOCE). ATP depletes the calcium store in ER, consequently elicits SOCE in microglia. Thus, we examined the effect of LPS and PRE-084 on SOCE. The amplitudes of SOCE (Δ[Ca2+]i) were significantly changed in the control group, LPS-treated group and the LPS + PRE-084 treated group (Fig. 6g, h, one-way ANOVA, F(2, 6) = 0.8462, p = 0.47) which suggested SOCE isn’t involved in the LPS-elicited fast microglia activation and the anti-inflammatory activity of PRE-084.
It has been reported that sigma-1 receptor inhibited diverse kinds of Ca2+ channels. It is possible that the inhibitory effect of PRE-084 on calcineurin is due to the decrease of basal intracellular Ca2+ in BV-2 cells. To exclude this possibility, we examined the change of the basal intracellular Ca2+ in BV-2 cells after the treatment of LPS or PRE-084. Interestingly, we didn’t observe the basal intracellular Ca2+ level increased in the 30 min after the application of LPS (Supplemental Material 1e repeated measures of ANOVA, p > 0.05). Thus, we proposed that the inhibition of calcineurin by PRE-084 is due to the inhibition of intracellular Ca2+ oscillation.
2-APB, an IP3 Receptor Inhibitor, Replicates Anti-Inflammatory Effect of PRE-084
It has been reported that the intracellular Ca2+ oscillation derives from the opening and closing of IP3 receptor on the endoplasmic reticulum in microglia [20]. Thus, we tested the hypothesis that the IP3 receptor inhibitor of 2-aminoethoxydiphenyl borate (2-APB) would replicate the effects of S1R activation. We found that 2-APB completely inhibited that intracellular Ca2+ oscillation (Data not shown). In the LPS + 2-APB treated BV-2 microglia, the mRNA level of TNF-α, IL-1β and iNOS were significantly lower those in the LPS treated group (Fig. 7a–c F(2, 6) = 17.67, p < 0.001, F(2, 6) = 28.6, p < 0.001, F(2, 8) = 32.51, p < 0.001, respectively). Moreover, 2-APB significantly inhibited the catalytic activation of calcineurin (Fig. 7d F(2, 6) = 9.216, p < 0.05), and promoted the S9 phosphorylation of GSK-3β (Fig. 7e, f F(2, 12) = 5.772, p < 0.05). These results indicated the inhibition of IP3 receptor can replicate the anti-inflammatory activity of PRE-084, and provided more evidence that the anti-inflammatory activity of S1R agonists are due to the inhibition of IP3 receptor.
IP3 receptor antagonists 2-APB mimicked the anti-inflammatory activity of PRE-084. a–c 2-APB decreased the mRNA level of TNF-α (a), IL-1β (b) and iNOS (c) in BV-2 microglia activated by LPS. (d). 2-APB reversed that elevated effect of LPS on calcineurin. (e, f). 2-APB reversed that inhibited effect of LPS on GSK-3β. BV-2 microglia were pretreated with 2-APB for 30 min, and then treated with LPS (200 ng/mL). The mRNA expressing levels of TNF-α, IL-1β and iNOS were quantified by Q-PCR. The calcineurin activity was assayed using a commercial colorimetric assay kit. p-GSK-3β, total GSK-3β and GAPDH was examined using Western blot. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests n = 3 independent experiments, except n = 5 in Subplot f
PRE-084 Therapeutically Inhibited the mRNA Expression of TNF-α, IL-β and iNOS
At last, we examined the effect of PRE-084 when BV2 cells were initially activated by LPS. In this study, RPE-084 was added 1 h after the treatment of LPS. We found the mRNA of TNF-α, IL-1β and iNOS were significantly lower than in the PRE-084 1 μM + LPS treated groups than the single LPS treated group (Fig. 8a–c, one-way ANOVA, F(2, 6) = 23.39, F(2, 6) = 19.29, F(2, 6) = 24.71, respectively, p < 0.05). Thus, we concluded that activation of S1R is an efficient anti-inflammatory strategy.
PRE-084 therapeutically inhibited the m RNA expression of TNF-α, IL-β and iNOS. aTNF-α (a), b IL-1β, c iNOS. BV-2 microglia were treated with LPS (200 ng/mL) for 1 h and treated with PRE-084. The mRNA expressing levels of TNF-α, IL-1β and iNOS were quantified by Q-PCR. Data were expressed as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple-comparison tests n = 3 independent experiments
Discussion
Deeply understanding the mechanism of microglia activation is very important for developing effective strategy for inhibiting over-activated neuroinflammation. In this study, we identified a noncanonical pro-inflammatory pathway of Ca2+ oscillation/calcineurin/GSK-3β, and found that the inhibition of this pathway is necessary for the anti-inflammatory activity of S1R activation.
