Abstract
Endoplasmic reticulum (ER) stress has been indicated to be involved in the pathogenesis of epilepsy. Sodium valproate (VPA), one of the most commonly used antiepileptic drugs, is reported to regulate ER stress in many neurological diseases. However, the effect of VPA on ER stress in epilepsy remains unclear. The current study was performed to investigate the role of ER stress in the neuroprotection of VPA against seizure induced by pentylenetetrzole (PTZ). Our results showed that VPA treatment could inhibit the increased expressions of ER stress proteins (GRP78 and CHOP), and significantly reduce neuronal apoptosis in the PTZ-induced experimental seizure model. In addition, Salubrinal, an ER stress inhibitor, was used as a positive control, and exhibited neuroprotective effects via inhibiting excessive ER stress in the seizure model, which further supported that the inhibition in ER stress by VPA treatment could exert neuroprotection in seizures. In summary, our work demonstrated for the first time that ER stress was involved in the neuroprotective potential of VPA for seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsies are a diverse group of brain disorders, which are characterized by recurrent and unpredictable epileptic seizures [1, 2]. There are approximately 65 million people suffering from epilepsy all over the world [3]. The main treatment strategies for epilepsy are antiepileptic drugs (AEDs), and AEDs can control seizures in most of patients with epilepsy. However, about 30% of epilepsy patients who are pharmacoresistant can’t benefit from the treatment with AEDs [4]. Therefore, it is necessary to constantly explore the pathogenesis of epilepsy, and provide new strategies or optimize current schemes for the treatment of epilepsy.
The endoplasmic reticulum (ER), an important organelle, is involved in protein secretion and modification. Dysregulated ER function can cause ER stress, which triggers the unfolded protein response (UPR) [5]. Proper ER stress is beneficial to reestablish cellular homeostasis, but excessive ER stress can lead to C/EBP homologous protein (CHOP)-mediated apoptosis [6]. Studies have indicated that ER stress is involved in many neurological diseases including epilepsy [7,8,9,10]. Prolonged ER stress is reported to be associated with the pathogenesis of epilepsy [11, 12]. Thus, ER stress becomes a target for the treatment of epilepsy, and ER stress inhibitors, such as Salubrinal (Sab) and 4-phenylbutyrate (4-PBA), have been shown to have therapeutic effects on epilepsy [13, 14].
Sodium valproate (VPA) is one of the most commonly used AEDs, which is able to suppress epileptic seizures, ameliorate neuron apoptosis, and restore blood brain barrier (BBB) function [15, 16]. The antiepileptic mechanisms of VPA are indicated to be involved in enhancing Na channel inactivation and promoting GABAergic neurotransmission release [17, 18]. In addition, VPA has been reported to exert neuroprotective effects through epigenetic mechanisms, such as histone modification, brain-derived neurotrophic factor (BDNF) and glial-cell-line-derived neurotrophic factor (GDNF) modulation [19]. In recent years, many studies have shown that VPA can regulate ER stress. VPA was reported to prevent ischemic retina apoptosis by increasing glucose-related protein (GRP78) expression and acetylation of histone H3, attenuating upregulation of CHOP [20]. Moreover, another study also found that the neuroprotective effect of VPA was associated with the inhibition of ER stress-induced apoptosis [21]. These studies provided a new perspective for the therapeutic potential of VPA in neuroprotection and neurological diseases. However, currently, no studies have been conducted to explore whether VPA can regulate ER stress in epilepsy. We hypothesized that ER stress might be involved in the neuroprotection of VPA against seizures. In this study, we used a mouse seizure model induced by pentylenetetrazol (PTZ), which is a convulsant that has been widely used in the study of screening new compounds with antiepileptic activity [22] to investigate our hypothesis. We found that VPA could inhibit the excessive ER stress and reduce neuronal apoptosis in the PTZ-induced seizure model.
