Abstract
Microglia as resident cells of the brain can regulate neural development and maintenance of neuronal networks. Any types of pathologic events or changes in brain homeostasis are involved in the activation of microglia. This activation depends on the context, type of the stressor, or pathology. Due to the release of a plethora of substances such as chemokines, cytokines, and growth factors, microglia able to influence the pathologic outcome. In Alzheimer's disease (AD) condition, the deposition of amyloid‐β (Aβ) result in provokes the phenotypic activation of microglia and their elaboration of pro-inflammatory molecules. New investigations reveal that cellular therapy with stem cells might have therapeutic effects in preventing the pathogenesis of AD. Although many strategies have focused on the use of stem cells to regenerate damaged neurons, new researches have demonstrated the immune-regulatory feature of stem cells which can modulate the activity state of microglia as well as mediates neuroinflammation. Hence, understanding the molecular mechanisms involved in the brain homeostasis by the protective features of mesenchymal stem cells (MSCs) could lead to remedial treatment for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD), one of the most prevalent neuronal disorders worldwide, clinically is characterized by impairments in memory, cognition, and intellectual disabilities. The extracellular plaques composed predominately of the amyloid‐β (Aβ) peptide, intra-neuronal tangles of hyper-phosphorylated tau, and synaptic and neuronal loss are the neuropathological hallmarks of the AD in the brain of patients [1, 2]. Moreover, inflammation in brain tissue plays a significant role in the neurodegeneration process. In the last 10 years, there was dramatic increase in the AD prevalence due to the advancing age of the human population, female sex, and genetic risk factors; to this end, it is estimated that in the future, there will be more people affected by this neuronal disorder in the world [3, 4]. Hence, this shows that there are no effective therapeutic strategies available to prevent, treat, or manage AD [4].
Based on studies, it has been approved that microglia play important roles in the developmental process in the brain, tissue homeostasis, and neurological disorders [5]. The dysregulation function of microglia is prominent property of most neurodegenerative disorders like AD with distinct etiologies. The reactive gliosis of AD histopathology which can reflect the abnormal morphology and proliferation of microglia is one of the critical problems in brain tissue [5]. Microglia as the brain's resident immune cells can perform housekeeping functions that are vital to neuronal health. Though, persistently activated microglia, which are increased in the pathological conditions in AD, contribute to a chronic state of cerebral inflammation leading to neuronal death [6, 7]. It has been shown that one of the most notable late 20th-century findings in neuropathological researches of AD was the large increase in numbers of microglia with an activated morphology and an increased expression of the major histocompatibility complex (MHC) class II in regions of neurodegeneration [8]. In addition to classical immune cell function of theirs, microglia act as guardians of the cerebral tissue by enhancing phagocytic clearance and providing trophic support to ensure tissue repair and maintain brain homeostasis. Conditions associated with a disturbance of homeostasis or tissue changes induce several dynamic microglial processes such as changes of cellular morphology, surface phenotype, secretory mediators, and proliferative responses. Activated microglia can introduce a common pathological property of many neurodegenerative disorders like AD [9, 10]. Recently, it has been reported that stem cell-based therapy has the potential to modify the pathological condition of the brain in AD. Stem cells from various diverse origins and cell types are widely studied in both animals and humans [11]. Stem cells able to induce direct regeneration of neurons and synapses [12]. Besides, they can also prevent activation of pro-inflammatory microglia, increase activation of anti-inflammatory microglia, inhibit astrogliosis, and promote nonreactive astrocytes. These effects in return may increase Aβ degradation, decrease the risk of the Aβ cascade, repair damaged neurons, and enhance synaptogenesis [13, 14]. Hence, stem cell-based therapy represents the great potential to become a prospective treatment for AD in the future. Here, we show, the latest evidence demonstrating the impact of stem cells on the brain homeostasis through modulating microglial functions, and on how this may be relevant in the enhancement of AD and for the identification of novel therapeutic approaches to treat this neurodegenerative disorder.