Based on the difference of biomarkers and function, activated microglia is typically classified into M1 and M2 phenotypes. M1 polarized microglia is pro-inflammatory and detrimental to the CNS, while M2 polarized microglia is anti-inflammatory and protective. But, the exact role which Sig-1R plays in regulating microglia polarization is kept skeptical. For instance, methamphetamine induces the expression of M1 markers (iNOS) and decreases the expression of M2 markers (Arginase) via the activation of sigma-1 receptor [21]. But, in mutant SOD1-G93A ALS mice, RPE-084 increases the numbers of cells positive for M2 phenotype marker CD206 in the white matter, in concomitant with the increases pan-macrophage marker CD68 immunoreactivity, while not altering the mRNA levels of TNF-α and IL-1β [22]. In our study, we found that PRE-084 only inhibited the expression of M1-phenotype related cytokines (TNF-α, IL-1β, iNOS), but exerts no effect on the M2-phenotype related cytokines (IL-10, TGF-β). Thus, we proposed that the exact pattern of S1R in regulating microglia polarization may depend on the milieu of stimuli.
GSK-3β is a constitutively active kinase, being deactivated when the S9 site is phosphorylated. By promoting the activation of downstream transcription factors (such as Fyn, Nrf2, C/EBPβ and NFAT), GSK-3β argument the inflammatory state [12, 23,24,25]. Meanwhile, the inhibition of GSK-3β decreases the production of pro-inflammatory cytokines. It has been suggested that the inhibition of GSK-3β may be an excellent strategy for anti-inflammation [13]. However, because the normal level GSK-3β activity is fundamental for various cellular functions and the GSK-3β activity should controlled in a narrow window, direct inhibitor of GSK-3β should be discreetly applied and alternative methods for targeting GSK-3β signaling needs be identified [26]. In this study, we found that PRE-084 inhibited that activation of GS-3β and this kind of inhibition was reversed by the GSK-3β agonist of pyrvinium. Thus, besides explaining a novel mechanism for anti-inflammatory action of PRE-084, we propose an applicable strategy for taking advantage of GSK-3β as a powerful anti-inflammatory target.
The activity of GSK-3β is mainly determined by the equilibrium between Ser 9 phosphorylation and dephosphorylation of GSK-3β. AKT is the prominent kinase that promotes the Ser 9 phosphorylation of GSK-3β. In our study, we didn’t find the change of AKT activity after the treatment of LPS and PRE-084. This suggested that the deactivation of GSK-3β by PRE-084 didn’t rely on the enhancement of phosphorylation, but on the inhibited de-phosphorylation process.
Recent studies reveal that calcineurin, a well-known signal molecule in the activating process of immune cells, directly interacts with GSK-3β, makes the Ser 9 dephosphorylated and consequently increases GSK-3β kinase activity in neuroblast-derived cells and T-cell acute lymphoblastic leukemia cells [15, 16]. In our study, we also found the calcineurin modulated activity of GSK-3β in BV-2 microglia, since the calcineurin activator chlorogenic acid decreased the phosphorylation of GSK-3β and the inhibitor FK-506 increased the phosphorylation of GSK-3β in BV-2 cells. Moreover, the anti-inflammatory activity of PRE-084 was reversed by chlorogenic acid. Based on these finding, we proposed that the deactivation of GSK-3β induced by PRE-084 relies on the inhibition of calcineurin.
The predominant activator of calcineurin is the intracellular Ca2+. Coordinating with calcineurin, Ca2+ binds with the subunit B of calcineurin, leading to activation of calcineurin and subsequent dephosphorylation of target proteins [27]. The high affinity and slow interaction between calcineurin and Ca2+ allowed calcineurin to respond gradually and consistently to the fast changing intracellular Ca2+. In our study, the activity of calcineurin rose 30 min after the stimulation of LPS. Thus, the change of intracellular Ca2+ should occur earlier. However, we also didn’t observe the basal intracellular Ca2+ level increased in the 30 min after the application of LPS. Similarly, it has been reported to cause a sustained increase in basal intracellular Ca2+ in microglia after a 24-h treatment. Therefore, another Ca2+ activating manner should exist.