Materials and Methods
PTZ-Induced Seizure Mouse Model and Drug Treatment
Adult male C57BL/6 mice (weight: 20–30 g) were used. Animals were maintained in the temperature controlled environment (20–24 °C) and provided with free access to food and water. All procedures were in accordance with the protocol approved by the Institutional Animal Care and Use Committee of University of Southern California. Acute epileptic seizures were induced by intraperitoneal injection of PTZ (P6500, Sigma-Aldrich, USA) at the dose of 60 mg/kg [23]. After the injection of PTZ, seizure behavior was monitored for 30 min using the Racine’s score as follows [24]: Score 0, no response; Score 1, mouth and facial twitching; Score 2, head nodding; Score 3, myoclonic jerks, clonic forelimb convulsions; Score 4, rearing with clonus; Score 5, generalized tonic–clonic seizures, loss of posture. In the VPA pretreatment groups, VPA (P4543, Sigma-Aldrich, USA) were intraperitoneally administrated by the doses of 100 mg/kg or 200 mg/kg [25] 30 min prior to PTZ injection. In the Sab group, mice were administrated intraperitoneal injections of 1 mg/kg Sab (SML0951, Sigma-Aldrich, USA), twice at 24 h and 30 min before the injection of PTZ [26]. The latency to the first generalized tonic–clonic seizure and the duration of the first generalized tonic–clonic seizure were recorded.
Western Blot
The lysates of hippocampal tissues were prepared in RIPA lysis buffer with protease and phosphatase inhibitors mixture. Protein concentrations were determined by using BCA protein assay. 30 μg of total protein lysate was added to each lane. Proteins were fractionated by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. Primary antibodies used were GRP78 (Mouse, 75 kDa, sc-376768, 1:500, SantaCruz, USA), phospho-eIF2α (Rabbit, 38 kDa, 9721, 1:500, Cell signaling, USA), eIF2α (Mouse, 38 kDa, 2103, 1:500, Cell signaling, USA), CHOP (Rabbit, 29 kDa, 1:300, ab10444, Abcam, USA), caspase-3 (Rabbit, 30, 19 kDa, 9665, 1:500, Cell signaling, USA), Actin (Mouse, 43 kDa, sc-8432, 1:500, SantaCruz, USA). Secondary antibodies used were HRP-conjugated goat anti-mouse (1:2500, Proteinte-ch) and goat anti-rabbit (1:2500, Cell Signaling Technology), accordingly. Protein was visualized by using a chemiluminescence kit (34577, Pierce, USA). Protein bands were detected by Odyssey infrared imaging (LI-COR Biosciences, Lincoln, NE). Optical densities of the protein bands were analyzed using image J software.
Immunochemistry
Mice were deeply anesthetized with the intraperitoneal injection of a ketamine/xylazine mixture (80/12 mg/kg) and intracardially perfused with 0.9% saline. The brains were then removed, embedded in O.C.T. medium, and frozen. Frozen mouse brain tissues were sectioned at 10 μm and then fixed in acetone. The slides were washed in PBS and blocked with Sea Block (37527, Thermo Scientific) for 20 min. Subsequently, IHC was performed according to a previous method [27]. Anti-NeuN antibody (Rabbit, 1:500, ab104225, Abcam, USA) used as primary antibody overnight, followed by incubation with goat anti-rabbit IgG (1:300, BA-1000,Vector Laboratories, USA) for 45 min. Then, samples were treated with avidin–biotin–peroxidase complex for 30 min, and amino-ethylcarbazol substrate kit for 1 min. A negative control was conducted using isotype-matched serum instead of primary antibody. Three mice were in each group, and at least three sections with 100 μm intervals per mouse were selected to quantitatively analyze NeuN immunoreactivity. NeuN-immunoreactive neurons of twelve slides from each group were counted in a 100 × 100 μm square in the hippocampus at 400 × magnification. Cell counts were obtained by averaging the counts from each mouse.
Tunel assay
DeadEnd™ Fluorometric TUNEL assay was conducted using a commercial kit according to manufacturer’s instructions (G3250, Promega, USA). 10 μm thick slices from each group were fixed with 4% paraformaldehyde for 15 min and permeabilized with 20 μg/ml proteinase K for 10 min. Subsequently, slides were covered with equilibration buffer for 10 min. Then, rTdT incubation buffer was added into each slide and slides were incubated in a humid chamber at 37 °C for 60 min. The reaction was terminated by immersing the slides in 2 × SSC for 15 min. The slides were rinsed in PBS and blocked with Sea Block (37527, Thermo Scientific) for 20 min. Subsequently, slides were incubated overnight with anti-NeuN antibody (Rabbit, 1:500, ab104225, Abcam, USA) at 4 °C. Then, slides were incubated with goat anti-rabbit IgG (Alexa Fluor® 647) (1:200, ab150083, Abcam, USA) at room temperature for 45 min, rinsed with PBS, and mounted in mounting media. The images from each group in the CA1 and DG regions of hippocampus were obtained using confocal microscopy. The quantitative analysis of Tunel-positive neurons was conducted by an independent investigator.
