Abstract
Alzheimer’s disease (AD) is a common neurodegenerative disease of progressive dementia which is characterized pathologically by extracellular neuritic plaques containing aggregated amyloid beta (Aβ) and intracellular hyperphosphorylated tau protein tangles in cerebrum. It has been confirmed that microglia-specific nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome-mediated chronic neuroinflammation plays a crucial role in the pathogenesis of AD. Stimulated by Aβ deposition, NLRP3 assembles and activates within microglia in the AD brain, leading to caspase-1 activation along with downstream interleukin (IL)-1β secretion, and subsequent inflammatory events. Activation of the NLRP3 inflammasome mediates microglia to exhibit inflammatory M1 phenotype, with high expression of caspase-1 and IL-1β. This leads to Aβ deposition and neuronal loss in the amyloid precursor protein (APP)/human presenilin-1 (PS1) mouse model of AD. However, NLRP3 or caspase-1 deletion in APP/PS1 mice promotes microglia to transform to an anti-inflammatory M2 phenotype, with decreased secretion of caspase-1 and IL-1β. It also results in improved cognition, enhanced Aβ clearance, and a lower cerebral inflammatory response. This result suggests that the NLRP3 inflammasome may be an appropriate target for reducing neuroinflammation and alleviating pathological processes in AD. In the present review, we summarize the generally accepted regulatory mechanisms of NLRP3 inflammasome activation, and explore its role in neuroinflammation. Furthermore, we speculate on the possible roles of microglia-specific NLRP3 activation in AD pathogenesis and consider potential therapeutic interventions targeting the NLRP3 inflammasome in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disease in older people that is characterized by progressive memory loss and cognitive disorder. The characteristic pathological changes of AD are extracellular senile plaques, which contain accumulated amyloid beta (Aβ), and intracellular neurofibrillary tangles (NFTs), which consist of hyperphosphorylated tau proteins. The abnormal accumulation of Aβ, especially oligomeric Aβ, in the brain is an early pathological feature of AD. It occurs years or even decades before the onset of any clinical symptoms, such as memory deterioration and cognitive dysfunction [1, 2]. The accumulation of Aβ is also accompanied by neuronal loss, microglial activation, inflammatory responses, oxidative stress, and other pathological changes [3]. Stimulated by Aβ plaques, microglia-specific nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome-mediated chronic neuroinflammation is involved in the pathogenesis of AD [4]. The NLRP3 inflammasome is an intracellular multimeric protein complex of innate immunity, and can be activated by many types of stimuli in response to cellular infection and stress or tissue damage. By increasing the expression of major histocompatibility complex II (MHC-II) on the cell surface, abnormal Aβ aggregation activates NLRP3 inflammasome in microglia. It also promotes caspase-1 activation, along with the subsequent secretion of interleukin (IL)-1β and IL-18, which eventually results in chronic inflammatory responses, neuronal death, and pyroptosis in the brain [4,5,6]. In the amyloid precursor protein (APP)/human presenilin-1 (PS1) mouse model of AD, aberrant NLRP3 activation mediates microglia to exhibit inflammatory M1 phenotype, which is characterized by increased Aβ deposits and a high expression of caspase-1 and IL-1β. However, APP/PS1 mice with microglial NLRP3 or caspase-1 deletion show less impaired spatial memory abilities and lower cerebral inflammatory responses. Furthermore, microglia exhibit anti-inflammatory M2 phenotype characterized by enhanced Aβ degradation and decreased caspase-1 and IL-1β release [7]. These results demonstrate that suppressing the activation of NLRP3 inflammasome may reduce neuroinflammation and alleviate the pathological processes of AD, and may therefore be a novel therapeutic strategy for this disease. In the current review, we summarize the generally accepted mechanisms of NLRP3 inflammasome activation and explore its role in neuroinflammation. Furthermore, we speculate on the possible roles of microglia-specific NLRP3 activation in AD pathogenesis, and raise potential therapies targeting the NLRP3 inflammasome.
The Activation of NLRP3 Inflammasome in AD
As the body’s leading line of defense, the innate immune system can detect various pathogens with a series of germline encoding pattern recognition receptors (PRRs). PRRs are mainly expressed by monocytes, macrophages, neutrophils, and dendritic cells, with the ability to defend against infection [8]. PPRs can detect pathogen-associated molecular patterns (PAMPs), including bacterial secretion systems, microbial nucleic acids, and danger-associated molecular patterns (DAMPs) such as adenosine tri-phosphate (ATP), uric acid crystals, heat shock proteins, and high mobility group box-1 (HMGB1) [9]. PPRs can be divided into two major classes depending on their subcellular localization. The first class of PRRs, termed Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), are membrane-spanning proteins located in the cytomembrane and endosomes, in which they can recognize extracellular PAMPs and DAMPs. The second class of PRRs reside in intracellular compartments and include RIG-I-like receptor (RLR), HIN-200 family member AIM2-like receptor (ALR), and NOD-like receptor (NLR) proteins [9]. A subset of PRRs (NLRs and ALRs) can assemble intracellular multimolecular complexes, named inflammasomes, which can generate potent inflammatory reactions in response to cellular infection and stress [8, 10]. Proteins in the NLR family are composed of a central NOD domain, C-terminal leucine-rich repeats (LRRs), and N-terminal caspase recruitment domains (CARD) or pyrin domains (PYD). Oligomerization of the NOD domain triggers inflammasome activation in an ATP-dependent manner. The CARD and PYD domains mediate interactions between homologous proteins for downstream signaling pathways [8, 9]. The inflammasome consists of a nucleotide binding and oligomerization domain (NACHT) or HIN domain, an apoptosis-associated speck-like protein containing a CARD (ASC), and a precursor of cysteine-containing aspartate-specific proteases (procaspase-1) [8]. To date, some inflammasomes have been confirmed, including NLRP1, NLRP3, AIM2, and IPAF inflammasomes. Of these, the most widely studied and characterized is the NLRP3 inflammasome, which consists of the NLRP3 scaffold, ASC adaptor, and procaspase-1 [8]. A variety of different stimulators have been confirmed to induce NLRP3-dependent caspase-1 activation, such as bacterial infection, endogenous metabolites (ATP, Aβ, urate crystals, and cholesterol crystals), and exogenous crystalline particles (alum, asbestos, and silica). The NLRP3 inflammasome engages in a variety of metabolic and inflammatory diseases, such as gout, diabetes, atherosclerosis and neurodegenerative diseases (especially AD and Parkinson's disease) [9]. Increasing evidence has indicated that misfolded protein aggregates, such as Aβ and alpha-synuclein, might stimulate NLRP3 activation in microglia [11, 12]. NLRP3 activation in protein-misfolding diseases of central nervous system is mainly concentrated in astrocytes and microglia [13].