Ca2+ oscillation is the fast fluctuation of intracellular Ca2+, without causing calcium overload. It has been reported that Ca2+ oscillation is an efficient signal that activates downstream pathways, such as NFAT [28]. The spontaneous Ca2+ oscillations were observed in microglia [20]. We found that the Ca2+ oscillation is augmented after the immediate stimulation of LPS, and PRE-084 inhibited the Ca2+ oscillation. Based on this finding, we concluded that the inhibition of Ca2+ oscillation can directly inhibit that action of calcineurin and GSK-3β.
S1R directly interacts with diverse ion channel, including IP3 receptor that resides on the membrane of endoplasmic reticulum [29]. It has been reported that the intracellular Ca2+ oscillation mainly derives from the periodic opening and closing of IP3 receptor on the endoplasmic reticulum [30]. In our study, we found that 2-APB, an IP3 receptor blocker, inhibited the Ca2+ oscillation, the activity of calcineurin and GSK-3β, mimicking the pharmacological effect of PRE-084. Based on these findings, we concluded that Ca2+/calcineurin/GSK-3β comprises a complete inflammatory pathway. By inhibiting this new-identified pathway, S1R agonists display their anti-neuroinflammatory activity.
Nevertheless, the complex interaction between sigma-1 receptor and IP3 receptor should be further discussed. S1R agonists enhance IP3-dependent calcium release from endoplasmic reticulum in MCF-7 cells when the cells are stimulated by bradykinin, vasopressin or ATP [31]. Hayashi and Su reported that S1R binds with mature IP3 R3 at MAM and selectively affect Ca2+ mobilization at mitochondrion [32]. By contrast, Tagashira proposed that S1R stimulation by agonist pharmacologically suppresses IP3-mediated Ca2+ release from the endoplasmic reticulum in the in hypertrophic cardiomyocytes [33]. In our study, it seems that the activation of sigma-1 receptor inhibited the IP3 receptor in BV-2 cells. Thus, we consider that this dissimilarity may come from the difference of stimuli and the cellular categories. Given that S1R modulates the target proteins in an allosteric manner [6], it is possible that S1R agonists show different activity in diverse micro-environment.
Another mechanism for microglia activation is store-operated Ca2+ entry (SOCE) that was mainly mediated by Stim1 and Orai1 [34]. It has been reported that ATP depletes the calcium store depletion, consequently elicits SOCE in microglia [34, 35]. Heo and co-workers found that LPS (3–24 h) raised the expression of Stim1 by about 1.5 folds in primary microglia [36]. In our study, we didn’t find that either LPS changed the process of SOCE. One possible reason is that the treatment time of LPS or PRE-084 is too short to change the expression or activity of STIM1 and Orai1. As to the effect of S1R on SOCE, it depends on the type of cells [37]. In HEK293 cells, continuous stimulation of S1R (at least > 1 h) competes with STIM1 for binding to Orai1, then inhibits SOCE [18]. Similarly, cocaine activates S1R and inhibits SOCE [38]. But, the S1R agonist (+)-pentazocine SOCE in MCF7 sigma1 and SK-N-SH cell lines. In our study, no difference of SOCE amplitude was found in the LPS treated group and LPS + PRE-084 treated group. Considering the complexity of proteins supporting SOCE and the wide action of S1R on cell functions, these contrasting results aren’t surprising [37].
Collectively, we identified a noncanonial pro-inflammatory pathway of Ca2+ oscillation/calcineurin/GSK-3β and this inhibition of this noncanonical pathway contributed to the anti-inflammatory activity of S1R activation.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2-APB:
-
2-Aminoethoxydiphenyl borate
- BD1047:
-
N′-[2-(3,4-dichlorophenyl)ethyl]-N,N, N′-trimethylethane-1,2-diamine
- CGA:
-
Chlorogenic acid
- ERK:
-
Extracellular-signal-regulated kinase
- JNK:
-
C-Jun N terminal kinase
- IKK:
-
IκB kinase
- iNOS:
-
Inducible nitric oxide synthase
- IκBα:
-
Inhibitor of nuclear factor-κB, α
- IL-1β:
-
Interleukin-1β
- IL-10:
-
Interleukin-10
- IP3 :
-
Inositol triphosphate
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MTT:
-
