Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by excessive accumulation of the amyloid-β peptide (Aβ) in the brain, which has been considered to mediate the neuroinflammation process. Microglial activation is the main component of neuroimmunoregulation. In recent years, exosomes isolated from human umbilical cord mesenchymal stem cells (hucMSC-exosomes) have been demonstrated to mimic the therapeutic effects of hucMSCs in many inflammation-related diseases. In this study, exosomes from the supernatant of hucMSCs were injected into AD mouse models. We observed that hucMSC-exosomes injection could repair cognitive disfunctions and help to clear Aβ deposition in these mice. Moreover, we found that hucMSC-exosomes injection could modulate the activation of microglia in brains of the mice to alleviated neuroinflammation. The levels of pro-inflammatory cytokines in peripheral blood and brains of mice were increased and the levels of anti-inflammatory cytokines were decreased. We also treated BV2 cells with hucMSC-exosomes in culture medium. HucMSC-exosomes also had inflammatory regulating effects to alternatively activate microglia and modulate the levels of inflammatory cytokines in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common dementia which severely damages memories and cognitive functions of patients. According to the statistical data published by WHO in 2015, more than 47 million people suffered from dementia and this number will triple by 2050 [1]. All forms of AD are characterized by excessive amyloid-β peptide (Aβ) accumulation in the brain, which triggers the neuroinflammation process in the central nervous system (CNS). Neuroinflammation is an obvious pathological feature of neurodegenerative diseases which has been indicated to mediate neuronal damage and also aggravate the backward accumulation of Aβ in AD [2, 3]. It is urgent to seek an effective strategy to treat this disease based on its pathophysiology.
In recent years, cell therapies have been demonstrated as potential approaches for AD treatment. In consideration of the ethical issues, the administration of mesenchymal stem cells (MSCs) in vivo has become an ideal choice. MSCs are considered to possess superiorities in extensive sources (such as human umbilical cord, bone marrow, and adipose tissues), low immunogenicity, and outstanding self-renewal characters [4, 5]. In previous researches, our team investigated the therapeutic effects of hucMSCs on AD in vivo. We demonstrated that the injection of hucMSCs reduced the accumulation of Aβ in the brains of AD mice through alleviating neuroinflammation [6, 7]. However, hucMSCs have relative limitations in the maintenance of biological activity, the quantification of bioactive substances, and the logistics of delivery in clinical therapies. Thus, there is an urgent need to find an effective cell-free approach to mimic the functions of hucMSCs and avoid their flaws.
A novel member of extracellular vesicles named exosomes has been considered as a promising cell-free therapy because of their multiple biological activities and function of cell-to-cell communication [8]. In recent years, numerous research teams have proved that as a new therapeutic method for many diseases, exosomes isolated from hucMSCs (hucMSC-exosomes) possess their particular morphological and functional characteristics. HucMSC-exosomes are 30–150 nm extracellular membrane vesicles with CD9, CD63 and CD81 positive. They serve as natural transporters of RNA, cytokines, and proteins from hucMSCs to other cells in different tissues to act on cell proliferation, immune-regulation and many other functions [9, 10]. In clinical treatment, hucMSC-exosomes express all of the advantages of hucMSCs and avoid their imperfections. They are also easier to be quantified and maintain their bioactivities in preservation and transport. Moreover, multiple researches have shown that exosomes can cross the blood–brain barrier (BBB), especially under pathological conditions such as AD and other neurodegenerative diseases [11,12,13]. They realize the intercellular communication from peripheral circulation to the CNS. In addition, because of the source is human, hucMSC-exosomes seem easier to be accepted in clinic treatment by both doctors and patients in the future. However, whether hucMSC-exosomes can be used in the treatment of AD remains unknown, especially concerning the anti-neuroinflammatory effects of hucMSC-exosomes. In this study, we isolated exosomes from the supernatant of hucMSCs, and injected the hucMSC-exosomes into AβPP/PS1 transgenic AD mouse models. The impacts of hucMSC-exosomes injection on neuroinflammation, microglial reaction, Aβ deposition, and cognitive functions in these mice were investigated. We also treated BV2 cells with hucMSC-exosomes in culture medium to detect the inflammatory regulating effects of hucMSC-exosomes on BV2 cells.
Methods
HucMSC Isolation and In Vitro Culture
With informed written consent approved by the Second Hospital of Shandong University ethical committee, we obtained fresh human umbilical cords of full-term births by cesarean section. The procedure was based on our previous research [14]. In short, after three washes with phosphate-buffered saline (PBS, Corning, USA), we removed the arteries and veins from the umbilical cords. The mesenchymal tissue, Wharton’s jelly, was exposed and cut into small pieces (0.5–1 cm3). Then we placed them in tissue culture dishes with Dulbecco modified Eagle’s medium with 1 g/L glucose, l-glutamine and sodium pyruvate (DMEM-1X; Corning, USA) containing 10% fetal bovine serum (FBS; Gibco), and 100 mg/mL penicillin for 7 days under incubating condition at 37 °C with 5% CO2 for cultivating the cells to migrate from the tissues. We changed the culture medium every 3 days. By using 0.25% trypsin–EDTA solution, we expanded the cells in culture up to passage-3 for further research. According to a previous study, we analyzed the cells by flow cytometry to indicate the immune-phenotype of hucMSCs [15]. The cells used in this study were positive for CD73, CD90, and CD105, but negative for CD34, CD45, and HLA-DR, in accordance with hucMSCs characteristics.
Isolation of hucMSC-Exosomes
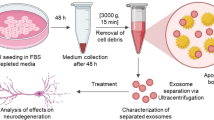
HucMSCs as previously described were cultured in fresh conditioned medium (DMEM-1X with 100 mg/mL penicillin) for 48 h. HucMSC-exosomes were extracted by employing ExoQuick-TC (System Bioscience, CA, USA) [16]. Based on the manufacturer’s protocol, supernatants of the cell culture were centrifuged at 3000×g for 15 min to eliminate cells and cell debris. ExoQuick-TC exosome precipitation solution was added into the supernatants (1:5) and the mixture was stored at 4 °C overnight. Then, the mixture was centrifuged at 1500×g for 30 min and the supernatants were discarded. After centrifugation, the hucMSC-exosomes were dissolved in 100–200 µL PBS and then stored at − 80 °C until further use. The total protein content of the exosomes was also quantified. In order to avoid the influence of substances in ExoQuick-TC in our test, we used ExoQuick-TC on fresh conditioned medium with the same process, added the same quantities of PBS and then stored the solution as the reagent of the control group at − 80 °C until further use.