Active caspase-1 and IL-1β are the products of NLRP3 inflammasome activation. Studies have demonstrated that active caspase-1 and IL-1β levels are increased in microglia in the brains of animal models and patients with AD [7, 12, 14]. Neuritic plaques in AD recruit microglia to phagocytose the aggregated Aβ, especially oligomer and fibrillar Aβ (fAβ). This then stimulates NLRP3 inflammasome activation, with a subsequent release of proinflammatory cytokines (IL-1β and IL-18) and potentially neurotoxic factors. This release enhances the neurotoxic effects of Aβ and aggravates the pathological processes of AD [12, 15,16,17]. In transgenic APP/PS1 mice, Aβ activates NLRP3 inflammasome in microglia and mediates microglia to exhibit inflammatory M1 phenotype. This phenotype microglia are characterized by a high expression of caspase-1 and IL-1β, which result in increased hippocampal and cortical Aβ deposition, neuronal loss, and cognitive impairment [7]. In contrast, NLRP3 or caspase-1 deletion in APP/PS1 mice causes microglia to exhibit anti-inflammatory M2 phenotype, with decreased caspase-1 and IL-1β secretion. NLRP3 or caspase-1 deletion also significantly reduced amyloid burden and clearly improved cognition (Fig. 1) [7, 18]. Notably, NLRP3 activation in APP/PS1 mice is limited to plaque-associated microglia in the brain [7], and does not occur in any other central nervous system cells; this indicates that microglia-specific NLRP3 activation contributes to AD pathogenesis. The intrahippocampal injection of ASC specks in APP/PS1 mice promotes Aβ plaque formation and accumulation. However, it fails to induce the spreading of Aβ pathology in ASC-deficient APP/PS1 mice [19]. We therefore conclude that abnormal microglia-specific NLRP3 activation induces chronic neuroinflammation in the pathological processes of AD, leading to microglial Aβ phagocytic dysfunction, peripheral neuronal damage, and serious pathological injury [20]. However, this process might be altered by the microglia-specific destruction of NLRP3 inflammasome. Furthermore, excessive NLRP3 activation and elevated IL-1β levels in microglia may also aggravate neuronal tau hyperphosphorylation, neurofibrillary tangles, and synaptic dysfunction in AD by inducing a detrimental chronic inflammatory reaction [21,22,23,24]. ASC or NLRP3 deficiency has been reported to decrease tau pathology and protect against cognitive impairment in tau transgenic mice [25, 26]. Furthermore, IL-1β suppression in the triple transgenic (3xTg) mouse model of AD leads to rescued cognition, attenuated tau pathology, and restored neuronal beta-catenin pathway function [27]. These studies offer some insights into the possible functional mechanisms of the NLRP3 inflammasome in AD. They also suggest that, by regulating NLRP3 inflammasome, we might be able to reduce inflammatory responses and alleviate the pathological processes of AD.
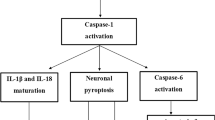
Proposed mechanisms of microglia-specific NLRP3 mediated neuroinflammation and neurotoxicity in APP/PS1 mice. In transgenic APP/PS1 mice, Aβ activates NLRP3 in microglia and induces microglia to exhibit inflammatory M1 phenotype (left). This phenotype microglia are characterized by a high expression of NOS2, activated caspase-1 and IL-1β, which result in increased hippocampal and cortical Aβ deposition, neuronal loss, and cognitive impairment. In contrast, NLRP3 or caspase-1 deletion in APP/PS1 mice causes microglia to exhibit anti-inflammatory M2 phenotype (right), with increased M2 markers (Fizz1, Arg-1) and decreased caspae-1 and IL-1β secretion. NLRP3 or caspase-1 deletion also leads to enhanced Aβ clearance, preserved neurons and improved cognition. APP amyloid precursor protein, PS1 human presenilin-1, NOS2 nitric oxide synthase 2, Fizz1 found in inflammatory zone 1, Arg-1 arginase-1
The Mechanisms of NLRP3 Inflammasome Activation
It is generally acknowledged that NLRP3 inflammasome activation, induced by multiple exogenous and endogenous activators, needs two signals. The first signal (the priming signal) is the nuclear factor kappa B (NF-κB)-dependent transcription of NLRP3 and pro-IL-1β, which is triggered by the binding of the TLR4 ligand lipopolysaccharide (LPS) to its receptor. The signal can then promote the expression of the inflammasome constituents: NLRP3, procaspase-1, and pro-IL-1β [9, 28, 29]. The first signal is initiated by TLR4 and is then relayed by its involved adaptor molecules, including MyD88, IRAK1, and IRAK4, without any requirement for the synthesis of new proteins [30,31,32]. The second signal (the activation signal) involves the assembly and activation of the NLRP3 complex, which is induced by extracellular ATP, certain bacterial toxins, crystalline and particulate matters. This signal results in the generation of active cleaved caspase-1. When microglia are activated by the NLRP3 activators as part of the second signal, NLRP3 carries out its own oligomerization via homotypic NACHT domain interactions and recruits PYD domains to interact with the PYD of ASC, which then triggers ASC fibrillar assembly [33]. Next, ASC assembly recruits CARD to interact with the CARD domain of procaspase-1, resulting in caspase-1 activation and the subsequent polymerization of ASC fibrils into a large filamentous protein complex, termed ASC speck [34]. Clustered procaspase-1 mediates its autocleavage and activation in the form of activated caspase-1. This active caspase-1 can then cleave the precursors of IL-1β and IL-18 to generate the activated forms IL-1β and IL-18, thereby generating inflammatory responses and driving pyroptosis [35, 36]. In this process, post-translational modifications of NLRP3, such as deubiquitination and phosphorylation, are necessary steps for NLRP3 inflammasome assembly and activation (the second signal) [31, 37]. It has been proposed that ubiquitin chains can be removed from the NLRP3 LRR motifs by the K63-specific deubiquitinase BRCC3 [38]; however, the exact mechanisms by which it promotes NLRP3 inflammasome assembly remain unknown. Upon triggering the activation of NLRP3, ASC speck formation can be considered as an upstream readout of NLRP3 activation [39]. There are currently three probable models that have been raised to explain the mechanisms of NLRP3-mediated caspase-1 activation (the second signal), and these may not be mutually exclusive (Fig. 2) [40].
