Abstract
Evidence has shown therapeutic potential of irisin in cerebral stroke. The present study aimed to assess the effects of recombinant irisin on the infarct size, neurological outcomes, blood–brain barrier (BBB) permeability, apoptosis and brain-derived neurotrophic factor (BDNF) expression in a mouse model of stroke. Transient focal cerebral ischemia was established by middle cerebral artery occlusion (MCAO) for 45 min and followed reperfusion for 23 h in mice. Recombinant irisin was administrated at doses of 0.1, 0.5, 2.5, 7.5, and 15 µg/kg, intracerebroventricularly (ICV), on the MCAO beginning. Neurological outcomes, infarct size, brain edema and BBB permeability were evaluated by modified neurological severity score (mNSS), 2,3,5-triphenyltetrazolium chloride (TTC) staining and Evans blue (EB) extravasation methods, respectively, at 24 h after ischemia. Apoptotic cells and BDNF protein were detected by TUNEL assay and immunohistochemistry techniques. The levels of Bcl-2, Bax and caspase-3 proteins were measured by immunoblotting technique. ICV irisin administration at doses of 0.5, 2.5, 7.5 and 15 µg/kg, significantly reduced infarct size, whereas only in 7.5 and 15 µg/kg improved neurological outcome (P < 0.001). Treatment with irisin (7.5 µg/kg) reduced brain edema (P < 0.001) without changing BBB permeability (P > 0.05). Additionally, irisin (7.5 µg/kg) significantly diminished apoptotic cells and increased BDNF immunoreactivity in the ischemic brain cortex (P < 0.004). Irisin administration significantly downregulated the Bax and caspase-3 expression and upregulated the Bcl-2 protein. The present study indicated that irisin attenuates brain damage via reducing apoptosis and increasing BDNF protein of brain cortex in the experimental model of stroke in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irisin is a novel peptide hormone derived from precursor fibronectin type III domain-containing protein 5 (FNDC5). It is mainly released from human and rodent skeletal muscle tissue in response to physical activity [1]. Recently, Roca-Rivada reported that irisin is also a new adipokine secreted from the adipose tissue [2]. In addition, it likely acts as a link between physical activity and the health state of the neural system in human [1]. However, the physiological and pathological roles of irisin are not completely clear. It has been showed that irisin is implicated in the regulation of the energy consumption, body weight regulation and metabolic diseases [1]. Moreover, there are some scientific reports that indicates irisin might have associated with the central nervous system diseases [1, 3] and myocardial infarction [4, 5]. A recent study indicated that irisin (100 mg/kg) remarkably reduces the myocardial infarct size and improves the ventricular function via reducing apoptosis and oxidative stress-induced damage in a Langendorff perfused heart and in vitro model of cultured myocytes [6]. Jiang et al. reported that irisin relaxed the mesenteric arteries via endothelium-dependent and endothelium-independent pathways in in vitro model [7].
An animal study reported that irisin and FNDC5 were expressed in cerebellum Purkinje cells in rats and mice [8]. Another experimental study has revealed that irisin or FNDC5 might contribute in regulating neural differentiation and proliferations of hippocampal neuronal cells [9, 10]. These documents suggest that irisin may have a therapeutic potential effect on managing degenerative diseases. Interestingly, Li et al. showed that intravenous administration of recombinant irisin at 30 min after MCAO markedly diminished infract size and improved neurological function via activating the Akt and ERK1/2 signaling pathways and attenuating the inflammatory cytokines in the mice model of stroke [11]. Moreover, a recent study reported that irisin protected neuronal cells against injury induced by exposure to oxygen–glucose deprivation in PC1-2 cell line [12]. Recently a number of clinical studies reported that there might be a link between plasma level of irisin and risk of cardiovascular and cerebrovascular diseases as a predictive factor [13,14,15].
However, more investigations are necessary to clarify the role of irisin in cerebral stroke and exploring possible molecular mechanisms. Therefore, the present study aimed to assess the effects of various doses of recombinant irisin on the ischemic injury, neurological outcomes, BBB permeability, apoptotic cells and apoptosis-related proteins and BDNF in a mouse model of stroke.
Materials and Methods
Animals and Ethics
Male Swiss albino mice (30–35 g) were obtained from the animal center of Semnan University of Medical Sciences (SUMS), Semnan, Iran. The mice were housed in standard condition and had unlimited access to food and water. All experiments were performed in accordance with the national guidelines on the care and use of laboratory animals in medical research and the institutional guidelines of SUMS Research Ethics Committee. Study protocol was approved by the SUMS institutional Committee of Research Ethics (ethical code number: IR.SUMUMS.REC.1394.127).
Animal Model of Stroke
MCAO model was used to produce focal ischemic stroke in mice. Under chloral hydrate (400 mg/kg, IP) anesthesia and LDF guide, a silicone-coated 8-0 monofilament was inserted into the internal carotid artery and advanced to the origin of MCA to blockage of cerebral circulation. After 45 min, the monofilament was extruded and the reperfusion was established for about 23 h in middle cerebral artery [16].
Local Cerebral Blood Flow (LCBF) Measurement
Under deep anesthesia and before starting the microsurgery, temporal bone in the right side was exposed, and LDF probe was fixed on the temporal bone to record the basal regional blood flow during MCAO and reperfusion. Cerebral blood flow continuously recorded every 5 min until 15 min after reperfusion. Local blood flow in the ischemic cortex was monitored for 15 min before MCAO, 45 min during MCAO and 15 min after reperfusion.