Microglia in Alzheimer’s Disease
Microglia can introduce approximately 10% of all the cells in the spinal cord and brain. During an inflammatory reaction, microglia is one of the first immune cells that get immunologically active, and then constitute the first line of cellular defense against invading pathogens and other types of the cerebral lesion [15, 16]. Despite the microglia vastly have been studied, their true origin remains a subject of debate. Primary evidence showed that microglia are able to differentiate in the bone marrow from embryonic hematopoietic pre-cursor cells, whereas new researches have demonstrated that these cells may, in fact, arise from progenitors in the embryonic yolk sac early during the developmental process [17, 18]. In normal conditions, microglia exist in a resting/quiescent state, and morphologically they are characterized by small-shaped cell body and highly ramified processes [17, 18]. One of the important functions of quiescent state microglia is to vigilantly monitor the central nervous system (CNS) for the detection of pathogens and host-derived ligands such as danger-associated molecular patterns and pathogen-associated molecular patterns [19, 20]. It has been reported that the expression of pattern recognition receptors on their molecular surface can make them well equipped for this aim [20, 21]. In response to invading pathogens, and undergo morphological changes like enlargement of their cell body and shortening of their cellular processes microglia can activate [21, 22]. Activated microglia play a critical role in the phagocytosis of pathogens and in the clearance of cellular debris and degenerating cells at the injury area [22,23,24].
According to basic molecular biology, it has been revealed that the dual roles of microglia in the pathogenesis of AD. For instance, microglia are involved in the pathogenesis of AD by producing inflammatory mediators like inflammatory cytokines, chemokines, complement components, and free radicals that are all known to contribute to the production and accumulation of Aβ [25, 26]. On the other hand, researches showed that microglia have an essential role in generating anti-Aβ antibodies and they can stimulate clearance of amyloid plaques. It was revealed that Aβ by inducing microglial activation is involved in neuroinflammation, which has been considered as an underlying and unifying factor in the progression of AD pathogenesis. Finally, a vicious cycle of inflammation is formed between the accumulated Aβ, the activated state of microglia, and microglial inflammatory mediators, which enhance Aβ deposition and neuroinflammation [25, 26].
Overall, microglia has unique roles not only to maintain immune homeostasis, but also being indispensable to cerebral development and cognitive ability. For example, microglia can drive neuronal activity, synaptogenesis, synaptic pruning, and neurogenesis [27, 28]. Unfortunately, abnormal function of microglia is implicated in some neurodevelopmental diseases, and dysregulation of microglial function is strongly linked to several pathologies like the decline of cognitive ability and AD [27, 28]. Despite great efforts toward relieving AD symptoms, there remains a lack of curable therapeutic strategy and current pharmaceutical treatments only alleviate the symptoms of diseases just for a short period time [29]. Therefore, there are no effective and curative therapeutic ways for AD [30]. In the past years, the advent of stem cell-based technology can represent a novel therapeutic tool to improve AD in animal models. Furthermore, researches have revealed that stem cell technology might enhance AD symptoms by regulating microglial functions, from detrimental to protective [30, 31]. Although primary strategies have focused on the application of stem cells to regenerate damaged cells, new researches have implied on stem cell-immuno-regulation property and they can modulate the activity state of microglia or astrocytes [29]. Stem cells also can mediate neuroinflammation process through some transcription factors molecular pathways, that can act as the main mechanism for the therapeutic efficacy of stem cell and be responsible for some of the satisfactory results [29].