The priming signal and activation signal of the NLRP3 inflammasome in microglia. The priming signal (blue line) is NF-κB-dependent transcription of NLRP3 and pro-IL-1β, which is triggered by the binding of the TLR4 ligand LPS to its receptors. It can promote the expression of NLRP3 and pro-IL-1β. This signal is initiated by TLR4 and in then relayed its adaptor molecules, including MyD88, IRAK1, and IRAK4. The activation signal (black line) involves the assembly and activation of the NLRP3 complex, which is induced by extracellular ATP, certain bacterial toxins, crystalline and particulate matters. This signal results in the generation of active cleaved caspase-1, which then promotes the cleavage of IL-1β and IL-18 and subsequent inflammatory response. Three probable models have been put forward to explain the mechanisms of NLRP3-mediated caspase-1 activation (the activation signal): (1) Extracellular ATP stimulates the P2X7 ATP-gated ion channel, which initiates a rapid K+ efflux and causes the formation of an endogenous membrane pore via the pannexin-1 hemichannel. Pannexin-1 hemichannel, as well as pores formed by bacterial toxins, permit the translocation of extracellular NLRP3 agonists to the cytoplasm where they can directly activate NLRP3. (2) ROS generation can be induced by all DAMPs and PAMPs. With increased intracellular ROS, TXNIP dissociates from TRX and interacts with NLRP3, resulting in NLRP3 activation. (3) The phagocytosis of crystalline or particulate by macrophages leads to lysosomal rupture and the release of cathepsin B into the cytoplasm, which induce NLRP3 activation. This progress can be blocked by cathepsin B inhibitor CA-074Me. NF-κB nuclear factor kappa B, IL-1β interleukin-1β, TLR4 toll-like receptor 4, LPS lipopolysaccharide, ROS reactive oxygen species, DAMPs danger-associated molecular patterns, PAMPs pathogen-associated molecular patterns, TXNIP thioredoxin-interacting protein, TRX thioredoxin
The Ion Channel Model
Substantial K+ efflux is a critical upstream process of NLRP3 activation in the first model, and can be induced by many NLRP3 activators, such as some small molecules (e.g., nigericin, ATP, or uric acid) [41]. ATP is a kind of NLRP3-activating DAMP, and is induced by cellular stress reaction or necrosis. Extracellular ATP activates the P2X7 ATP-gated ion channel, which initiates a rapid K+ outflow and causes the formation of an endogenous membrane pore via the hemichannel pannexin-1 [28, 42]. Pannexin-1 is a gap junction protein that can carry ions and microbial molecules between the cytoplasmic and extracellular regions [28, 42]. It has been suggested that pannexin-1 hemichannels, as well as pores formed by bacterial toxins, permit the translocation of extracellular bacterial products, such as muramyl dipeptide (MDP), to the cytoplasm where they can directly activate NLRP3 [43]. These pores also allow K+ efflux from the cytoplasm. These results indicate that low concentration of cytosolic K+ or membrane pore formation can be recognized by NLRP3 [8]. Nevertheless, macrophages stimulated with ATP alone could trigger K+ outflow without triggering NLRP3-mediated caspase-1 activation. Furthermore, unless they are prestimulated with the microbial ligand LPS, neither silica, asbestos, nor aluminiun hydroxide can induce NLRP3 activation in macrophages [44, 45]. These results manifest that low intracellular K+ concentration is in sufficient to activate NLRP3, and that microbial molecules may be an essential substance that cooperates with P2X7R and bacterial toxins to induce NLRP3 activation. However, given that there is no evidence of a direct link between NLRP3 and its stimuli, and considering the diversity and complexity of NLRP3 activators, it is difficult to suppose that NLRP3 can recognize more extracellular activators directly. In addition, a number of NLRP3 agonists are too large to translocate to the cytoplasm through ion channels or membrane pores [46]. Thus, the ion channel model cannot explain NLRP3 activity in response to all NLRP3 agonists, and K+ efflux is independent but is not specific for NLRP3 activation [47].
The Reactive Oxygen Species (ROS) Model
The second model of NLRP3-mediated caspase-1 activation holds that ROS production, especially from mitochondria and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, is an important upstream signal of NLRP3 inflammasome activation [44, 48,49,50,51]. ROS generation can be induced by all DAMPs and PAMPs, including ATP and particulate/crystalline agonists that require phagocytosis [44]. Research has demonstrated that numerous NLRP3 agonists can stimulate mitochondria-derived ROS generation [52]. While, ROS generation can be stimulated by NADPH oxidases only upon particle phagocytosis. Notably, NLRP3 inflammasome mediated IL-1β up-regulation is influenced by mitochondrial damage and increased ROS levels in the cytoplasm and mitochondria [52]. Moreover, both ROS scavengers and suppression of the common p22 subunit of NADPH oxidase can block the activation of NLRP3 by various agonists, thus demonstrating that ROS production is essential for NLRP3 activation [44]. Nevertheless, the specific process by which NLRP3 inflammasome recognize ROS generation remains unclear. However, there is evidence indicating that NLRP3 inflammasome cannot be activated by some ROS-inducing substances, such as cytokines, suggesting that ROS may be necessary even though ROS itself is insufficient to stimulate NLRP3 activation [8]. Furthermore, high concentrations of ROS inhibitors prevent the initial step of NLRP3 inflammasome activation, but do not affect the nigericin- and silica-stimulated direct activation of NLRP3 [53].