Statistical Analysis
Statistical significance was conducted using the Student two-tailed t test. A p value with < 0.05 was considered statistically significant.
Results
Expressions of ER Stress Proteins at Different Time Points in a Mouse Seizure Model Induced by PTZ
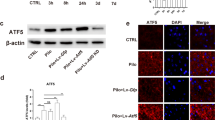
We first examined the expressions of ER stress proteins (GRP78, phosphorylation-elF2a (p-elF2a), elF2a, and CHOP) following PTZ injection (Fig. 1). At 2 h after the first generalized tonic–clonic seizure induced by PTZ treatment, the expressions of GRP78 and CHOP were significantly increased compared to those of controls (Fig. 1a, b, d), while p-elF2a was unaltered at 2 h and the most obvious increase was at 15 h (Fig. 1a, c). Since GRP78 is a major chaperone protein in sustained ER stress and CHOP is a downstream pro-apoptotic pathway effector of ER stress [28, 29], the expressions of GRP78 and CHOP proteins at 2 h after the first tonic–clonic seizure were selected to evaluate in the subsequent experiments with VPA and Sab treatments.
Expressions of proteins associated with ER stress. Hippocampal lysates were prepared from different time points after PTZ injection as indicated. a Western blot analysis of GRP78, p-elF2a, elF2a and CHOP. b–d Quantification was performed to show the densitometry values for GRP78 and CHOP were normalized to actin and expressed as fold change. Densitometry values for p-eIF2a was normalized to elF2a. Data (n = 3 per each time point) are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group
VPA Ameliorates Seizure Behavior in a PTZ-Induced Seizure Model
As shown in Fig. 2, the latency to the first generalized tonic–clonic seizure in the 200 mg/kg VPA group was significantly delayed (23.60 ± 2.79 min, p < 0.001) compared with that in the PTZ group (3.57 ± 0.55 min). In contrast, the latency to the first generalized tonic–clonic seizure in the 100 mg/kg of VPA group was not significantly different (4.52 ± 0.56 min, p > 0.05) from that in the PTZ group. In addition, compared to mice in the PTZ group (13.40 ± 2.38 s), both 100 mg/kg and 200 mg/kg of VPA treatments decreased the duration of the first generalized tonic–clonic seizure induced by PTZ (4.80 ± 1.07 s, p < 0.05; 0.60 ± 0.40 s, p < 0.001; respectively). Of note, decline in duration in the 200 mg/kg VPA group was more significant than that in the 100 mg/kg VPA group (p < 0.01). Therefore, compared to 100 mg/kg of VPA-treated group, 200 mg/kg VPA exerted a stronger effect on the improvement of seizure behavior in PTZ-induced epileptic mice. In the subsequent experimental investigations, the dose of 200 mg/kg VPA was selected.
VPA treatment ameliorated pentylenetetrazol (PTZ)-induced seizure behavior in a mouse seizure model. C57BL/6 mice were intraperitoneally injected with PTZ at the dose of 60 mg/kg or with PTZ + VPA (100 mg/kg or 200 mg/kg). The evaluation of seizure behavior including latency. a Duration, b of seizures. Values were shown as mean ± SEM (n = 5). *p < 0.05, **p < 0.01 and ***p < 0.001
VPA Inhibits the Increased Levels of ER Stress Proteins in the PTZ-Induced Seizure
Compared with the PTZ group, the administration of VPA could reduce the increased levels of GRP78 and CHOP at 2 h after the first tonic–clonic seizure (p < 0.01) (Fig. 3a–c). In addition, the administration of VPA without PTZ did not alter the expressions of GRP78 and CHOP, compared to the Control group (p > 0.05) (Fig. 3a–c).