Transmission Electron Microscopy
We used differential ultracentrifugation for fine purification of hucMSC-exosomes. Then the samples were loaded onto a formvar-coated grid and negatively stained with neutral 1% aqueous phosphotungstic acid. The electron microscopic images were captured and analyzed by a transmission electron microscope at 80 kV [17].
BV2 Cells Culture
Murine microglia cell line BV2 cells were cultivated in DMEM/F12 (Corning, USA) containing 10% fetal bovine serum (FBS; Gibco), and 100 mg/mL penicillin in a humidified incubator at 37 °C with 5% CO2. We washed the cells with PBS and added serum-free medium before treatment. In this study, we treated BV2 cells (5 × 104 cells per well in 24-well plate) with Aβ25–35 peptide (20 µmol/L) which was resuspended in sterile distilled water and kept for one week at 37 °C before use. Two different groups of cells were set. We treated the first group of cells with Aβ25–35 peptide and hucMSC-exosomes (30 µg/mL), and the control group with Aβ25–35 peptide and the reagent of the control group (RC) for 24 h incubation [18].
Animals
We used a transgenic mouse model of AD to estimate the therapeutic functions of hucMSC-exosomes injection. Heterozygous AβPPswe/PS1dE9 double-transgenic mice with C57BL/6 background were obtained from Beijing HFK Bio-Technology Co., Ltd., Institute of Laboratory Animal Science, Chinese Academy of Medical Science (Beijing, China). This type of mouse has been widely used in the research of AD [19, 20] because of possessing the representative features of AD and expressing an early accumulation of Aβ plaque and enhanced activation of “M1 microglia” [21]. In addition, we used wild-type mice (WT) with a C57BL/6 background as the proper controls in behavior tests. In this study, we used 18 APP/PS1 mice for the hucMSC-exosomes group and 18 APP/PS1 mice for the control group. In the hucMSC-exosomes group and the control group, 6 mice per group were used for immunofluorescent staining and the other 12 mice per group were used for PCR, ELISA and Western blot. We removed the cortex and hippocampus from every hemi-cerebrum of the mice, hen flash-froze the mixtures of cortex and hippocampus respectively. They were stored at − 80 °C until PCR, ELISA and Western blot. In these tests, the mixture of cortex and hippocampus were used as the brain sample (n = 6). We only used male mice for the gender specific differences in the performance of Aβ accumulation [22]. The animals were kept in a temperature-controlled room of 20–22 °C with a controlled humidity of 50–60%, lights on\off in a 12 h cycle, and freely available food and water. All procedures in this study were approved by the Ethical Committee for Animal Experiments of Shandong University.
Injection of hucMSC-Exosomes in AβPP/PS1 Transgenic Mice
We conducted this study with male 7 months old AβPP/PS1 transgenic mice. The hucMSC-exosomes suspension or RC as previously mentioned was injected into them through tail veins, every 2 weeks, four times altogether. After quantification of the total protein content of the hucMSC-exosomes, the hucMSC-exosomes solution (30 µg dissolved in 100 µL PBS), or 100 µL RC was injected into the mice each time.
Behavior Test
Three weeks after the last injection, we evaluated the spatial memory performance of mice by using the modified Morris water-maze (MWM) test based on a previously published study [23, 24]. On the first day of the test, the mice were placed in the water to adapt to the environment. During the next 5 days, the mice were released and swam around the water tank searching for a platform for up to 60 s. The starting locations were semi-randomly selected at the northern, eastern, southeastern, or northwestern corner. If a mouse failed to arrive at the platform in 60 s, it would be placed on the platform by the experimenter. We monitored every mouse with four trials every day and located the platform in the southwestern quadrant of the pool. Finally, we had the spatial probe test on the last day of the test without a platform. In this test the time spent in the platform quadrant and the times passing the location of the platform were measured as an evaluation of the memory of the mice. Each group contained 12 mice. The time to find the platform, swim speed, and other variables were recorded by a video tracking system.
Immunofluorescent Staining
After the MWM tests, we anesthetized the mice (six mice per group) with chloral hydrate and then rapidly infused their hearts with 0.9% saline solution and then with 4% paraformaldehyde (PFA, pH 7.4). After the infusion sacrifice, we removed the brains of the mice and immersed them into PFA at 4 °C overnight, and then the tissues were dehydrated in 30% sucrose at 4 °C to be equilibrated. A freezing microtome was used to cut the tissues into 10 µm coronal sections. All sections were stored at − 20 °C until the further experiments.
Immunofluorescent staining was conducted on cortex and hippocampus sections in 10 µm which were incubated with the primary antibody against Iba-1 (rabbit IgG, 1:500, Wako, Richmond, VA, USA) at 4 °C overnight, and then with the fluorescent dye-conjugated secondary antibodies (IgG-FITC). The staining was captured and analyzed by Image Pro Plus 6 (Media Cybernetics, Rockville, MD, USA).
We plated BV2 cells on glass coverslips and fixed them with 4% PFA for 20 min. The cells were permeabilized with 0.1% TritonX-100 for 20 min and then blocked with goat serum for 1 h at room temperature. Primary antibodies (Anti-Ym-1 + Ym-2 antibody, rabbit IgG, 1:100, Abcam) were reacted with the cells at 4 °C overnight. Then we used secondary antibodies (Goat Anti-Rabbit IgG, 1:200, Alexa Fluor 488, Abcam) to react with the cells. Nuclei were counterstained by 40,6-diamidino- 2-phenylindole (DAPI, Abcam).