Both ROS production and K+ outflow often co-exist in ROS-dependent NLRP3 activation [54]. The interactions between ROS generation and K+ efflux remain undefined, but it is likely that either low cytoplasmic K+ concentration triggers ROS generation, or that low cytoplasmic K+ concentration is required for NLRP3 activation, without depending on ROS. ROS-dependent NLRP3 activation and K+ outflow by NLRP3 activators, except ATP, do not depend on the activity of the P2X7 ion channel. The ROS-dependent NLRP3 ligand thioredoxin-interacting protein (TXNIP) participates in NLRP3 inflammasome activation. The association of TXNIP with NLRP3 in human macrophages is triggered by NLRP3 agonists [55]. In macrophages with no such stimulation, TXNIP binds to the oxidoreductase thioredoxin and can also be suppressed by this enzyme. With increased intracellular ROS, TXNIP dissociates from oxidized thioredoxin (TRX) in a ROS-dependent manner and interacts with NLRP3 (mainly with the LRRs), resulting in NLRP3 inflammasome activation [55]. TXNIP deficiency or deletion inhibits NLRP3 and caspase-1 activation as well as subsequent IL-1β secretion [55]. Conversely, NLRP3 activation can be improved by knocking down the TXNIP inhibitor thioredoxin [44]. Thus, it is clear that TXNIP acts as an upstream activating ligand of the NLRP3 inflammasome. It is also noteworthy that capase-1 activation cannot be completely inhibited by a lack of TXNIP [46]. Other regulatory factors of NLRP3 activity, or other pathways that act together with the ROS signaling pathway to trigger NLRP3 inflammasome activation, may therefore exist.
The Lysosomal Rupture Model
The third model of lysosomal rupture applies to NLRP3 activation that is induced by crystalline and particulate activators, and closely considers the size of the stimuli [12, 36]. When large particulate or crystalline activators (such as silica, asbestos, or Aβ) are endocytosed by macrophages, the phagolysosome loses stability. This results in lysosomal rupture and the release of proteinase cathepsin B into the cytoplasm, which induce NLRP3 inflammasome activation [36, 52, 56]. This result is consistent with a study reporting that multiple cathepsins (B, L, C, S and X) promote NLRP3-dependent IL-1β activation [57]. It has also been demonstrated that NLRP3 activation can be impaired by cathepsin B inhibitor CA-074Me, through the inhibition of lysosomal phagocytosis [58]. However, cathepsin B deletion in mouse macrophages has no effect on particulate activator-induced NLRP3 activation or IL-β secretion, implying that the cathepsin B inhibitor has an unidentified off-target effect on NLRP3 [44]. However, no direct ligand-receptor interactions between NLRP3 and cathepsin B have yet been identified. In addition, some particulates, such as silica, calcium pyrophosphate crystals (CPPD), L-leucyl-L-leucine methyl ester (LL-OMe) and Al(OH)3, are phagocytosed by bone marrow-derived macrophages (BMDMs). This can lead to lysosomal damage and trigger K+ efflux by opening membrane pores, thus inducing NLRP3 inflammasome activation [28, 59]. Nevertheless, the exact mechanisms of particulate-induced lysosomal damage and K+ outflow remain to be clarified.
Is there relationship between these three models? The ROS and lysosomal rupture models might both be useful to explain the activation of NLRP3 inflammasome by particulates. Furthermore, NADPH oxidases and mitochondrial damage are potential sources of ROS in macrophages, and the inefficient removal of large phagocytosed particulates may induce excessive ROS generation on the way to lysosomes, where they can give rise to lysosomal rupture [44]. Thus, lysosomal rupture induced by phagocytosis can be identified as a common part of the ROS pathway [46]. In addition, tiny stimuli, such as ATP, might also stimulate ROS generation by activating P2X7 receptors differently in the ion channel model. ROS generation is considered a common upstream signal of ATP- and particulate-induced NLRP3 activation [44].
Microglia-Specific NLRP3 Activation in AD
In the process of microglial NLRP3 activation in AD, lysosomal rupture has been reported, as well as subsequent Aβ-stimulated ROS generation [60, 61]. However, the precise mechanisms of microglia-specific NLRP3 inflammasome activation in AD have not yet been fully elucidated. Aβ oligomers and fibrils directly interact with NLRP3 and promote NLRP3 and ASC interaction in a cell free system [60]. In the priming signal (the first signal) of Aβ-induced NLRP3 activation, the microglial surface receptors CD36 and TLR4 are required [62]. Aggregated Aβ (especially oligomeric Aβ and fAβ) binds to the class B scavenger receptor CD36, and then forms complexes with TLR4 and TLR6 [63,64,65,66,67]. This process triggers the priming of NLRP3 activation and promotes the translocation of NF-κB from the cytoplasm to the nucleus, leading to the transcription of NLRP3 and pro-IL-1β.
In the activation signal (the second signal), NLRP3 assembly and NLRP3-mediated caspase-1 activation in AD most probably occurs via the lysosomal rupture pathway and the ROS generation pathway (Fig. 3) [61]. When fAβ is phagocytosed by microglia, fAβ aggregates in the phagosome and promotes phagosome to combine with lysosome, which result in lysosomal rupture and subsequent cathepsin B release into the cytoplasm [12]. Lysosomal rupture stimulates NLRP3 complex assembly, caspase-1 maturation, and IL-1β secretion. This proposed pathway is in accordance with a previous research that found high levels of cathepsin B in Aβ plaques [68]. In addition, cathepsin B inhibitors CA-074Me and E64d have been demonstrated to reduce memory deficits and decrease Aβ plaque loads in transgenic AD mice [69]. Furthermore, a study has confirmed that oligomeric Aβ induces the high expression of active NLRP3 and caspase-1 via mitochondrial ROS generation, as well as partially through NADPH oxidase-derived ROS production in AD [61]. Thus, the inefficient removal of fAβ probably stimulates excessive ROS generation on the way to lysosomes, giving rise to NLRP3 activation in a ROS-dependent manner [44]. Both pathways promote the generation of activated caspase-1 and the subsequent secretion of inflammatory factors (e.g., IL-1β and IL-18) in AD, leading to pyroptosis and neuronal death [70]. However, the mechanisms of fAβ-induced activation of the NLRP3 inflammasome cannot be explained in just one pattern, and more signaling pathways may be involved. More detailed and precise mechanism studies are therefore required, to clarify the relationships and interactions between NLRP3 activation and other signaling pathways in AD.