VPA suppressed the increased levels of GRP78 and CHOP in a mouse seizure model. a–c Western blot analysis of GRP78 and CHOP, which indicated that increased levels of GRP78 and CHOP in PTZ-induced seizures were significantly diminished by VPA treatment. The administration of VPA without PTZ did not alter the expressions of GRP78 and CHOP compared to the Control group. Values were shown as mean ± SEM (n = 3). **p < 0.01 and ***p < 0.001; ns, not significant (p > 0.05)
VPA Reduces Neuronal Apoptosis in the PTZ-Induced Seizure
Neuronal apoptosis in the PTZ-induced seizure was evaluated by Tunel staining and the level of cleaved caspase-3/caspase-3 in hippocampal samples. In addition, we also measured the neuronal loss by the analysis of NeuN immunoreactivity. The PTZ-induced seizure model demonstrated the significant neuronal apoptosis in the CA1 and DG regions of hippocampus (Fig. 4a–c). VPA administration obviously reduced the apoptotic rates of neurons in the hippocampal CA1 and DG regions of mice treated with PTZ (p < 0.001; Fig. 4a–c). In addition, the level of cleaved caspase-3/caspase-3 in the hippocampus of mice treated with PTZ was also significantly inhibited after VPA treatment (p < 0.01; Fig. 4d, e). Of note, the number of NeuN-positive neurons in the hippocampus of the PTZ-treated mice was similar to those of the control and VPA-treated animals (p > 0.05; Fig. 4f–h).
VPA reduced neuronal apoptosis in PTZ-induced seizure model. a Representative co-labeling TUNEL/NeuN merge images of the hippocampus of mice treated with PTZ and VPA. The arrowheads indicate double-positive neurons. Scale bar, 50 μm. b, c Statistical analysis of TUNEL-positive neurons in the CA1 and DG regions of hippocampus of mice treated with PTZ and VPA, expressed as percentage of total (NeuN+) cells. d, e Showed that VPA inhibited the increased level of cleaved caspase-3/caspase-3 in PTZ-induced seizures. f Showed the representative images of the CA1 and DG regions of hippocampus of mice treated with PTZ and VPA by NeuN Immunochemistry staining. Scale bar, 50 μm. g, h Statistical analysis of NeuN positive cells in the CA1 and DG regions of hippocampus of mice treated with PTZ and VPA. Values were shown as mean ± SEM (n = 3). **p < 0.01, ***p < 0.001 and ****p < 0.0001
Salubrinal, an ER Stress Inhibitor, Suppresses the Increased Levels of ER Stress Proteins and Reduces Neuronal Apoptosis in the PTZ-Induced Seizure
In order to further determine that the inhibition in ER stress by VPA treatment could exhibit neuroprotective effects in the PTZ-induced seizure model, we also detected the effect of Sab (an ER stress inhibitor) on neuronal apoptosis in the PTZ-induced seizure. As shown in Fig. 5, the increased levels of GRP78 and CHOP in mice treated with PTZ were suppressed after Sab treatment (p < 0.01). Additionally, the administration of Sab without PTZ did not influence the expressions of GRP78 and CHOP, compared to the Control group (p > 0.05; Fig. 5a–c). Sab could also reduce the apoptotic levels of neurons in the hippocampal CA1 and DG regions of mice treated with PTZ (p < 0.05 and p < 0.001, respectively; Fig. 6a–c). These results suggested that the inhibition of excessive ER stress in the PTZ-induced seizure model promoted neuronal survival.
Sab inhibited the increased levels of GRP78 and CHOP in a mouse seizure model. a–c Western blot analysis of GRP78 and CHOP, which indicated that increased levels of GRP78 and CHOP in PTZ-induced seizures were significantly diminished by Sab treatment. The administration of Sab without PTZ did not alter the expressions of GRP78 and CHOP compared to the Control group. Values were shown as mean ± SEM (n = 3). **p < 0.01, ***p < 0.001 and ****p < 0.0001; ns not significant (p > 0.05)
Sab reduced neuronal apoptosis in PTZ-induced seizure model. a Showed the representative co-labeling TUNEL/NeuN merge images of the hippocampus of mice treated with PTZ and Sab. The arrowheads indicate double-positive neurons. Scale bar, 50 μm. b, c Statistical analysis of TUNEL-positive neurons in the CA1 and DG regions of hippocampus of mice treated with PTZ and Sab, expressed as percentage of total (NeuN+) cells. Values were shown as mean ± SEM (n = 3). *p < 0.05, ***p < 0.001 and ****p < 0.0001
Discussion
Our current study indicated that VPA could exert neuroprotective effects through inhibiting the excessive ER stress in the PTZ-induced seizure model. Of note, this was the first time that VPA was reported to be able to regulate ER stress in seizure.