The Role of Stem Cell-Microglia Interaction in Brain Homeostasis
In the past decade, comprehensive single-cell RNA sequencing analysis of AD and aging demonstrated a new and rare subset of microglia, disease-associated microglia, conserved in humans and animals [32]. For different CNS pathologies such as AD and senile dementia, the presence of activated microglia is a common property [33, 34]. Microglia can regulate Aβ deposition by phagocytosis with a potentially protective impact on AD progression [35]. Microglia as the brain’s resident immune cell, can take part in the establishment of normal neuronal connectivity and regulatory processes and they are critical for the CNS developmental process such as synaptic pruning [36]. Disease-associated microglia are characterized molecularly as immune cells expressing typical microglial markers, Iba1, Cst3, and Hexb, coincident with the downregulation of ‘‘homeostatic’’ microglial genes, including P2ry12, P2ry13, Cx3cr1, CD33, and Tmem119 [37]. Microglia are unique tissue-resident macrophage populations of the CNS and they can involve in immune reactions and inflammatory disorders as primary cells [38]. Microglia has the ability of response to the Aβ peptides and it can increase their clearance by the release of cytotoxic factors, which, in turn, promote the phagocytosis of these peptides. Therefore, if, on the one hand, phagocytosis of Aβ peptides can enhance disease, on the other hand, the release of pro-inflammatory mediators may be enhancing the disease [39]. Microglial has the ability to sense neuronal activity, that allows them to regulate synaptic plasticity, learning, and memory mechanisms, and hence determine cognitive functions. As such, brain-derived neurotrophic factor (BDNF) produced by microglia had shown to modulate motor learning-dependent synapse formation in animals [40]. While microglia can exert neuroprotection in AD, especially through their phagocytic clearance of Aβ, a loss of their beneficial functions can exacerbate amyloidosis, leading to subsequent inflammation, synaptic loss, and neuronal damage. Vice versa, microglial inability to clear abnormally aggregated Aβ results in the activation of pro-inflammatory signalling pathways, furthering inflammation, oxidative stress, and neurodegeneration in AD [41]. Also, the CR3/C3 signalling pathway implicates in early synapse loss in mouse models of AD, indicating that inappropriate activation of microglia by pathogenic proteins results in aberrant phagocytosis of functional synapses [42]. In addition, CX3CR1CreER/ROSA26iDTR-flox mice treated with tamoxifen to induce expression of the diphtheria toxin receptor selectively in microglia, and receiving diphtheria toxin to deplete these cells, showed decreased motor learning-induced dendritic spine formation, and reduced levels of synaptic proteins VGlut1 and GluA2 from glutamatergic synapses in the motor cortex [40]. CX3CR1 as a specific to microglia in the nervous system and subset of peripheral monocytes, and response to pruning cues from surrounding neurons. Scientists show the removal of CX3CR1 results in a brief reduction in microglia and subsequent deficit in synaptic pruning cause an excess of excitatory synapses, as well as an increase in spine density and PSD95 expression [43]. In AD patients and animal models of AD, reduction in the Smad3 signalling pathway, likely result in the pathological activation of microglia [44]. Beside, IFN-γ as a potent activator of microglia and initiator of pro-inflammatory transcription of the gene is increased in the aged cerebral tissue [45]. In the pro-inflammatory state, a variety of inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-6, IL-12, IL-23, IL-1β, as well as reactive oxygen species (ROS) and nitric oxide (NO) released by microglia result in promoting neurotoxicity and reinforce the inflammatory response. According to the researches, the accumulation of reactive oxidative species can damage the neurons and triggers apoptosis [46]. Furthermore, scientists attempt to understand the heterogeneity of activated microglia (M1 and M2). The M1 type is classically activated microglia and produces inflammatory cytokines and ROS, whereas M2 microglia was in a state of alternative activation that shows an anti-inflammatory phenotype [47]. M1 microglia showed notable infiltration in the nervous tissues of patients with AD who showed pathological changes. While a number of studies have reported that inflammatory processes are highly correlated with cognitive deficits in AD brain, many studies have suggested that acute beneficial effects of stem cells depend instead on their paracrine signalling actions in brain homeostasis. For instance, human MSCs can ameliorate the symptoms of AD by decreasing the levels of inflammatory cytokines and regulating the expression of Aβ‑related genes [48, 49]. Based on a new study, researchers have proven that stem cell factor or paracrine signalling actions can induce polarization of microglia to the M2 phenotype [50]. Stem cell factor signalling can modulate microglial functions and is involved in neuron-microglia interactions. Stem cell factor and its receptor, c-kit reported being express in the CNS. Stem cell factor mainly expressed in neurons, whereas c-kit expressed largely by glial cells and weakly by neurons. After brain injury, the c-kit expression is up-regulated in microglia, and stem cell factor expression is elevated in neuron and astrocyte [50,51,52]. Other reports showed that stem cell factor/c-kit signalling stimulates neurogenesis and survival for neuronal stem cells. Stem cell factor can modulate microglial functions and induces activation of the neuroprotective effects of microglia, which that be use for treatment of neuronal diseases. Also other study revealed that MSCs can induce immunosuppressive effects in microglia, representing an attractive source for the control of CNS chronic inflammation [53, 54]. MSCs have shown to prevent lymphocytes, dendritic cells and microglia proliferation as well as modulate the cytokine secretion profile of monocytes and macrophages, prevent graft rejection and support post-traumatic-stroke regeneration. Cellular therapy using MSCs in neurodegenerative diseases results in encourage repair, decrease apoptosis, regulate inflammation [53,54,55]. Based on Yarúa Jaimes et al. (2017) study, modulation of microglia involves the prevention of mitogen activated protein kinase phosphorylation, therewith leading to lower transcription of genes associated with inflammation [54, 55]. Collectively, microglia can play a coordinated and sophisticated role to deactivate the initial immune response and lead to processes for tissue repair. These findings could open a new way for novel cell therapeutics to prevent CNS chronic inflammation.