Quantitative Real-Time PCR
The trizol method (Invitrogen, USA) was used to extract the total RNA from brains and BV2 cells. Reverse transcription was done in every 1 µg total RNA within a final volume of 20 µL according to the manufacturer’s protocol (DRR047A, TAKARA, Japan). We used an amount of 2 µL cDNA for real-time PCR with the SYBR Premix Ex Taq (DRR041A, TAKARA, Japan) in a procedure which was 40 cycles of 95 °C for 15 s, 95 °C for 5 s, and 60 °C for 30 s. All PCR reactions were conducted in triplicate. We analyzed the fold change in expression of the target gene based on the 2−ΔΔCT method [25], with GAPDH as a normalization control. The following primers were used:
Arg-1 forward: 5′-TCATGGAAGTGAACCCAACTCTTG-3′,
Arg-1 reverse: 5′-TCAGTCCCTGGCTTATGGTTACC-3′ [NM_007482.3, NCBI];
Ym-1 forward: 5′-AGACTTGCGTGACTATGAAGCATTG-3′;
Ym-1 reverse: 5′-GCAGGTCCAAACTTCCATCCTC-3′ [NM_009892.2, NCBI];
CD163 forward: 5′-GGGTCATTCAGAGGCACACTG-3′,
CD163 reverse: 5′-CTGGCTGTCCTGTCAAGGCT-3′; [NM_001170395.1,NCBI]
MRC1 forward: 5′-CTTCTGGGCCTGCTGTTCA-3′,
MRC1 reverse: 5′-CCAGCCTACTCATTGGGATCA-3′; [NM_008625.2,NCBI]
FIZZ1 forward:5′-TCCAGCTAACTATCCCTCCACTGT-3′,
FIZZ1 reverse: 5′-GGCCCATCTGTTCATAGTCTTGA-3′. [NM_020509.3,NCBI]
Enzyme-Linked Immunosorbent Assay
Inflammation-related factors from mice brains, peripheral blood (PB) and culture medium of BV2 cells, transforming growth factor-β (TGF-β), Interleukin-10 (IL-10), Tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β) were analyzed by enzyme-linked immunosorbent assays (ELISAs) which were performed based on the manufacturer’s protocol by using ELISA kits (ZCi Bio, Shanghai, China). The mouse brains and PB for ELISAs were homogenized in ten volumes of ice-cold PBS and then centrifuged at 5000×g at 4 °C for 5–10 min. The supernatant was used for the ELISA assays.
Aβ40/Aβ42 ELISAs were conducted based on the manufacturer’s Aβ40/Aβ42 protocol of ELISA kits (Invitrogen, Carlsbad, CA, USA). Tissue for ELISAs was homogenized in ten volumes of ice-cold guanidine buffer (5.0 M guanidine-HCl, 50 mM Tris–HCl, pH 8.0). The mixtures were settled by standing for 4 h at room temperature and then stored at − 20 °C until the further tests. The sample was diluted in 20 volumes of ice-cold reaction buffer BSAT-DPBS (Dulbecco phosphate-buffered saline, with 5% BSA, 0.03% Tween-20, 0.2 g/L KCl, 0.2 g/L KH2PO4, 8.0 g/L NaCl, 1.150 g/L Na2HPO4, pH 7.4) containing 1 × protease inhibitor cocktail (PMSF, aprotinin, leupeptin, EDTA, pepstatin A, NaF, and NaVO3) and then centrifuged at 15,000×g at 4 °C for 20 min. The supernatant was used for the ELISA assays.
Western Blot Analysis
The hucMSC-exosomes were stored at − 80 °C until homogenization. We homogenized the hucMSC-exosomes and the mixture of cortex and hippocampus in ice-cold RIPA lysing buffer (Beyotime, Shanghai, China) for 30 min, and then centrifuged the solutions at 12,000×g for 10 min at 4 °C. The supernatant was used for Western blot analysis. The proteins in the sample were separated with SDS–PAGE and electrophoretically transferred to PVDF membranes. Then we pretreated them were with a blocking solution (5% nonfat dry milk in 0.1% Tween 20 in PBS) for 1 h and incubated them with primary antibodies against CD9 (rabbit IgG, 1:2000, Abcam), CD63 (rabbit IgG, 1:1000, Abcam), Neprilysin (NEP, goat IgG, 0.2 µg/mL, R&D, Minneapolis, MN, USA), Insulin-degrading enzyme (IDE, rabbit IgG, 1:5000, Abcam), and β-actin (mouse IgG, 1:500, Abcam). Then they were rinsed with a washing solution (0.1% Tween 20 in PBS) three times and incubated with secondary antibodies (Goat anti-mouse IgG/HRP, 1:5000, Golden Bridge International, Beijing, China), (Goat anti-rabbit IgG/HRP, 1:5000, Golden Bridge International), and (Rabbit anti-goat IgG/HRP, 1:5000, Golden Bridge International) for 1 h. The intensity of the protein bands was analyzed by using ImageJ (Bethesda, MD, USA).
Thioflavin S Staining
We cut the brains into floating sections of 30 µm. The sections were stained with 0.5% thioflavin S (Solarbio, Beijing, China) dissolved in 50% ethanol for 5 min followed by rinsing twice with 50% ethanol for 5 min, and finally rinsed with distilled water for 5 min [26]. Green fluorescent-stained plaques in the cortex and the hippocampus were observed under a fluorescent microscope. Previous research showed that these regions commonly have an increased number of Aβ plaques in patients with AD which are related to memory disfunction [27].
Statistical Analysis
All data were recorded as mean ± standard error of the mean (SEM). A value of P < 0.05 was considered as statistically significant. A one-way Anova was used to measure the difference between three groups in behavior test. A t test was conducted to detect the difference between two groups. Data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Injection of hucMSC-Exosomes Increases Spatial Learning and Memory Function of AβPP/PS1 Mice
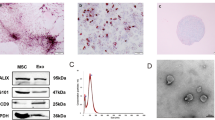
To investigate the potential therapeutic effects of hucMSC-exosomes on AD, we estimated the effects of hucMSC-exosomes on the behavior of AD mice. We first isolated exosomes from the culture supernatants of hucMSCs as described in the “Methods” section. Under transmission electron microscopy, the purified exosomes exhibited a round shape with a hypodense center and a diameter ranging from 30 to 150 nm (Fig. 1a, b). To examine whether they were successfully purified, we performed Western blot analyses and found that the hucMSC-exosomes contained subsets of proteins that are commonly found in exosomes, such as CD63 and CD9. (Fig. 1c).