ER is a dynamic organelle, which plays a critical role in multiple cellular activities, such as folding and quality control of proteins, lipid biosynthesis, Ca2+ buffering [30]. Disturbance of ER function could cause unfolded or misfolded proteins to accumulate in the ER lumen, which is known as ER stress [31, 32]. Proper ER stress could maintain ER homeostasis through reducing protein synthesis and promoting degradation [33]. However, excessive ER stress could lead to apoptotic cell death, which is caused by activation of ER stress sensors, upregulating expression of CHOP [34], GRP78 [35], and cleaved caspase-3 [36]. ER stress has been indicated to be associated with pathogenesis of epilepsy. One study reported that the expression of CHOP was associated with neuronal death in the status epilepticus model [37]. In detail, this study indicated that the expression of CHOP reached the peak at 24 h after status epilepticus induced by PTZ, and CHOP expression correlated with apoptotic neurons. Furthermore, they also found that Sab, an ER stress inhibitor, could reduce neuronal apoptosis through the downregulation of CHOP expression [13]. In contrast, one study by Engel et al. [38] indicated that CHOP expression peaked at 8 h after status epilepticus induced by kainic acid, and CHOP was anti-apoptotic, which was required for neuronal survival after seizure. Moreover, they observed that Sab increased the level of CHOP in the hippocampus, and decreased neuronal death. The underlying mechanism may be that CHOP activated the transcription of murine double minute 2 that could inhibit p53 function, and thus prevented neuronal injury caused by increased p53 level. However, our present study showed that the highest expression of CHOP protein occurred at 2 h after PTZ-induced generalized tonic–clonic seizure. In addition, both VPA and Sab downregulated the expression of CHOP, but they still played neuroprotective roles in seizure. The discrepancy in CHOP expression and function from different studies may be due to different seizure models and detection platforms. Moreover, roles of ER stress in epilepsy could be versatile, which are linked with complicated interconnections between signaling pathways.
VPA is one of the conventional AEDs, which exhibits a broad spectrum of antiepileptic properties, and is the first choice for treatment of generalized seizures [39]. VPA is classified as a sodium channel-blocking AED, but VPA has been found to exert therapeutic effects in epilepsy via regulating various signaling pathways. For instance, one recent study demonstrated that VPA could ameliorate neuronal apoptosis in KA-induced seizure via augmenting PKC-dependent GABAAR γ2 phosphorylation at serine 327 residue [16]. Our study indicated for the first time that VPA could inhibit excessive ER stress in PTZ-induced seizures. Previous studies have indicated that VPA could enhance GRP78 expression [20, 21]. However, our study found that VPA downregulated the increased expression of GRP78 in the PTZ-induced seizure model. Effects of VPA on the GRP78 expression may be associated with various mechanisms in different diseases. One study investigated the mechanism of epilepsy-induced ER stress, and they found that PTZ kindling-induced hippocampal ER stress may be dependent on neuronal nitric oxide synthase (nNOS) activity [12]. Furthermore, nNOS deficiency inhibited PTZ kindling-induced the increased levels of GRP78 and CHOP. Interestingly, VPA was reported to be able to reduce nNOS expression [40]. Therefore, we speculate that nNOS may be involved in the regulation of ER stress by VPA in epilepsy, which requires further exploration. Of note, our study showed that the administration of VPA without PTZ did not decrease the expressions of GRP78 and CHOP, which indicated that the effects of VPA with PTZ on ER stress were not due to VPA itself but the effects of markedly decreased seizure activity caused by the VPA pretreatment.
It is widely accepted that prolonged and repeated seizures lead to neuronal damage, and even brief seizures could also cause neuronal apoptosis [41]. However, brief epileptic seizures may not necessarily result in neuronal loss. Consistent with previous studies [42, 43], our study also indicated that acute seizures induced by a single injection of PTZ did not lead to significant neuronal loss, despite causing neuronal apoptosis. It is reported that apoptosis may be one of the mechanisms contributing to neuronal loss [44]. Therefore, VPA and Sab pretreatments reduced early neuronal apoptosis in acute seizures, which was beneficial for preventing neuronal loss thereafter.
In conclusion, our current study revealed that VPA could inhibit excessive ER stress and exert neuroprotective effects in acute seizures induced by PTZ. Our present work provided a new mechanical explanation for the therapeutic effects of VPA on seizure. Future research can be conducted to explore the combined effects of ER stress inhibitors and VPA on epileptic seizures.