Many investigations including those on the human post-mortem AD brain, as well as neuroimaging analysis in patients and rodent transgenic models, have represented evidence that microglia are attracted to and surround senile plaques in cerebral tissue [56, 57]. Scientific researches showed the existence of an age-dependent phenotypic change of microglial activation in the hippocampus of an AD animal model, from an alternative (expressing IL-4) activation state to a classic cytotoxic (expressing IL-1β and TNF-α) phenotype [58]. Previous studies revealed that intracerebral transplantation of bone marrow stem cells could increase microglial activation and reduce Aβ deposits in an acutely induced AD model. The activated microglia were located near the Aβ deposits, and their morphology was changed from ramified to ameboid as an action of the microglial phagocytosis [59, 60]. New developments in stem cell-based technology using MSCs raise the prospect of cellular therapy for AD. MSCs transplantation into amyloid precursor protein (APP) and presenilin1 (PSEN1) double-transgenic animals significantly improved spatial learning and memory decline. It has been reported that the alternatively activated microglia preferentially surrounded and infiltrated into Aβ deposits in the brains of APP/PSEN1 transgenic animals after MSCs transplantation. Furthermore, Aβ deposition, β-secretase 1 (BACE-1) levels, and tau hyper-phosphorylation were dramatically reduced in MSCs transplanted APP/PSEN1 animals [61]. Interestingly, these effects were associated with reversal of disease-associated microglial neuroinflammation, as evidenced by decreased microglia-induced pro-inflammatory cytokines, elevated alternatively activated microglia, and increased anti-inflammatory cytokines. During microglial activation, MSCs-transplanted animals exhibited not only decreased neurotoxic cytokine expression levels but also increased expression levels of anti-inflammatory cytokines, indicative of alternative microglial activation. These findings lead us to suggest that MSCs produced their sustained neuroprotective effect by inducing a feed-forward loop involving alternative activation of microglial neuroinflammation, thereby ameliorating disease pathophysiology and reversing the cognitive decline associated with Aβ deposition in AD animals [62, 63]. The effect of MSCs on reducing Aβ accumulation is likely attributable to the inhibition of BACE-1 expression via immunomodulation [61, 63]. Experimentally, MSCs transplantation could modulate microglial activation in AD animals, mitigate AD symptoms, and alleviate cognitive decline, all of which suggest MSCs transplantation as a promising choice for AD therapy. MSCs secrete various cytotropic factors that may exert beneficial effects in AD animals through various mechanisms such as reducing amyloid burden, decreasing inflammation, or increasing endogenous neurogenesis [64, 65]. Also in the last 10 years, several human clinical trials have suggested that MSCs are effective in slowing down the course of neurodegenerative diseases (Fig. 1) [66,67,68]. Since 2010, scientists began clinical trials investigating the use of stem cells for AD. Two-phase I trials on moderate AD have been completed; though they revealed no severe acute or long-term side effects, no significant clinical efficacy was observed [69, 70]. The phase 1 clinical trial demonstrated that surgical stereotactic administration of MSCs into the hippocampus and precuneus is feasible, safe, and well-tolerated in patients with mild-to-moderate AD dementia [67]. A meta-analysis of MSCs clinical trials approved that MSCs therapy does not increase the risk of acute infusional toxicity, arrhythmia, cardiac dysfunction, gastrointestinal or renal dysfunction, infection, death, or tumor development [71]. Several clinical trials, which involve more sophisticated study designs using different injection routes, well-established scales, and biomarkers, are planned for mild to moderate AD patients (Table 1; "Clinical trials.gov") [69,70,71,72]. Taken together, MSCs based therapies will play a critical role in the treatment of neurodegenerative diseases such as AD in the future.