Characterization of hucMSC-exosomes. a, b The ultrastructure of exosomes was analyzed by transmission electron microscopy [reference bar, 500 nm (a), 200 nm (b)]. HucMSC-exosomes showed round membrane vesicles with a hypodense center and a diameter ranging from 30 to 150 nm. c Expressions of protein markers CD63 and CD9 which are commonly found in exosomes were determined by Western blot
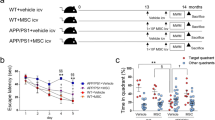
MWM was conducted to assess the spatial learning and memory function on the post-injection day. The mice treated with hucMSC-exosomes performed significantly better in the MWM test than the mice in treated with the reagent of control group. The mice treated with hucMSC-exosomes had a significantly shorter mean escape latency than the control group. Compare with WT group, the mice treated with hucMSC-exosomes had a longer mean escape latency in the second and third days but had no significant difference in the fourth and fifth days (Fig. 2a). The mice spatial memory was evaluated by performing probe trials 24 h after the last training. The mice treated with hucMSC-exosomes accomplished a larger number of platform location crossing times and a longer time spent in the target quadrant than the mice from the control group but had no significant difference compared with WT group (Fig. 2b, c), suggesting that the hucMSC-exosomes injection increased the behavioral performance of the mice. The swimming speed was similar between the two groups (Fig. 2d), proposing that the improved behavioral performance had nothing to do with the noncognitive components.
Injection of hucMSC-exosomes increases spatial learning and memory function of AβPP/PS1 mice. a The mice treated with hucMSC-exosomes had a significantly shorter mean escape latency than the control group. Compare with WT group, the mice treated with hucMSC-exosomes had a longer mean escape latency in the second and third days but had no significant difference in the fourth and fifth days. b and c The mouse spatial memory was evaluated by performing probe trials 24 h after the last training. The mice treated with hucMSC-exosomes accomplished a larger number of platform location crossing times b and a longer time spent in the target quadrant c than the mice from the control group but had no significant difference compared with WT group. d The swimming speed was not significantly different between three groups. (n = 12 in each group) Data are presented as mean ± SEM; */#P < 0.05, **P < 0.01
Effects of HucMSC-Exosomes Injection on Aβ Deposition and Soluble Aβ Quantities
The injection of hucMSC-exosomes was found to increase spatial learning and memory function of AβPP/PS1 mice. Accumulation of Aβ in the brain is the most common pathological feature of AD which triggers disfunction of cognitive behavior. So we used thioflavin S staining to detected the effects of hucMSC-exosomes injection on Aβ deposition in AD mice. We found that the number of Aβ plaques in the cortex and the hippocampus areas of the brain was significantly lower in the hucMSC-exosomes-injected group than in the control group (Fig. 3a, b). Furthermore, ELISA was performed to explore soluble Aβ40 and Aβ42 quantities in the brains of AD mice which confirmed that hucMSC-exosomes injection reduced both Aβ40 and Aβ42 levels in the mice (Fig. 3c). Our data suggested that hucMSC-exosomes injection reduced Aβ deposition in AD mice.
Effects of HucMSC-exosomes injection on Aβ deposition and soluble Aβ quantities. a Thioflavin S staining was used to evaluated Aβ deposition according to the description in the “Methods” section. Images were captured with a camera system connected to a fluorescence microscope (Olympus 1 × 71S1F-3, Japan). Scale bar, 200 µm. b Quantification of thioflavin S staining. The Aβ plaque burden was calculated as the percentage of the thioflavin S staining area over the total area. (n = 6 in each group) Image Pro Plus 6 (Media Cybernetics) was used to analyze the images. c ELISA kits (Invitrogen) were performed to quantify soluble Aβ40 and Aβ42 levels in the mice. (n = 6 in each group) Data are presented as mean ± SEM; *P < 0.05; the hucMSC-exosomes transplantation group versus the control group
HucMSC-Exosomes Promote the Secretion of Aβ-Degrading Enzymes
Two main Aβ-degrading enzymes IDE and NEP are related to clearance of Aβ deposition in the brain. Since we found hucMSC-exosomes could reduce accumulation of Aβ, we next detected whether hucMSC-exosomes could regulate IDE and NEP. We found that the levels of IDE and NEP were higher in the mice treated with hucMSC-exosomes than in the control group (Fig. 4). Our data indicated that hucMSC-exosomes could increase the levels of Aβ-degrading enzymes in vivo.
HucMSC-exosomes promote the secretion of Aβ-degrading enzymes. Expression of Aβ-degrading enzymes in brains of AD mice, including IDE and NEP was determined by Western blot with hucMSC-exosomes transplantation group or the control group; The relative densities of all proteins bands were normalized to β-actin in the same samples. (n = 6 per group) Data were presented as mean ± SEM. *P < 0.05; the HUMSC-exosomes transplantation group versus the control group
HucMSC-Exosomes Injection Reduces the Inflammatory Reaction of Microglia and Induces Alternative Microglial Activation In Vivo and In Vitro
All forms of AD are characterized by excessive Aβ accumulation in the brain [2, 28]. Neuroinflammation which is mediated by accumulation of Aβ manifested an increased number of activated microglia in the brain [29]. Therefore, as the resident innate immune cells in CNS, microglia are always counted for evaluation of neuroinflammation. In our tests, the total number of activated microglia was counted for investigating the effect of hucMSC-exosomes injection in regulation of neuroinflammation. The density of Iba-1-positive microglia presented by quantitative image analysis was lower in the hucMSC-exosomes injection group than in the control group (Fig. 5a, b). These data suggested that in the AD mouse model, hucMSC-exosomes injection produced a lower number of activated microglial cells.