References
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472
Kumar R (2000) Classification and the need to classify epilepsy. Indian J Pediatr 67:S4–S11
Moshé SL, Perucca E, Ryvlin P, Tomson T (2015) Epilepsy: new advances. Lancet 385:884–898
Wahab Abdul (2010) Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals 3:2090–2110
Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang X, Xiang X, Qu K, Liu C, Zhang J (2018) Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid Med Cell Longev 2018:4756846
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102
Fan LF, He PY, Peng YC, Du QH, Ma YJ, Jin JX, Xu HZ, Li JR, Wang ZJ, Cao SL, Li T, Yan F, Gu C, Wang L, Chen G (2017) Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med 112:336–349
Han Y, Yi W, Qin J, Zhao Y, Zhang J, Chang X (2015) Carbon monoxide offers neuroprotection from hippocampal cell damage induced by recurrent febrile seizures through the PERK-activated ER stress pathway. Neurosci Lett 585:126e131
Ko AR, Kim JY, Hyun HW, Kim JE (2015) Endoplasmic reticulum (ER) stress protein responses in relation to spatio-temporal dynamics of astroglial responses to status epilepticus in rats. Neuroscience 307:199–214
Yuan Y, Yang J, Chen J, Zhao S, Wang T, Zou H, Wang Y, Gu J, Liu X, Bian J, Liu Z (2019) Alpha-lipoic acid protects against cadmium-induced neuronal injury by inhibiting the endoplasmic reticulum stress eIF2α-ATF4 pathway in rat cortical neurons in vitro and in vivo. Toxicology 414:1–13
Zhu X, Dong J, Xia Z, Zhang A, Chao J, Yao H (2017) Repeated restraint stress increases seizure susceptibility by activation of hippocampal endoplasmic reticulum stress. Neurochem Int 110:25–37
Zhu X, Dong J, Han B, Huang R, Zhang A, Xia Z, Chang H, Chao J, Yao H (2017) Neuronal nitric oxide synthase contributes to PTZ Kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front Cell Neurosci 11:377
Chen J, Zheng G, Guo H, Shi ZN, Jiang J, Wang XY, Yang X, Liu XY (2018) The effect of metformin treatment on endoplasmic reticulum (ER) stress induced by status epilepticus (SE) via the PERK-eIF2α-CHOP pathway. Bosn J Basic Med Sci 18:49–54
Yokoi N, Fukata Y, Kase D, Miyazaki T, Jaegle M, Ohkawa T, Takahashi N, Iwanari H, Mochizuki Y, Hamakubo T, Imoto K, Meijer D, Watanabe M, Fukata M (2015) Chemical corrector treatment ameliorates increased seizure susceptibility in a mouse model of familial epilepsy. Nat Med 21:19–26
Danjo S, Ishihara Y, Watanabe M, Nakamura Y, Itoh K (2013) Pentylentetrazole-induced loss of blood-brain barrier integrity involves excess nitric oxide generation by neuronal nitric oxide synthase. Brain Res 1530:44–53
Li Q, Li QQ, Jia JN, Cao S, Wang ZB, Wang X, Luo C, Zhou HH, Liu ZQ, Mao XY (2018) Sodium valproate ameliorates neuronal apoptosis in a kainic acid model of epilepsy via enhancing PKC-dependent GABAAR γ2 serine 327 phosphorylation. Neurochem Res 43:2343–2352
Macdonald RL, Kelly KM (1994) Mechanisms of action of currently prescribed and newly developed antiepileptic drugs. Epilepsia 35:S41–S50
Loscher W, Schmidt D (1980) Increase of human plasma GABA by sodium valproate. Epilepsia 21:611–615
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi P, Costa C (2018) Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. https://doi.org/10.2174/1570159X17666181227165722
Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, Wu X (2011) Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett 504:88–92
Li Z, Wu F, Zhang X, Chai Y, Chen D, Yang Y, Xu K, Yin J, Li R, Shi H, Wang Z, Li X, Xiao J, Zhang H (2017) Valproate attenuates endoplasmic reticulum stress-induced apoptosis in SH-SY5Y cells via the AKT/GSK3β signaling pathway. Int J Mol Sci 18:E315
Funck VR, de Oliveira CV, Pereira LM, Rambo LM, Ribeiro LR, Royes LF, Ferreira J, Guerra GP, Furian AF, Oliveira MS, Mallmann CA, de Mello CF, Oliveira MS (2011) Differential effects of atorvastatin treatment and withdrawal on pentylenetetrazol-induced seizures. Epilepsia 52:2094–2104