Possible mechanisms of microglia-specific NLRP3 activation in Alzheimer’s disease (AD) and potential therapeutic interventions for AD. In the priming signal (the first signal), aggregated Aβ binds to the class B scavenger receptor CD36 on microglial surface and then forms complexes with TLR4 and TLR6, which triggers the transcription of NLRP3 and pro-IL-1β in a NF-κB-dependent manner. In the activation signal (the second signal), NLRP3 assembly and NLRP3-mediated caspase-1 activation in AD most probably occurs via lysosomal rupture pathway and ROS generation pathway. The phagocytosis of aggregated fAβ by microglia promotes lysosomal rupture and subsequent cathepsin B release into the cytoplasm, thereby stimulating NLRP3 assembly and caspase-1 activation. Furthermore, Inefficient removal of fAβ probably induces mitochondrial ROS generation, as well as partially NADPH oxidase-derived ROS production, which promotes NLRP3 activation in a ROS-dependent way. Both pathways promote the generation of activated caspase-1 and the subsequent secretion of inflammatory factors (e.g., IL-1β and IL-18) in AD. Overview the potential drug interventions for AD: (1) IL-1β antibodies and IL-1R antagonists inhibit the binding of IL-1β to IL-1RI and block IL-1β signals. (2) MCC950 and CY-09 specifically inhibit NLRP3 activation by interacting with NACHT domain and blocking ATP hydrolysis (which is essential for NLRP3 oligomerization), thus maintaining NLRP3 in an inactive state. (3) POPs interact with ASC and directly block the combination of ASC to NLRP3, thus hindering subsequent caspase-1 activation. (4) The effect of NSAIDs on NLRP3 is probably via the reversible inhibition of membrane volume-regulated anion channel. NSAIDs can also inhibit the nuclear translocation of NF-κB, which is essential for transcription of NLRP3 and pro-IL-1β. (5) Damaged or depolarized mitochondria release ROS and oxidized mitochondrial DNA, which are NLRP3 inflammasome agonists and can be removed by autophagy and autophagy-related proteins. TLR4 toll-like receptor 4, NADPH nicotinamide-adenine dinucleotide phosphate, IL-1RI type I IL-1 receptor, NACTH nucleotide-binding and oligomerization, ASC apoptosis-associated speck-like protein containing a caspase recruitment domain, POPs PYD-only proteins, NSAIDs nonsteroidal anti-inflammatory drugs, COX cyclooxygenase
Potential Therapeutic Interventions
As mentioned in the previous paragraphs, microglial NLRP3 inflammasome activation is a crucial characteristic of AD pathogenesis. Research has shown that NLRP3 or caspase-1 deficiency dramatically reduces the amyloid burden and results in less cognitive impairment in mouse model [7]. Furthermore, it has been reported that IL-1β inhibition in 3 × Tg-AD mice significantly decreases brain neuroinflammation, relieves cognitive impairment, and partially reduces Aβ deposition [27]. These findings indicate that the inhibition of NLRP3 inflammasome activation at the molecular level may be a novel therapeutic intervention for AD. Therapeutic strategies aimed at inflammasome constituents or downstream products can therefore be designed to tackle neuroinflammation and slow the progression of AD (Fig. 3).
IL-1β Antibodies and IL-1R Antagonists
As the important downstream molecule of activated NLRP3 inflammasome, IL-1β is a possible target for AD treatment. Currently, IL-1β suppression is a therapeutic tactic in different kinds of diseases [71]. The mediators of IL-1β signal transduction are type I IL-1 receptor (IL-1RI) and type II IL-1 decoy receptor (IL-1RII). When IL-β binds to IL-1RI, the inflammatory signaling pathway is triggered, resulting in the secretion of inflammatory mediators, cytokines, and chemokines. In contrast, although it has an analogous affinity for IL-1β to IL-1RI, IL-1RII cannot initiate or even attenuate IL-1β signal transduction events [72]. An endogenous IL-1 receptor antagonist (IL-1Ra) may also modulate IL-1β function by inhibiting the binding of IL-1β to IL-1RI and blocking IL-1 signals [73].
To date, three medications directed against IL-1β have been applied as therapies for inflammatory diseases: the IL-1R antagonist anakinra, the soluble decoy receptor rilonacept, and the neutralizing monoclonal anti-IL-1β antibody canakinumab [74, 75]. Using anakinra to specifically block IL-1β activity has been successful in the treatment of multiple inflammatory and non-inflammatory diseases, including rheumatoid arthritis, familial Mediterranean fever, cryopyrin-associated periodic syndrome, gout [71], type 2 diabetes mellitus (T2DM) [76], and heart failure [2]. Anakinra has a marked effect on blood glucose control and islet beta cell function, and it can reduce inflammatory factors when combined with canakinumab in the treatment of T2DM patients [22]. Moreover, some studies have emphasized that IL-1β may be a potential therapeutic strategy for AD. For example, when exposed to exogenous Aβ, IL-1Ra knockout mice exhibit enhanced microglial activation and neuronal impairment [77]. Another study demonstrated that chronic dosing of 3 × Tg-AD mice with IL-1R blocking antibody markedly reduces cerebral inflammation, improves cognition, and alleviates tau pathology, as well as partly decreasing fAβ levels [27]. However, IL-1β is not the only product of NLRP3 inflammasome, and it participates in many other inflammatory responses. This means that IL-1β inhibitors lack specificity, which may result in unintended immunosuppressive effects and many side effects. Thus, although IL-1β inhibitors are promising for the treatment of AD, they may not be well tolerated by all individuals.
Specific Inhibitors of the NLRP3 Inflammasome
The integrity of NLRP3 constituents as well as the assembly of the NLRP3 complex is necessary for inflammasome activation. Hence, other potential therapeutic targets include the constituents of the NLRP3 inflammasome (including the NACHT domain, ASC, and caspase-1) and NLRP3 assembly. Pharmacological inhibitors specific to the NLRP3 inflammasome may be an optimal treatment for AD. Here, we discuss several pharmacological inhibitors of NLRP3 inflammasome activation and their therapeutic targets.