3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyl tetrazoliumbromide
- Nrf2:
-
Nuclear factor erythroid-2 related factor 2
- NO:
-
Nitric oxide
- PRE-084:
-
2-Morpholin-4-ylethyl 1-phenylcyclohexane-1-carboxylate
- ROS:
-
Reactive oxygen species
- S1R:
-
Sigma-1 receptor
- SKF83959:
-
3-Methyl-6-chloro-7,8-hydroxy-1-(3-methylphenyl)-2, 3, 4, 5-tetrahydro-1H-3-benzazepine
- SOCE:
-
Store-operated Ca2+ entry TGF-β, transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
References
Regen F, Hellmann-Regen J, Costantini E, Reale M (2017) Neuroinflammation and Alzheimer’s disease: implications for microglial activation. Curr Alzheimer Res 14:1140–1148
Lenart N, Brough D, Denes A (2016) Inflammasomes link vascular disease with neuroinflammation and brain disorders. J Cereb Blood Flow Metab 36:1668–1685
Eyo UB, Murugan M, Wu LJ (2017) Microglia-neuron communication in epilepsy. Glia 65:5–18
Subhramanyam CS, Wang C, Hu Q, Dheen ST (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120
Penke B, Fulop L, Szucs M, Frecska E (2018) The role of sigma-1 receptor, an intracellular chaperone in neurodegenerative diseases. Curr Neuropharmacol 16:97–116
Su TP, Su TC, Nakamura Y, Tsai SY (2016) The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol Sci 37:262–278
Jia J, Cheng J, Wang C, Zhen X (2018) Sigma-1 receptor-modulated neuroinflammation in neurological diseases. Front Cell Neurosci 12:314
Dong H, Ma Y, Ren Z, Xu B, Zhang Y, Chen J, Yang B (2016) Sigma-1 receptor modulates neuroinflammation after traumatic brain injury. Cell Mol Neurobiol 36:639–645
Wu Z, Li L, Zheng LT, Xu Z, Guo L, Zhen X (2015) Allosteric modulation of sigma-1 receptors by SKF83959 inhibits microglia-mediated inflammation. J Neurochem 134:904–914
Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith SB, Bollinger KE (2014) Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci 55:3375–3384
Rosen DA, Seki SM, Fernandez-Castaneda A, Beiter RM, Eccles JD, Woodfolk JA, Gaultier A (2019) Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med 11:5266
Baumgart S, Chen NM, Zhang JS, Billadeau DD, Gaisina IN, Kozikowski AP, Singh SK, Fink D, Strobel P, Klindt C, Zhang L, Bamlet WR, Koenig A, Hessmann E, Gress TM, Ellenrieder V, Neesse A (2016) GSK-3beta governs inflammation-induced NFATc2 signaling hubs to promote pancreatic cancer progression. Mol Cancer Ther 15:491–502
Feng X, Guan W, Zhao Y, Wang C, Song M, Yao Y, Yang T, Fan H (2019) Dexmedetomidine ameliorates lipopolysaccharide-induced acute kidney injury in rats by inhibiting inflammation and oxidative stress via the GSK-3beta/Nrf2 signaling pathway. J Cell Physiol 234:18994–19009
Wang Y, Guo L, Jiang HF, Zheng LT, Zhang A, Zhen XC (2016) Allosteric modulation of sigma-1 receptors elicits rapid antidepressant activity. CNS Neurosci Ther 22:368–377
Kim Y, Lee YI, Seo M, Kim SY, Lee JE, Youn HD, Kim YS, Juhnn YS (2009) Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J Neurochem 111:344–354
Tosello V, Bordin F, Yu J, Agnusdei V, Indraccolo S, Basso G, Amadori A, Piovan E (2016) Calcineurin and GSK-3 inhibition sensitizes T-cell acute lymphoblastic leukemia cells to apoptosis through X-linked inhibitor of apoptosis protein degradation. Leukemia 30:812–822
Wang Y, Gao C, Gao T, Zhao L, Zhu S, Guo L (2021) Plasma exosomes from depression ameliorate inflammation-induced depressive-like behaviors via sigma-1 receptor delivery. Brain Behav Immun 94:225–234
Srivats S, Balasuriya D, Pasche M, Vistal G, Edwardson JM, Taylor CW, Murrell-Lagnado RD (2016) Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J Cell Biol 213:65–79
Li J, Shi C, Ding Z, Jin W (2020) Glycogen synthase kinase 3beta promotes postoperative cognitive dysfunction by inducing the M1 polarization and migration of microglia. Mediators Inflamm 2020:7860829
Wu X, Pan L, Liu Y, Jiang P, Lee I, Drevensek-Olenik I, Zhang X, Xu J (2013) Cell-cell communication induces random spikes of spontaneous calcium oscillations in multi-BV-2 microglial cells. Biochem Biophys Res Commun 431:664–669
Chao J, Zhang Y, Du L, Zhou R, Wu X, Shen K, Yao H (2017) Molecular mechanisms underlying the involvement of the Sigma-1 receptor in methamphetamine-mediated microglial polarization. Sci Rep 7:11540