Ashhar MU, Ahmad MZ, Jain V, Agarwal NB, Ahmad FJ, Jain GK (2017) Intranasal pitavastatin attenuates seizures in different experimental models of epilepsy in mice. Epilepsy Behav 75:56–59
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Deng XH, Zhang X, Wang J, Ma PS, Ma L, Niu Y, Sun T, Zhou R, Yu JQ (2017) Anticonvulsant effect of swertiamarin against pilocarpine-induced seizures in adult male mice. Neurochem Res 42:3103–3113
Kim JS, Heo RW, Kim H, Yi CO, Shin HJ, Han JW, Roh GS (2014) Salubrinal, ER stress inhibitor, attenuates kainic acid-induced hippocampal cell death. J Neural Transm 121:1233–1243
Virrey JJ, Golden EB, Sivakumar W, Wang W, Pen L, Schönthal AH, Hofman FM, Chen TC (2009) Glioma-associated endothelial cells are chemoresistant to temozolomide. J Neurooncol 95:13–22
Qie X, Wen D, Guo H, Xu G, Liu S, Shen Q, Liu Y, Zhang W, Cong B, Ma C (2017) Endoplasmic reticulum stress mediates methamphetamine-induced blood–brain barrier damage. Front Pharmacol 8:639
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Song S, Tan J, Miao Y, Li M, Zhang Q (2017) Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977–2984
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283:2640–2652
Pinkaew D, Changtam C, Tocharus C, Thummayot S, Suksamrarn A, Tocharus J (2015) Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Abeta25-35. Neurochem Int 80:110–119
Vembar SS, Brodsky JL (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9:944–957
Chen CM, Wu CT, Chiang CK, Liao BW, Liu SH (2012) C/EBP homologous protein (CHOP) deficiency aggravates hippocampal cell apoptosis and impairs memory performance. PLoS ONE 7:e40801
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Gong N, Wu JH, Liang ZS, Jiang WH, Wang XW (2015) Role of salubrinal in protecting cardiomyocytes from doxorubicin-induced apoptosis. Genet Mol Res 14:12377–12385
Chen J, Guo H, Zheng G, Shi ZN (2013) Region-specific vulnerability to endoplasmic reticulum stress-induced neuronal death in rat brain after status epilepticus. J Biosci 38:877–886
Engel T, Sanz-Rodgriguez A, Jimenez-Mateos EM, Concannon CG, Jimenez-Pacheco A, Moran C, Mesuret G, Petit E, Delanty N, Farrell MA, O’Brien DF, Prehn JH, Lucas JJ, Henshall DC (2013) CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain 136:577–592
Societas Neurologica Japonica (2010) Guideline for epilepsy diagnose and treatment in Japan. Igaku-Shoin Ltd., Tokyo
Wang X, Guo J, Song Y, Wang Q, Hu S, Gou L, Gao Y (2018) Decreased number and expression of nNOS-positive interneurons in basolateral amygdala in two mouse models of autism. Front Cell Neurosci 12:251
Kalantaripour TP, Esmaeili-Mahani S, Sheibani V, Asadi-Shekaari M, Pasban-Aliabadi H (2016) Anticonvulsant and neuroprotective effects of apelin-13 on pentylenetetrazole-induced seizures in male rats. Biomed Pharmacother 84:258–263
Vasilev DS, Tumanova NL, Kim KK, Lavrentyeva VV, Lukomskaya NY, Zhuravin IA, Magazanik LG, Zaitsev AV (2018) Transient morphological alterations in the hippocampus after pentylenetetrazole-induced seizures in rats. Neurochem Res 43:1671–1682
Zaitsev AV, Kim KK, Vasilev DS, Lukomskaya NY, Lavrentyeva VV, Tumanova NL, Zhuravin IA, Magazanik LG (2015) N-methyl-d-aspartate receptor channel blockers prevent pentylenetetrazole-induced convulsions and morphological changes in rat brain neurons. J Neurosci Res 93:454–465
Cotman CW, Su JH (1996) Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol 6:493–506
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declared no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, J., Peng, L., Wang, W. et al. Sodium Valproate Reduces Neuronal Apoptosis in Acute Pentylenetetrzole-Induced Seizures via Inhibiting ER Stress. Neurochem Res 44, 2517–2526 (2019). https://doi.org/10.1007/s11064-019-02870-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02870-w