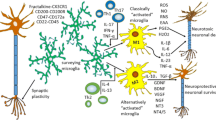
Microglial activation is involved in the progression of Alzheimer's disease (AD). a AD pathogenesis is associated to Aβ plaques deposition in extracellular space due to an increased production and/or lack of clearance of Aβ peptides derived from APP cleavage and by abnormal intra-neuronal accumulation of hyper-phosphorylated tau protein. In pathological conditions, intracellular accumulation of hyper-phosphorylated tau disorganizes microtubules can impairing the cytoskeleton. This has been linked to neurodegeneration and cognitive impairment. Formation of Aβ plaques can triggers microglial activation. Finally, a vicious cycle of inflammation can be form between Aβ accumulation, activated microglia, and microglial inflammatory mediators, which enhance Aβ deposition and neuro-inflammation. b Cell-based therapy using MSCs can induce immunosuppressive effects in microglia result in control of CNS chronic inflammation. Stem cell factor signalling can modulates microglial functions and is involved in neuron-microglia interactions. Stem cell factor mainly expressed in neurons. After brain injury, c-kit expression is up-regulate in microglia, and stem cell factor expression is elevated in neuron. Stem cell factor can modulate microglial functions and induces activation of the neuroprotective effects of microglia. Also, MSCs can reduce Aβ accumulation is likely attributable to the inhibition of BACE-1 expression via immunomodulation
Future researches about the pathogenesis of AD have needed to fully disclose the association between multiple pathogenesis at the same time. In sum, the novel regulatory mechanism and biological pathways about the microglial function have been discovered, but prevention of the disease is still a challenge ahead. Thereby we suggest that continuation of the investigations of gene mutations and the elucidation of the missing genetic etiology in patients still have a vast potential to deliver novel crucial pieces that will lead to a better understanding of the complex puzzle of AD at large. An understanding of genetic mechanisms underlying AD pathogenesis and the potentially implicated pathways will lead to the development of novel treatment for this devastating disease.
Challenges and Future Directions for Stem Cell Technology
Taken together, the experimental and preclinical effort performed to date reveal that stem cell-based technology has considerable potential to enhance cerebral function and regenerate viable neural cells. It is clear that to unleash the full potential of regenerative medicine, cellular therapy, and proceed toward clinical translation, many major unresolved issues will have to be resolved; for instance, what are the optimal cell dose, the optimal cell type(s), the optimal route of cell administration, and the optimal frequency of treatment? These questions should be answered just by performing careful preclinical and clinical researches. Hence, due to the uniqueness of regenerative medicine and cellular therapies, regulatory agents are needed to develop new regulatory policies to foster their appropriate development and success.
In conclusion, our review strongly suggest that cellular therapy can provide the basis for a novel immune-modulatory strategy for AD. In vivo transplantation of MSCs has showed a beneficial effect through endogenous microglia activation in the AD brains. Therefore, we proposed MSCs are potent regulators of microglia activation and brain homeostasis. But the safe and ethical future of stem cell therapies, especially for AD, will likely be slow, expensive, and tightly controlled.