HucMSC-exosomes transplantation reduces the inflammatory reaction of microglia and induces alternative microglial activation in vivo. a As described in the “Methods” section, Immunofluorescence staining with anti-Iba1 was performed to detect microglia in both the hippocampus and cortex of mice. The processed tissue sections were stained with rabbit anti-mouse Iba-1 IgG (1:500, Wako), and then visualized by the fluorescent dye-conjugated secondary antibodies IgG-FITC. Scale bar, 20 µm. b Quantification of the staining images. The Iba-1 burden was calculated as the percentage of Iba-1-positive area over the total area by using the software Image Pro Plus 6 (Media Cybernetics). (n = 6 in each group) Data are presented as mean ± SEM. *P < 0.05; the hucMSC-exosomes transplantation group versus the control group. c mRNA levels of Arg-1, YM-1, MRC1, FIZZ1, and CD163 in brains of AD mice were evaluated by quantitative RT-PCR. GAPDH was measured as the reference gene. (n = 6 in each group)
Microglia contain different states in neuroinflammation: proinflammatory “M1 microglia” and anti-inflammatory “M2 microglia”. “M2 microglia” are indicated to increase the expression of the two main Aβ-degrading enzymes in the brain [30, 31]. Therefore, to explore whether hucMSC-exosomes injection induces alternative microglial activation (M2-like microglia) can further estimate the role of hucMSC-exosomes injection in neuro-immuno-regulation, we used the following markers for M2-like microglia: Chitinase 3-like 3 (YM-1), arginase-1 (Arg-1), and AMCase, mannose receptors C type 1 (MRC1), found in inflammatory zone 1 (FIZZ1), and the haptoglobin/hemoglobin scavenger receptor (CD163). It was demonstrated by the qRT-PCR assay that gene-expression levels of YM-1, Arg-1, MRC1, FIZZ1, and CD163 in the brains of the mice with hucMSC-exosomes injection were higher than those in the mice treated with the reagent of the control group (Fig. 5c). This suggested that hucMSC-exosomes injection increased the number of “M2 microglia” in AD mice.
To investigate the influence of hucMSC-exosomes on microglial alternative activation in vitro, Aβ25–35 pretreated BV2 cells were treated either with hucMSC-exosomes (30 µg/mL) or with the reagent of the control group for 24 h. The assessment of the alternative activation relied on molecule markers Arg1 and Ym1. In our tests of immunofluorescent staining, Ym-1 staining intensity was increased in the hucMSC-exosomes group compared with the control group (Fig. 6a). This suggested that hucMSC-exosomes treatment induced the alternative activation of BV2 cells. Using qRT-PCR the up-regulation of Arg1 and Ym1 was also determined. A significant increase in Arg1 and Ym1 RNA levels was observed after treatment with hucMSC-exosomes (Fig. 6b).
HucMSC-exosomes induces alternative microglial activation in vitro. a Immunofluorescence staining for Ym-1 demonstrated increased staining intensity for Ym-1 after treatment with hucMSC-exosomes. Scale bars indicate 100 µm. b Quantitative RT-PCR showed a significant increase in Arg1 and Ym1 RNA levels of BV2 after treatment with hucMSC-exosomes. (n = 6 in each group) Data are presented as mean ± SEM. *P < 0.05, **P < 0.01
The above data indicated that hucMSC-exosomes could alleviate acute inflammation and induced alternative activation of microglia in vivo and in vitro.
Effects of hucMSC-Exosomes on the Levels of Inflammatory Cytokines In Vivo and In Vitro
Along with an alleviation of acute inflammation and an increase of microglial alternative activation, the total number of microglia is reduced and “M2 microglia” is growing. One can imagine, “M1 microglia” will decrease accordingly. “M2 microglia” is launched by as well as lead to the up-regulation of anti-inflammatory cytokines, TGF-β and IL-10. Conversely, pro-inflammatory cytokines, IL-1β and TNF-a are secreted by “M1 microglia”. We used ELISA kits to detect the inflammatory cytokines in both PB and brains of mice treated with hucMSC-exosomes and the ones treated with the reagent of the control group. The levels of the pro-inflammatory cytokines IL-1β and TNF-α were lower in the hucMSC-exosomes injection group than in the control group in PB and brains respectively (Fig. 7a, b). In contrast, the levels of the anti-inflammatory cytokines IL-10 and TGF-β were higher in the hucMSC-exosomes injection group than in the control group (Fig. 7a, b).
HucMSC-exosomes modulate neuroinflammation by regulating the secretion of inflammatory cytokines in vivo and in vitro. a Pro-inflammatory cytokines IL-1β and TNF-a, and the anti-inflammatory cytokines TGF-β and IL-10 in brains of mice were analyzed by ELISAs according to the manufacturer’s protocol by using ELISA kits (ZCi Bio, ShangHai, China) (n = 6 in each group). b Pro-inflammatory cytokines IL-1β and TNF-a, and anti-inflammatory cytokines, IL-10 and TGF-β, in PB of mice were analyzed by ELISAs according to the manufacturer’s protocol by using ELISA kits (ZCi Bio, ShangHai, China) (n = 6 in each group). c Pro-inflammatory cytokines IL-1β and TNF-a, and the anti-inflammatory cytokines IL-10 and TGF-β in the culture medium of BV2 cells (pretreated with Aβ25–35) were analyzed by ELISAs according to the manufacturer’s protocol by using ELISA kits (ZCi Bio, Shanghai, China) (n = 6 in each group). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01; the hucMSC-exosomes-treated group versus the control group
We also used ELISA kits to detect the inflammatory cytokines in the culture medium of BV2 cells (pretreated with Aβ25–35) treated with hucMSC-exosomes (30 µg/mL) and the ones treated with the reagent of the control group (Fig. 7c). The levels of the proinflammatory cytokines, IL-1β and TNF-a, were lower in the hucMSC-exosomes group than in the normal control group. In contrast, the level of the anti-inflammatory cytokines IL-10 and TGF-β were higher in the hucMSC-exosomes group than in the normal control group.