MCC950
A selective small molecule inhibitor, termed MCC950, specifically suppresses NLRP3-induced ASC oligomerization and activation by interacting with the NACHT domain and blocking ATP hydrolysis (which is essential for NLRP3 oligomerization), thus maintaining NLRP3 in an inactive state [78, 79]. In mouse model of multiple sclerosis, MCC950 reduced IL-1β secretion and alleviated the severity of experimental autoimmune encephalomyelitis [80]. However, the priming process and TLR signaling of NLRP3 activation were not suppressed by MCC950, and K+ efflux and NLRP3-ASC interactions were not affected [80]. Hence, it is likely that MCC950 combines with NLRP3 and regulates its activation via post-translational modifications [59, 80]. In a mouse model of Parkinson’s disease, treatment with oral MCC950 inhibited α-synuclein-mediated NLRP3 activation, and also reduced motor dysfunction, α-synuclein aggregates and dopaminergic neurodegeneration. Moreover, MCC950 promoted Aβ clearance and cognitive function by suppressing the NLRP3 inflammasome in APP/PS1 mice [81]. Additionally, in tau transgenic mice, chronic intracerebral injection of MCC950 inhibited exogenous tau pathology [26]. MCC950 is therefore a potential treatment strategy to target the NLRP3 inflammasome in AD, and deserves to be further evaluated in animal experiments and clinical trials.
PYD-Only Proteins (POPs)
ASC is an adaptor protein that is necessary for inflammasome function. In ASC−/− mice, the ASC deletion results in the inhibition of caspase-1 activation and mature IL-1β secretion induced by pathogens, asbestos, silica, or monosodium urate crystals (MSU) [44, 82, 83]. Moreover, it has been reported that ASC specks promote Aβ deposition in APP/PS1 mice. However, ASC deficiency or the application of ASC antibodies led to less Aβ deposition in the brain and improvements in spatial-memory in APP/PS1 mice [84]. PYD-only protein (POP)1 and POP2 inhibit inflammasome activation by directly interacting with ASC and blocking PYD interactions between NLRP3 and ASC, thus hindering the combination of ASC to NLRP3 as well as subsequent caspase-1 activation [8, 85,86,87,88,89,90]. Furthermore, NF-κB activation can also be suppressed by POP1 and POP2; thus, the initial signal of inflammasome activation is inhibited [89, 91]. POPs may be a potential regulatory mechanism to “fine-tune” NLRP3 inflammasome activation in AD.
CY-09
The compound CY-09 has potent anti-inflammatory activity for the NLRP3 inflammasome, and its suppressive effect does not depend on priming signals or post-translational modifications [59]. The ATPase activity of NLRP3 is crucial for NLRP3 oligomerization and activation [92]. CY-09 specifically inhibits NLRP3 assembly and activation by directly interacting with the ATP-binding motif of the NLRP3 NACHT domain and inhibiting the ATPase activity that is necessary for NLRP3 oligomerization [92, 93]. It has been confirmed that CY-09 has therapeutic effects in mouse models of both cryopyrin-associated periodic syndrome and T2DM [93]. Additionally, CY-09 efficiently suppressed NLRP3 activation in monocytes from healthy individuals or synovial cells from patients with gout [93]. Furthermore, CY-09 markedly decreased collagen- and ADP-induced human platelet aggregation via the inhibition of NLRP3 inflammasome [94]. Together, these results suggest that CY-09 may be a novel therapeutic approach for NLRP3-associated diseases. The effects of CY-09 have not yet been reported in any AD models, and its application in AD models should be performed as soon as possible.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs suppress the generation of prostaglandins and thromboxane by inhibiting their main targets, cyclooxygenase (COX)-1 and COX-2 [95], which ultimately reduces the inflammatory response. COX-2 overexpression in neurons resulted in neuronal apoptosis and cognitive disorder in COX-2 transgenic mouse model [96]. Furthermore, Aβ plaque deposition was increased in a cross of COX-2 transgenic mice with APP/PS1 mice [97], indicating that COX-2 aggravates the pathological features of AD. Therefore, the pathological reaction of AD might be reduced by the anti-inflammatory action of NSAIDs. The chronic treatment of Tg2576 mice with ibuprofen for 6 months decreased the number and total area of Aβ deposition, activated IL-1β, and microglial activation [98]. The reduction in Aβ plaques in this mouse model was roughly equivalent to the reduced numbers of activated microglia [98]. Some widely used NSAIDs (e.g., mefenamic acid) can selectively inhibit NLRP3 inflammasome activation (e.g., ASC speck, active caspase-1, and IL-1β) in macrophages and mitigate cognitive impairment in a mouse model of AD [99]. The effect of NSAIDs on NLRP3 is probably via the reversible inhibition of membrane volume-regulated anion channel (VRAC), independent of COX enzymes [99]. Nevertheless, there may be some discordant consequences. For example, cerebral prostaglandins were reduced by 90% in Tg2576 mice treated with indomethacin, but hippocampal Aβ was decreased by only 20% and cortical Aβ was unchanged [100]. The reasons for the different effects of NSAIDs on Aβ might be a result of the off-target effects of most NSAIDs, which do not only involve COX-1 or COX-2. NSAIDs can inhibit the nuclear translocation of NF-κB, which is essential for the transcription of NLRP3 and pro-IL-1β [95]. Furthermore, γ-secretase and Aβ generation can be inhibited or modulated by certain NSAIDS [101, 102]. In view of the multiple targets of NSAIDs, it is difficult to link them to anti-inflammatory effects alone. Thus, if NSAIDs are useful for AD treatment, their effects are most likely achieved through their multi-target mechanisms.