Peviani M, Salvaneschi E, Bontempi L, Petese A, Manzo A, Rossi D, Salmona M, Collina S, Bigini P, Curti D (2014) Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol Dis 62:218–232
Pan J, Shen F, Tian K, Wang M, Xi Y, Li J, Huang Z (2019) Triptolide induces oxidative damage in NRK-52E cells through facilitating Nrf2 degradation by ubiquitination via the GSK-3beta/Fyn pathway. Toxicol In Vitro 58:187–194
Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G (2015) Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun 6:8272
Liu SS, Lv XX, Liu C, Qi J, Li YX, Wei XP, Li K, Hua F, Cui B, Zhang XW, Yu JJ, Yu JM, Wang F, Shang S, Zhao CX, Hou XY, Yao ZG, Li PP, Li X, Huang B, Hu ZW (2019) Targeting degradation of the transcription factor C/EBPbeta reduces lung fibrosis by restoring activity of the ubiquitin-editing enzyme A20 in macrophages. Immunity 51:522–534
Jaworski T (2020) Control of neuronal excitability by GSK-3beta: epilepsy and beyond. Biochim Biophys Acta Mol Cell Res 1867:118745
Park YJ, Yoo SA, Kim M, Kim WU (2020) The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front Immunol 11:195
Hannanta-Anan P, Chow BY (2016) optogenetic control of calcium oscillation waveform defines NFAT as an integrator of calcium load. Cell Syst 2:283–288
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610
Zhang S, Fritz N, Ibarra C, Uhlen P (2011) Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations. Neurochem Res 36:1175–1185
Wu Z, Bowen WD (2008) Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem 283:28198–28215
Hayashi T, Su TP (2003) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther 306:726–733
Tagashira H, Bhuiyan MS, Shioda N, Fukunaga K (2014) Fluvoxamine rescues mitochondrial Ca2+ transport and ATP production through sigma(1)-receptor in hypertrophic cardiomyocytes. Life Sci 95:89–100
Michaelis M, Nieswandt B, Stegner D, Eilers J, Kraft R (2015) STIM1, STIM2, and Orai1 regulate store-operated calcium entry and purinergic activation of microglia. Glia 63:652–663
Gilbert DF, Stebbing MJ, Kuenzel K, Murphy RM, Zacharewicz E, Buttgereit A, Stokes L, Adams DJ, Friedrich O (2016) Store-operated Ca(2+) entry (SOCE) and purinergic receptor-mediated Ca(2+) homeostasis in murine bv2 microglia cells: early cellular responses to ATP-mediated microglia activation. Front Mol Neurosci 9:111
Heo DK, Lim HM, Nam JH, Lee MG, Kim JY (2015) Regulation of phagocytosis and cytokine secretion by store-operated calcium entry in primary isolated murine microglia. Cell Signal 27:177–186
Gasparre G, Abate C, Carlucci R, Berardi F, Cassano G (2017) The sigma1 receptor agonist (+)-pentazocine increases store-operated Ca(2+) entry in MCF7sigma1 and SK-N-SH cell lines. Pharmacol Rep 69:542–545
Brailoiu GC, Deliu E, Console-Bram LM, Soboloff J, Abood ME, Unterwald EM, Brailoiu E (2016) Cocaine inhibits store-operated Ca2+ entry in brain microvascular endothelial cells: critical role for sigma-1 receptors. Biochem J 473:1–5
Funding
This project was supported by grants from the National Natural Science Foundation of China (Grant No. 81872847, 82173803). Supports provided from the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD) and Jiangsu Overseas Visiting Scholar Program for University Prominent Young and Middle-Aged Teacher and President are also appreciated.
Author information
Authors and Affiliations
Contributions
Investigation, writing—original draft: [TG]; investigation: [CG]; investigation: [ZL]; conceptualization, funding acquisition: [YW]; investigation: [XJ]; investigation: [HT]; supervision, writing—review & editing: [QL]; conceptualization, funding acquisition, supervision, writing—review & editing: [LG].
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 649 kb)
Supplementary file3 (AVI 2106 kb)
Rights and permissions
About this article
Cite this article
Gao, T., Gao, C., Liu, Z. et al. Inhibition of Noncanonical Ca2+ Oscillation/Calcineurin/GSK-3β Pathway Contributes to Anti-Inflammatory Effect of Sigma-1 Receptor Activation. Neurochem Res 47, 264–278 (2022). https://doi.org/10.1007/s11064-021-03439-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03439-2