References
Ashe KH (2007) Cognitive impairment in transgenic Aβ and tau models of Alzheimer’s disease. Alzheimer’s disease. Springer, Boston, pp 77–91
LaFerla FM, Oddo S (2005) Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med 11:170–186
Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W, Oddo S (2019) Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 18:e13037
Velazquez R, Ferreira E, Winslow W, Dave N, Piras IS, Naymik M, Huentelman MJ, Tran A, Caccamo A, Oddo S (2019) Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol Psychiatry 8:1–10
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217:459–472
Tremblay MÈ, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16079
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77:10–18
Lue LF, Beach TG, Walker DG (2019) Alzheimer’s disease research using human microglia. Cells 8:838
Song WM, Colonna M (2018) The microglial response to neurodegenerative disease. Advances in immunology, vol 139. Academic Press, Cambridge, pp 1–50
Dansokho C, Heneka MT (2018) Neuroinflammatory responses in Alzheimer’s disease. J Neural Transm 125:771–779
Bagheri-Mohammadi S, Karimian M, Alani B, Verdi J, Tehrani RM, Noureddini M (2019) Stem cell-based therapy for Parkinson’s disease with a focus on human endometrium-derived mesenchymal stem cells. J Cell Physiol 234:1326–1335
Bagheri-Mohammadi S, Alani B, Karimian M, Moradian-Tehrani R, Noureddini M (2019) Intranasal administration of endometrial mesenchymal stem cells as a suitable approach for Parkinson’s disease therapy. Mol Biol Rep 46:4293–4302
Wang SM, Lee CU, Lim HK (2019) Stem cell therapies for Alzheimer’s disease: is it time? Curr Opin Psychiatry 32:105–116
Sun Y, Zhang X, Li H, Xu S, Zhang X, Liu Y, Han M, Wen J (2018) Stemazole promotes survival and preserves stemness in human embryonic stem cells. FEBS J 285:531–541
Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol 16:508–518
Ulland TK, Song WM, Huang SCC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A et al (2017) TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170:649–663
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Front Cell Neurosci 7:45
Hristovska I, Pascual O (2016) Deciphering resting microglial morphology and process motility from a synaptic prospect. Front Integr Neurosci 9:73
Fakhoury M (2016) Immune-mediated processes in neurodegeneration: where do we stand? J Neurol 263:1683–1701
Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16
Town T, Nikolic V, Tan J (2005) The microglial" activation" continuum: from innate to adaptive responses. J Neuroinflamm 2:24
Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay MÈ (2014) Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. https://doi.org/10.1155/2014/610343
Shaked I, Porat Z, Gersner R, Kipnis J, Schwartz M (2004) Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J Neuroimmunol 146:84–93
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6:193
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimer’s Dement 12:719–732
Sominsky L, De Luca S, Spencer SJ (2018) Microglia: key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol 94:56–60
Milinkeviciute G, Henningfield CM, Muniak MA, Chokr SM, Green KN, Cramer KS (2019) Microglia regulate pruning of specialized synapses in the auditory brainstem. Front Neural Circuits 13:55
Zhang L, Dong ZF, Zhang JY (2020) Immunomodulatory role of mesenchymal stem cells in Alzheimer’s disease. Life Sci 246:117405
Shen Z, Li X, Bao X, Wang R (2017) Microglia-targeted stem cell therapies for Alzheimer disease: a preclinical data review. J Neurosci Res 95:2420–2429
Claes C, Van den Daele J, Verfaillie CM (2018) Generating tissue-resident macrophages from pluripotent stem cells: lessons learned from microglia. Cell Immunol 330:60–67
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290
Wojtera M, Sikorska B, Sobow T, Liberski PP (2005) Microglial cells in neurodegenerative disorders. Folia Neuropathol 43(4):311–321
Cunningham C (2013) Microglia and neurodegeneration: the role of systemic inflammation. Glia 61:71–90
Thei L, Imm J, Kaisis E, Dallas ML, Kerrigan TL (2018) Microglia in Alzheimer’s disease: a role for ion channels. Front Neurosci 12:676
Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest 127:3240–3249
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE et al (2014) Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci 17:131
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171
Mammana S, Fagone P, Cavalli E, Basile MS, Petralia MC, Nicoletti F, Bramanti P, Mazzon E (2018) The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci 19:831
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609
Bisht K, Sharma K, Tremblay MÈ (2018) Chronic stress as a risk factor for Alzheimer’s disease: roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol Stress 9:9–21
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA et al (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458