Autophagy and Autophagy-Related Proteins
Autophagy is a physiological cellular self-protective process whereby aggregated proteins, pathogens or impaired organelles are aggregated in intracellular autophagosomes, and then degraded in lysosomes by their hydrolytic enzymes. Microglia can degrade fAβ through lysosomal mediated autophagy. Autophagy dysfunction leads to impaired Aβ clearance and is involved in AD pathogenesis [103]. Autophagy has been demonstrated to modulate inflammasome activation, including by eliminating inflammasome-activating stimuli and by degrading inflammasome constituents [104]. Moreover, autophagy promotes the degradation of extracellular Aβ fibrils by microglia, and it also inhibits Aβ-induced NLRP3 inflammasome activation [105]. Autophagy also plays a crucial role in regulating the subsequent release of IL-1β [104]. Aβ-induced NLRP3-dependent IL-1β expression in astrocytes can be efficiently inhibited by the autophagy agonist rapamycin [106]. In addition, deficiency of autophagic proteins (ATG3, ATG5, ATG7, ATG16L1, Beclin1, and LC3B) can increase caspase-1 activation and mature IL-1β secretion [107,108,109]. Some researchers have concluded that autophagy-related proteins regulate NLRP3 inflammasome by suppressing mitochondrial damage [108]. Damaged or depolarized mitochondria release ROS and oxidized mitochondrial DNA, which are NLRP3 inflammasome agonists and can be removed by autophagy [50, 108]. Furthermore, deficiency of the gene encoding the autophagy adaptor SQSTM1/p62 in macrophages leads to high amounts of impaired mitochondria and exaggerated inflammasome-mediated inflammatory response [110]. These results suggest that functional autophagy indirectly inhibits inflammasome activation. There is also evidence that autophagosomes may target inflammasomes for degradation [111]. The autophagy-lysosome pathway is a specific molecular mechanism that regulates NLRP3 activation in a multi-target manner. We presume that the enhancement of microglial autophagy may be a potential therapeutic strategy for AD. However, the exact mechanisms of autophagy-dependent inflammasome suppression remain unclear now. Further study is needed to illuminate the interactions between the inflammasome activation pathway and the autophagy-lysosome pathway.
Conclusions and Future Directions
It is generally accepted that chronic neuroinflammation, mediated by microglia-specific activation of NLRP3 inflammasome plays a crucial role in AD pathogenesis. The deletion of NLRP3 or caspase-1 in the APP/PS1 mouse model of AD leads to significantly improved cognition, enhanced Aβ clearance, and decreased caspase-1 activation along with IL-1β release. These findings indicate that the NLRP3 inflammasome may be a promising target for AD treatment. Nevertheless, many issues remain to be resolved, such as the clarification of the exact mechanisms regulating NLRP3 activation, as well as the interactions between NLRP3 activation and other signaling pathways in AD pathology. Furthermore, it remains unclear whether changes occur in the activated states of NLRP3 inflammasome in microglia during the progress of AD. A more accurate understanding of the complex regulatory mechanisms of NLRP3 activated signaling pathways is required to precisely fine-tune the inflammatory responses, and might offer further clues for treatment strategies in AD.
References
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer's disease. Lancet 368:387–403
Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW (2010) Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 105:1371–1377.e1371
Balducci C, Forloni G (2011) APP transgenic mice: their use and limitations. Neuromol Med 13:117–137
Cameron B, Landreth GE (2010) Inflammation, microglia, and alzheimer's disease. Neurobiol Dis 37:503–509
Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154:204–219
Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Schroder K, Tschopp J (2010) The Inflammasomes. Cell 140:821–832
Lamkanfi M, Dixit Vishva M (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022
Lamkanfi M, Kanneganti TD, Franchi L, Nunez G (2007) Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol 82:220–225
Masters SL, O'Neill LA (2011) Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med 17:276–282
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Shi F, Kouadir M, Yang Y (2015) NALP3 inflammasome activation in protein misfolding diseases. Life Sci 135:9–14
Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL 3rd, Araoz C (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in down syndrome and Alzheimer disease. Proc Natl Acad Sci USA 86:7611–7615
Prinz M, Priller J, Sisodia SS, Ransohoff RM (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14:1227–1235
Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122
Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT (2008) Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature 451:720–724
Goldmann T, Tay TL, Prinz M (2013) Love and death: microglia, NLRP3 and the Alzheimer's brain. Cell Res 23:595–596
Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT (2017) Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer's disease. Nature 552:355–361
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci 28:8354–8360
Heneka MT (2017) Inflammasome activation and innate immunity in Alzheimer's disease. Brain Pathol 27:220–222
Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T (2012) Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126:2739–2748
Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci 25:8843–8853
Sheng JG, Zhu SG, Jones RA, Griffin WS, Mrak RE (2000) Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol 163:388–391
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, Griep A, Santarelli F, Brosseron F, Opitz S, Stunden J, Merten M, Kayed R, Golenbock DT, Blum D, Latz E, Buée L, Heneka MT (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575:669–673
Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, Lodder C, Brône B, Huaux F, Octave JN, Terwel D, Dewachter I (2019) Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol 137:599–617
Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, Cribbs DH, LaFerla FM (2011) Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer's disease model. J Immunol 187:6539–6549
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith Brenna L, Rajendiran Thekkelnaycke M, Núñez G (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791
Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES (2013) Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol 191:3995–3999
Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287:36617–36622
Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA, Pasare C (2014) IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci USA 111:775–780
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156:1193–1206
Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 274:33835–33838
Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9:847–856
Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D (2013) Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem 288:2721–2733
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49:331–338
Stutz A, Horvath GL, Monks BG, Latz E (2013) ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 1040:91–101
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10:241–247
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589
Deplano S, Cook HT, Russell R, Franchi L, Schneiter S, Bhangal G, Unwin RJ, Pusey CD, Tam FWK, Behmoaras J (2013) P2X7 receptor-mediated Nlrp3-inflammasome activation is a genetic determinant of macrophage-dependent crescentic glomerulonephritis. J Leukoc Biol 93:127–134
Kanneganti TD, Lamkanfi M, Nunez G (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27:549–559
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677
Franchi L, Nunez G (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol 38:2085–2089
Tschopp J, Schroder K (2010) NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10:210–215
Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Cikovic T, Hartjes L, Smollich J, Robertson AAB, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Gross O (2016) K(+) efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45:761–773
Brown DI, Griendling KK (2009) Nox proteins in signal transduction. Free Radic Biol Med 47:1239–1253
Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD (2013) Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191:5230–5238
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225