The above data indicated that hucMSC-exosomes were associated with up-regulation of the levels of the anti-inflammatory cytokines and down-regulation of those of pro-inflammatory cytokines in vivo and in vitro, suggesting that hucMSC-exosomes played an important role in modulating neuroinflammation by regulating the secretion of inflammatory cytokines.
Discussion
Exosomes have a round morphology with a hypodense center and a diameter ranging from 30 to 150 nm. The outer membranes of exosomes, which are composed of lipids and proteins, express the specific surface markers CD9 and CD63. In recent years, many researches have demonstrated that exosomes carry multiple kinds of functional proteins, DNAs, RNAs, lipids, and microRNAs to conduct cell-to-cell communication [32,33,34]. As an important form of paracrine secretion, the proteins, DNAs, lipids and RNAs are encapsulated in cholesterol-rich phospholipid vesicles and transported through out bodies without enzymolysis [35]. The exosomes derived from MSCs can mimic the therapeutic effects of MSCs and avoid their imperfections in clinical treatment. Especially the exosomes from human umbilical cord mesenchymal stem cells have a more extensive application prospect in promising clinic treatment than those from mice. HucMSC-exosomes transfer a large number of therapeutic factors from hucMSCs and manifest no tumorigenicity, low immunogenicity, outstanding self-renewal and immunoregulation character. Because of the source in human, hucMSC-exosomes seem easier to be accepted in clinical treatment by both doctors and patients in the future. However, there has been no research about effects of hucMSC-exosomes in AD. In this study, the hucMSC-exosomes were injected into the mice through tail veins and rapidly moved forward with the blood flow without uptake or destruction by tail endothelial cells. When they are carried to the tissues such as the brain, the blood flow slows down which facilitates hucMSC-exosomes fusing with the target cells. Moreover, exosomes are able to easily cross the BBB, especially under pathological conditions such as AD and other neurodegenerative diseases [11,12,13]. Therefore hucMSC-exosomes replaced hucMSCs to communicate with cells such as microglia, astrocytes and neurons in the brains of the AD mice.
It has been widely reported that exosomes derived from MSCs possess immunomodulatory effects and relieve inflammation in different disease [36, 37]. Exosomes from MSCs can inhibit the proliferation of T cells and significantly reduce IFN-γ production [38]. In our research, we detected a similar immunoregulatory function of hucMSC-exosomes in AD mice and BV2 cells.
Neuroinflammation which is mediated by accumulation of Aβ is an obvious pathological feature of AD. Microglial activation generally induced by Aβ plaque in AD is the main component of neuro-immuno-regulation [29]. As for the lymphocyte system, a dichotomy has been proposed for macrophage activation: classic versus alternative, also M1 and M2, respectively [39, 40]. Similar to peripheral macrophages, several studies have demonstrated that microglia, like peripheral macrophages, exhibit two entirely different functional activation states [41,42,43]. In vitro, BV2 cells also have two types of polarization, M1/M2 [44, 45]. In the pro-inflammatory activated state, microglial cells are related to the up-regulation of pro-inflammatory cytokines such as IL-1β(a dominating cytokine secreted by classic activated microglia and related to neuroinflammation) and TNF-α (another dominating cytokine secreted by classic activated microglia and also involved in neuroinflammation). These pro-inflammatory cytokines cause neurodegeneration by inducing Aβ accumulation and reducing Aβ clearance [46,47,48]. Alternatively activated microglia, which was demonstrated to be neuroprotective by alleviating acute inflammation and degrading abnormal Aβ accumulation are launched by as well as lead to the up-regulation of anti-inflammatory cytokines including IL-10 (a prominent anti-inflammatory cytokine) and TGF-β (a pleiotropic cytokine with an anti-inflammatory function) [3, 41]. These anti-inflammatory cytokines lead to wound healing, alleviates pro-inflammatory immune-responses, and return tissue homeostasis [42, 49,50,51].
In addition, growing evidence suggests that CNS and peripheral inflammation might present common features in the disease. Recent findings indicate that microglia in AD is senescent whereas peripheral blood mononuclear cells (PBMCs) could infiltrate the brain to phagocyte amyloid deposits. Autophagy is a physiological degradation of proteins and organelles and can be enhanced by pro-inflammatory cytokines. Inflammation would induce autophagy in the PBMCs of AD patients while an anti-inflammatory environment could inhibit their autophagic response [52, 53].
In this study, we observed an upregulation of TGF-β and IL-10 expression and a downregulation of IL-1β and TNF-α in the PB and brains of hucMSCs-exosomes-injection mice and in the culture medium of BV2 treated with hucMSCs-exosomes. These results indicated that hucMSCs-exosomes had immunoregulatory functions in CNS and in vitro. Furthermore, the reduction of peripheral inflammation resulted from hucMSCs-exosomes injection might decrease the autophagy of PBMCs. Consequently, more Aβ deposits could be phagocyted by PBMCs which infiltrated into the brain.
In addition, in the review by Ransohoff, by approaches such as genome-wide transcriptomics, epigenomics and proteomics, the M1/M2 categories of microglial polarization are fine in vitro, but fail to yield a meaningful account of the transcriptional profile of the cell in vivo [54]. This new standpoint provided us innovative and meaningful research views and methods of microglia and enlightened us much on the future study of neuroinflammation. Meanwhile, we also noticed that in a great many studies of inflammation in vivo, the “M1/M2” was still applied extensively in researches of microglia, and the markers of M1/M2 such as arginase-1 and Ym1 were still used in studies about microglial activation [40, 55, 56]. In our future research, we will definitely think over the new viewpoint and reconsider the phenotypes of microglia.