Wehrspaun CC, Haerty W, Ponting CP (2015) Microglia recapitulate a hematopoietic master regulator network in the aging human frontal cortex. Neurobiol Aging 36:2443-e9
Klegeris A, Bissonnette CJ, McGeer PL (2005) Modulation of human microglia and THP-1 cell toxicity by cytokines endogenous to the nervous system. Neurobiol Aging 26:673–682
Clayton KA, Van Enoo AA, Ikezu T (2017) Alzheimer’s disease: the role of microglia in brain homeostasis and proteopathy. Front Neurosci 11:680
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm 11:98
Zhang Q, Wu HH, Wang Y, Gu GJ, Zhang W, Xia R (2016) Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J Neurochem 136:815–825
Wei Y, Xie Z, Bi J, Zhu Z (2018) Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp Ther Med 16:5015–5020
Terashima T, Nakae Y, Katagi M, Okano J, Suzuki Y, Kojima H (2018) Stem cell factor induces polarization of microglia to the neuroprotective phenotype in vitro. Heliyon 4:e00837
Bagheri-Mohammadi S, Moradian-Tehrani R, Noureddini M, Alani B (2020) Novel application of adipose-derived mesenchymal stem cells via producing antiangiogenic factor TSP-1 in lung metastatic melanoma animal model. Biologicals. https://doi.org/10.1016/j.biologicals.2020.09.004
Zhang SC, Fedoroff S (1999) Expression of stem cell factor and c-kit receptor in neural cells after brain injury. Acta Neuropathol 97:393–398
Zhang SC, Fedoroff S (1997) Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J Neurosci Res 47:1–15
Jin K, Mao XO, Sun Y, Xie L, Greenberg DA (2002) Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest 110:311–319
Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, Emmrich F (2017) Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells 35:812–823
van Groen T, Kadish I, Wiesehan K, Funke SA, Willbold D (2009) In vitro and in vivo staining characteristics of small, fluorescent, Aβ42-binding D-enantiomeric peptides in transgenic AD mouse models. ChemMedChem 4:276–282
Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST, Mathis CA (2009) Carbon 11–labeled Pittsburgh compound b and carbon 11–labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 66:60–67
Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J (2008) Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci 28:11650–11661
Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A (2009) Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13–induced tissue responses and apoptosis. J Exp Med 206:1149–1166
Lee JK, Jin HK, Bae JS (2009) Bone marrow-derived mesenchymal stem cells reduce brain amyloid-β deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett 450:136–141
Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS (2012) Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging 33:588–602
Yun HM, Kim HS, Park KR, Shin JM, Kang AR, Il Lee K, Song S, Kim YB, Han SB, Chung HM, Hong JT (2013a) Placenta-derived mesenchymal stem cells improve memory dysfunction in an A β 1–42-infused mouse model of Alzheimer’s disease. Cell Death Dis 4:e958–e958
Ma T, Gong K, Ao Q, Yan Y, Song B, Huang H, Zhang X, Gong Y (2013) Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant 22:113–126
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW (2012) Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ 19:680–691
Kim JY, Kim DH, Kim DS, Kim JH, Jeong SY, Jeon HB, Lee EH, Yang YS, Oh W, Chang JW (2010) Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-β42 neurotoxicity in vitro. FEBS Lett 584:3601–3608
Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, Jin HK, Bae JS (2010) The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett 481:30–35
Kim HJ, Seo SW, Chang JW, Lee JI, Kim CH, Chin J, Choi SJ, Kwon H, Yun HJ, Lee JM, Kim ST (2015) Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimer’s Dement 1:95–102
Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, Totey SM (2010) Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res 155:62–70
Kang JM, Yeon BK, Cho SJ, Suh YH (2016) Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimer’s Dis 54:879–889
Duncan T, Valenzuela M (2017) Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther 8:111
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ (2012) Safe ty of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE 7:47559
Yun HM, Kim HS, Park KR, Shin JM, Kang AR, Il Lee K, Song S, Kim YB, Han SB, Chung HM, Hong JT (2013b) Placenta-derived mesenchymal stem cells improve memory dysfunction in an A β 1–42-infused mouse model of Alzheimer’s disease. Cell Death Dis 4:958–958
Acknowledgements
This work was supported by grants from the Vice Chancellor for Research and Technology, Kashan University of Medical Sciences, Kashan, Iran; and Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Dr. SB-M design and prepare the paper.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagheri-Mohammadi, S. Microglia in Alzheimer's Disease: The Role of Stem Cell-Microglia Interaction in Brain Homeostasis. Neurochem Res 46, 141–148 (2021). https://doi.org/10.1007/s11064-020-03162-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03162-4