Aviello G, Knaus UG (2018) NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol 11:1011–1023
Jabaut J, Ather JL, Taracanova A, Poynter ME, Ckless K (2013) Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1beta secretion in association with alterations in cellular redox and energy status. Free Radic Biol Med 60:233–245
Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V (2011) Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 187:613–617
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11:136–140
Rubartelli A (2012) Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol 92:951–958
Orlowski GM, Colbert JD, Sharma S, Bogyo M, Robertson SA, Rock KL (2015) Multiple cathepsins promote Pro-IL-1beta synthesis and NLRP3-mediated IL-1beta activation. J Immunol 195:1685–1697
Jin C, Flavell RA (2010) Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 30:628–631
Yang Y, Wang H, Kouadir M, Song H, Shi F (2019) Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 10:128
Nakanishi A, Kaneko N, Takeda H, Sawasaki T, Morikawa S, Zhou W, Kurata M, Yamamoto T, Akbar SMF, Zako T, Masumoto J (2018) Amyloid beta directly interacts with NLRP3 to initiate inflammasome activation: identification of an intrinsic NLRP3 ligand in a cell-free system. Inflamm Regen 38:27
Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzumura A (2013) Oligomeric amyloid beta induces IL-1beta processing via production of ROS: implication in Alzheimer's disease. Cell Death Dis 4:e975
Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14:812–820
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11:155–161
El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD (2003) CD36 mediates the innate host response to beta-amyloid. J Exp Med 197:1657–1666
Labzin LI, Heneka MT, Latz E (2018) Innate immunity and neurodegeneration. Annu Rev Med 69:437–449
Dansokho C, Heneka MT (2018) Neuroinflammatory responses in Alzheimer's disease. J Neural Transm (Vienna) 125:771–779
Su F, Bai F, Zhou H, Zhang Z (2016) Microglial toll-like receptors and Alzheimer's disease. Brain Behav Immun 52:187–198
Eren E, Ozoren N (2019) The NLRP3 inflammasome: a new player in neurological diseases. Turk J Biol 43:349–359
Hook VY, Kindy M, Hook G (2008) Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem 283:7745–7753
Venegas C, Heneka MT (2017) Danger-associated molecular patterns in Alzheimer's disease. J Leukoc Biol 101:87–98
Dinarello CA, Simon A, van der Meer JW (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11:633–652
O'Neill LA (1996) Interleukin I receptors and signal transduction. Biochem Soc Trans 24:207–211
Osborn L, Kunkel S, Nabel GJ (1989) Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA 86:2336–2340
Esser N, Paquot N, Scheen AJ (2015) Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs 24:283–307
Ozaki E, Campbell M, Doyle SL (2015) Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 8:15–27
Akash MS, Shen Q, Rehman K, Chen S (2012) Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. J Pharm Sci 101:1647–1658
Craft JM, Watterson DM, Hirsch E, Van Eldik LJ (2005) Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. J Neuroinflammation 2:15
Tapia-Abellan A, Angosto-Bazarra D, Martinez-Banaclocha H, de Torre-Minguela C, Ceron-Carrasco JP, Perez-Sanchez H, Arostegui JI, Pelegrin P (2019) MCC950 closes the active conformation of NLRP3 to an inactive state. Nat Chem Biol 15:560–564
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K (2019) MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 15:556–559
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O'Neill LAJ, Lynch MA (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316
Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, Yamamoto M, Akira S, Nakanishi K, Noda T, Taniguchi S (2004) ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9:1055–1067
Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218
Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT (2017) Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer's disease. Nature 552:355–361
Stehlik C, Dorfleutner A (2007) COPs and POPs: modulators of inflammasome activity. J Immunol 179:7993–7998
Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell 11:591–604
Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner DL (2006) The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci USA 103:9982–9987
Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer HD, Grutter C, Grutter M, Tschopp J (2007) The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ 14:1457–1466
Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC (2003) The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J 373:101–113
Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C (2007) Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect Immun 75:1484–1492
Bedoya F, Sandler LL, Harton JA (2007) Pyrin-only protein 2 modulates NF-kappaB and disrupts ASC:CLR interactions. J Immunol 178:3837–3845
Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP (2007) Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA 104:8041–8046
Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R (2017) Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 214:3219–3238
Qiao J, Wu X, Luo Q, Wei G, Xu M, Wu Y, Liu Y, Li X, Zi J, Ju W, Fu L, Chen C, Wu Q, Zhu S, Qi K, Li D, Li Z, Andrews RK, Zeng L, Gardiner EE, Xu K (2018) NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 103:1568–1576
Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12:1005–1015
Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL (2001) Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci 21:8198–8209
Xiang Z, Ho L, Yemul S, Zhao Z, Qing W, Pompl P, Kelley K, Dang A, Qing W, Teplow D, Pasinetti GM (2002) Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr 10:271–278
Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci 20:5709–5714
Daniels MJ, Rivers-Auty J, Schilling T, Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS, Baldwin AG, Freeman S, Wong R, Latta C, Yu S, Jackson J, Fischer N, Koziel V, Pillot T, Bagnall J, Allan SM, Paszek P, Galea J, Harte MK, Eder C, Lawrence CB, Brough D (2016) Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nat Commun 7:12504
Quinn J, Montine T, Morrow J, Woodward WR, Kulhanek D, Eckenstein F (2003) Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer's disease. J Neuroimmunol 137:32–41
Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE (2003) NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest 112:440–449
Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E (2004) Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem 88:337–348
Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N (2015) Autophagy in Alzheimer's disease. Rev Neurosci 26:385–395
Harris J, Lang T, Thomas JPW, Sukkar MB, Nabar NR, Kehrl JH (2017) Autophagy and inflammasomes. Mol Immunol 86:10–15
Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, Kim HM, Kim DH, Yoon SY (2014) Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10:1761–1775
Hong Y, Liu Y, Yu D, Wang M, Hou Y (2019) The neuroprotection of progesterone against Abeta-induced NLRP3-Caspase-1 inflammasome activation via enhancing autophagy in astrocytes. Int Immunopharmacol 74:105669
Santeford A, Wiley LA, Park S, Bamba S, Nakamura R, Gdoura A, Ferguson TA, Rao PK, Guan JL, Saitoh T, Akira S, Xavier R, Virgin HW, Apte RS (2016) Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 12:1876–1885
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12:222–230
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456:264–268
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, McGeough MD, Ellisman MH, Seki E, Gustafsson AB, Hoffman HM, Diaz-Meco MT, Moscat J, Karin M (2016) NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164:896–910
Harris J, Hope JC, Lavelle EC (2009) Autophagy and the immune response to TB. Transbound Emerg Dis 56:248–254
Author information
Authors and Affiliations
Contributions
GY contributed to the review idea, YZ contributed to the paper draft and revision, YZ and JZ contributed to the literature search. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Declarations
We declare that this manuscript was original research and has not been published previously, and not under consideration for publication elsewhere.
Informed Consent
The manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhao, Y., Zhang, J. et al. Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer’s Disease. Neurochem Res 45, 2560–2572 (2020). https://doi.org/10.1007/s11064-020-03121-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03121-z