In AD, Aβ shows a significant imbalance between biosynthesis and elimination [57]. The predominant protease which has been reported to conduct cleavage of Aβ is NEP, a type II integral membrane protein. It has been demonstrated that the intracerebral expression of NEP obviously decreases in AD and the up-regulation of NEP expression in brains is related to lower Aβ deposition [30, 58]. IDE is another zinc metallopeptidase associated with the clearance of Aβ in brains [31]. “M2 microglia” was indicated to increase expression of IDE and NEP in vivo [26, 59, 60]. We found that hucMSC-exosomes injection significantly enhanced expression of IDE and NEP in AD mice by alternatively activating microglial cells. We also detected that hucMSC-exosomes injection significantly reduced Aβ deposition and soluble Aβ levels. Increasing evidence suggests that Aβ plaque deposition surrounding microglia in brains is responsible for neuronal and synaptic loss which leads to the damages of memories, cognitive functions, and personalities of the patients [61, 62]. In this study, we found that hucMSC-exosomes injection enhanced spatial learning and alleviated memory decline in AD mice.
Several previous studies suggested that exosomes derived from MSCs conduct their immunomodulatory functions by miRNA. miR-21 has been reported to regulate immunoreaction through the mechanisms of inhibiting the NF-κB pathway and reducing STAT3 expression [63]. miR-181c also acts an important role in modulating inflammation by suppressing the TLR4 signaling pathway and weakening NF-κB activation. Both miRNAs were proposed to be found in exosomes from MSCs and applied in the treatment of AD and other inflammation-related diseases [37, 64]. In the study by Cui et al. exosomes from C57BL/6 mice bone marrow mesenchymal stromal cells were injected into APP/PS1 double transgenic mice. Their findings suggested that the exosomes from C57BL/6 mice bone marrow mesenchymal stromal cells could repair cognitive decline and regulate neuroinflammation. In our study, we used hucMSC-exosomes to inject into APP/PS1double transgenic mice and explored the effects on cognitive decline and neuroinflammation. For the ultimate purpose of clinical treatment of AD patient, the use of exosomes derived from human cells is more progressive and has higher clinical value. In addition, we also detected the effects of hucMSC-exosomes on BV2 cells which confirmed the function of neuroinflammatory modulation in vitro. Our findings contributed new evidence and foundation to the field of cell-free treatment of AD. However the exact miRNA or other substance transported by hucMSC-exosomes, through which hucMSC-exosomes attenuated neuroinflammation, needs further exploration.
Abbreviations
- AD:
-
Alzheimer disease
- Aβ:
-
Amyloid β- peptides
- hucMSC-exosomes:
-
Exosomes isolated from human umbilical cord mesenchymal stem cells
- CNS:
-
Central nervous system
- BBB:
-
Blood–brain barrier
- PBS:
-
Phosphate-buffered saline
- RC:
-
Reagent of the control group
- MWM:
-
Morris water-maze
- PB:
-
Peripheral blood
- TGF-β:
-
Transforming growth factor-β
- IL-10:
-
Interleukin-10
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- ELISA:
-
Enzyme-linked immunosorbent assay
- NEP:
-
Neprilysin
- IDE:
-
Insulin-degrading enzyme
- YM-1:
-
Chitinase 3-like 3
- Arg-1:
-
Arginase-1
- CD163:
-
Haptoglobin/hemoglobin scavenger receptor
- FIZZ1:
-
Found in inflammatory zone 1
- MRC1:
-
Mannose receptors C type 1
References
Moonga J, Likupe G (2016) A systematic literature review on nurses’ and health care support workers’ experiences of caring for people with dementia on orthopaedic wards. J Clin Nurs 25(13–14):1789–1804
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-β peptide and Alzheimer’s disease. Pharmacol Ther 108(2):129–148
Krause DL, Müller N (2010) Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s disease. Int J Alzheimer’s Dis. https://doi.org/10.4061/2010/732806
Pelekanos RA, Li J, Gongora M, Chandrakanthan V, Scown J, Suhaimi N, Brooke G, Christensen ME, Doan T, Rice AM (2012) Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res 8(1):58–73
Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR (2008) Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 26(11):2865–2874
Yang H, Yang H, Xie Z, Wei L, Bi J (2013) Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AβPPswe/PS1dE9 transgenic mice. PLoS ONE 8(7):e69129
Xie Z-H, Liu Z, Zhang X-R, Yang H, Wei L-F, Wang Y, Xu S-L, Sun L, Lai C, Bi J-Z (2016) Wharton’s Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-β deposition in an APP/PS1 transgenic mouse model. Clin Exp Med 16(1):89–98
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9(6):871–881
Zhang B, Shen L, Shi H, Pan Z, Wu L, Yan Y, Zhang X, Mao F, Qian H, Xu W (2016) Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem Cells Int. https://doi.org/10.1155/2016/1929536
Wood MJ, O’Loughlin AJ, Lakhal S (2011) Exosomes and the blood–brain barrier: implications for neurological diseases. Ther Deliv 2(9):1095–1099
Matsumoto J, Stewart T, Banks W, Zhang J (2017) The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des 23(40):6206–6014
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX, Zimak J, Ségaliny A (2016) Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32(6):2003–2014
Endo F, Komine O, Fujimori-Tonou N, Katsuno M, Jin S, Watanabe S, Sobue G, Dezawa M, Wyss-Coray T, Yamanaka K (2015) Astrocyte-derived TGF-β1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep 11(4):592–604
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K, Kim Y-G, Jang J-Y, Kim C-W (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 8(12):e84256
Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid A-A, Mardani K (2012) Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 147(1–2):47–54
Raha S, Lee HJ, Yumnam S, Hong GE, Saralamma VVG, Ha YL, Kim JO, Kim YS, Heo JD, Lee SJ (2016) Vitamin D2 suppresses amyloid-β 25–35 induced microglial activation in BV2 cells by blocking the NF-κB inflammatory signaling pathway. Life Sci 161:37–44
Ruan L, Kang Z, Pei G, Le Y (2009) Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res 6(6):531–540
Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24(3):516–524
Huang P, Lin LM, Wu XY, Tang QL, Feng XY, Lin GY, Lin X, Wang HW, Huang TH, Ma L (2010) Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem 109(4):747–754
Ordonez-Gutierrez L, Fernandez-Perez I, Herrera JL, Anton M, Benito-Cuesta I, Wandosell F (2016) AβPP/PS1 transgenic mice show sex differences in the cerebellum associated with aging. J Alzheimers Dis 54(2):645–656
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848
Nichols JE, Niles JA, DeWitt D, Prough D, Parsley M, Vega S, Cantu A, Lee E, Cortiella J (2013) Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+ CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther 4(1):3
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25(4):402–408
Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae J (2010) Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells 28(2):329–343
Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE (2003) Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci 100(8):4837–4842
Salloway S, Mintzer J, Weiner MF, Cummings JL (2008) Disease-modifying therapies in Alzheimer’s disease. Alzheimer’s Dement 4(2):65–79
Reale M, Brenner T, Greig NH, Inestrosa N, Paleacu D (2010) Neuroinflammation, AD, and dementia. Int J Alzheimer’s Dis. https://doi.org/10.4061/2010/97402
Wang S, Wang R, Chen L, Bennett DA, Dickson DW, Wang DS (2010) Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer’s brain. J Neurochem 115(1):47–57
Edbauer D, Willem M, Lammich S, Steiner H, Haass C (2002) Insulin-degrading enzyme rapidly removes the β-amyloid precursor protein intracellular domain (AICD). J Biol Chem 277(16):13389–13393
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564
Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M (2014) Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep 8(5):1432–1446
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Lanzón MP, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N (2015) Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6(1):127
Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK (2009) Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38(1):215–224
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H (2016) Exosome derived from human umbilical cord mesenchymal stem cell mediates mir-181c attenuating burn-induced excessive inflammation. EBioMedicine 8:72–82
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X, Xu W (2017) Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res Int. https://doi.org/10.1155/2017/5356760
Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Álvarez V, Tarazona R, Casado JG (2014) Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol 5:556
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Investig 122(3):787–795
Franco R, Fernandez-Suarez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Colton CA, Wilcock DM (2010) Assessing activation states in microglia. CNS & Neurol Disord-Drug Targ 9(2):174–191
Mammana S, Fagone P, Cavalli E, Basile MS, Petralia MC, Nicoletti F, Bramanti P, Mazzon E (2018) The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s Disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci 19(3):831
Chen J, Sun Z, Jin M, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y, Pi R (2017) Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-κB pathway. J Neuroimmunol 305:108–114
Gong L, Wang H, Sun X, Liu C, Duan C, Cai R, Gu X, Zhu S (2016) Toll-Interleukin 1 Receptor domain-containing adaptor protein positively regulates BV 2 cell M1 polarization. Eur J Neurosci 43(12):1674–1682
Luccarini I, Grossi C, Traini C, Fiorentini A, Dami TE, Casamenti F (2012) Aβ plaque-associated glial reaction as a determinant of apoptotic neuronal death and cortical gliogenesis: a study in APP mutant mice. Neurosci Lett 506(1):94–99
Lull ME, Levesque S, Surace MJ, Block ML (2011) Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP (751) SL mice. PLoS ONE 6(5):e20153
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28(33):8354–8360
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4(4):399–418
Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T (1999) Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem 72(4):1466–1471
Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M (2005) Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Mol Cell Neurosci 29(3):381–393
François A, Julian A, Ragot S, Dugast E, Blanchard L, Brishoual S, Chassaing D, Page G, Paccalin M (2015) Inflammatory stress on autophagy in peripheral blood mononuclear cells from patients with Alzheimer’s disease during 24 months of follow-up. PLoS ONE 10(9):e0138326
Macchi B, Marino-Merlo F, Frezza C, Cuzzocrea S, Mastino A (2014) Inflammation and programmed cell death in Alzheimer’s disease: comparison of the central nervous system and peripheral blood. Mol Neurobiol 50(2):463–472
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665
Tang J, Yu W, Chen S, Gao Z, Xiao B (2018) Microglia polarization and endoplasmic reticulum stress in chronic social defeat stress induced depression mouse. Neurochem Res. https://doi.org/10.1007/s11064-018-2504-0
Wang D-S, Dickson DW, Malter JS (2006) β-Amyloid degradation and Alzheimer’s disease. BioMed Res Int. https://doi.org/10.1155/JBB/2006/58406
Hellström-Lindahl E, Ravid R, Nordberg A (2008) Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiol Aging 29(2):210–221
Malito E, Hulse RE, Tang W-J (2008) Amyloid β-degrading cryptidases: insulin degrading enzyme, presequence peptidase, and neprilysin. Cell Mol Life Sci 65(16):2574–2585
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ (2003) Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40(6):1087–1093
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Rowan M, Klyubin I, Wang Q, Hu N, Anwyl R (2007) Synaptic memory mechanisms: Alzheimer’s disease amyloid β-peptide-induced dysfunction. Biochem Soc Trans 35:1219–1223
Das A, Ganesh K, Khanna S, Sen CK, Roy S (2014) Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 192(3):1120–1129
Cui G-H, Wu J, Mou F-F, Xie W-H, Wang F-B, Wang Q-L, Fang J, Xu Y-W, Dong Y-R, Liu J-R (2017) Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J 32(2):654–668
Funding
This study was supported by National Natural Science Foundation of China (Grant Nos. 81401052, 81571052), The Special fund for basic research and business funds of Chinese Centre Universities (Grant No. 2018BTS01), Science and Technology Development Plan Project of Shandong Province (Grant Nos. 2017GSF218036, 2017GSF218046), The Fundamental Research Funds of Shandong University (Grant No. 2016JC022), Youth Talent Fund of the 2nd Hospital of Shandong University (Grant No. 2018YT09) and Focus on research and development projects in Shandong province (Grant No. 2015GSF118056).
Author information
Authors and Affiliations
Contributions
MD designed and performed the experiments and wrote the manuscript. YS, ZHX participated in designing the experiments. PW, SLX, and ZYZ provided assistance for data analysis, mouse-injection experiments, and ELISA assay, respectively. YW, YTL, LLX and DWW were responsible for mouse-behavior observation. HY and JZB participated in designing the experiments and drafting the manuscript. All authors read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Shandong University and the ethical committee of the Second Hospital of Shandong University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable Shandong University and the ethical committee of the Second Hospital of Shandong University guidelines for the care and use of animals were followed.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ding, M., Shen, Y., Wang, P. et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem Res 43, 2165–2177 (2018). https://doi.org/10.1007/s11064-018-2641-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2641-5