Experimental Protocol and Design
The experiments were carried out in the following seven steps, respectively. At the first step, to evaluate the effects of various doses of irisin peptide (orb180476, Biorbyt, Cambridge, UK) on brain damage and neurological function, 42 mice were randomly divided into six equal groups (n = 7 each); (1) control (PBS) group received PBS as vehicle, (2) 0.1 µg/kg irisin, (3) 0.5 µg/kg irisin, (4) 2.5 µg/kg irisin, (5) 7.5 µg/kg irisin and (6) 15 µg/kg of irisin. Then groups were compared for neurological outcomes and infarct size defined by TTC staining. The dose of 7.5 µg/kg had significantly improved the neurological outcomes and reduced the infarct size compared with the lower doses and the control group. The dose of 15 µg/kg of irisin peptide was significantly effective in improving the neurological outcomes and reduction of infarct size when compare with other doses except the dose of 7.5 µg/kg. Therefore, we used the dose of 7.5 µg/kg as the therapeutic dose for the treatment of the animals in future experiments and investigating the mechanisms of the action of the peptide.
In the next phase of the study, we studied the effect of the time interval of irisin (7.5 µg/kg, ICV) administration after ischemia on the infarct size. For this set of experiments, 28 mice were randomly divided into four equal groups (n = 7, each). In control (PBS) group, PBS as the vehicle was injected 2 µl into right ventricle at the onset of ischemia, and other animals received irisin (IR) at immediately after ischemia (time 0), and 1 and 3 h after ischemia. The best result was observed when irisin administered at time 0.
Then for mechanistic studies we used the following experimental set; the animals were divided into three groups: (1) sham operated (no MCAO), (2) control (PBS), (3) treatment (irisin at a dose of 7.5 µg/kg). Administration of the PBS and irisin (7.5 µg/kg) was immediately after MCAO in groups 2 and 3, respectively. Following variable were studied in these three groups: brain edema (n = 7, each), blood–brain barrier (BBB) permeability (n = 7, each), apoptotic cells (TUNEL positive cells) (n = 6, each), BDNF protein expression (n = 6, each) and Bax, Bcl-2 and caspase-3 protein expression (n = 5, each).
Administrations of the PBS or irisin were in volumes of 2 µl into the right lateral ventricle (0.1-mm posterior relative to the bregma, 0.9-mm right relative to midline and 3.1-mm deep).
Infarct Size Measurement
About 24 h after ischemia and examination of neurological disorders, the mice were sacrificed and their brains were separated and transferred to cold saline (4 °C) for 5 min. Then, five serial 2-mm-thick sections were prepared using of a mice brain matrix. The sections were stained with TTC solution (T8877, Sigma, Germany). TTC-stained slices were digitalized and subsequently infarct area was calculated by image analyzer software (NIH image analyzer). The volume of the lesion was calculated by multiplying the infarct area in the thickness of each section. Finally, data of infarct size was obtained with summing of the infarct volume of five sections and expressed as volume (mm3) corrected for edema.
Brain Edema Measurement
About 24 h after MCAO, the percent of brain water content (BWC%) as an index of brain edema was determined. Briefly, the animals were sacrificed and their brains were isolated and weighed to obtain their wet weight (WW). Subsequently, the brains were dried at 110 °C for 24 h to obtain their dry weight (DW). Brain water content percentage was obtained using the following formula: (WW − DW)/WW × 100 [17].
BBB Permeability
BBB disruption was determined by quantifying the EB leakage into ischemic brain tissue [18]. Briefly, Evans blue (E2129 Sigma, EB 2% in saline, 2 ml/kg) was injected through the tail vein at 1 h after ischemic reperfusion (I/R). Afterward, 24 h after ischemia and under deep anesthesia, chest wall was unscrewed and 200 ml saline solution transcardially infused to eliminate the cerebral circulation of EB. Subsequently, animals were decapitated. Brains were removed, and hemispheres were separated and weighed. Each isolated hemisphere was homogenized in 2.5 ml PBS and, then 2.5 ml of 60% trichloroacetic acid (CAS 76-03-9 Merck, Germany) was added to precipitate the protein. Afterward, samples were centrifuged for 30 min at 3500 rpm. The supernatants were measured at 610 nm for the absorbance of EB using a spectrophotometer (UV VIS spectrophotometer, Spekol 2000, Analytik Jena, AG, Germany). The data were presented in units of microgram per gram of the wet brain tissue and were calculated through a standard curve
Neurobehavioral Assessment
Sensory and motor functions were evaluated by modified neurological severity score (mNSS) at 24 h after MCAO as described in Table 1[18]. The scoring is as 10–14 severe; 5–9 moderate; and 1–4 mild injury. An operator who was unaware of the animal groups’ allocation performed the neurological test.
Histological Procedure, TUNEL and BDNF Immunohistochemistry
About 24 h after ischemia, animals were sacrificed and their brain isolated and paraffin embedded. Three coronal sections (5 µm thick) were prepared with a 2000-µm interval from bregma − 2 to + 2 for TUNEL and BDNF positive cells assay.
Cell apoptosis was assayed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique. TUNEL staining was performed using In Situ Cell Death Detection kit, Fluorescein (11684817910, Roche Diagnostic GmbH, Germany) according to the manufacturer’s protocol. The nuclei were stained by incubating the tissue by with Propidium Iodide (PI) (1 mg/ml) for 30 s. TUNEL-positive cells (green) were detected using a fluorescence microscopy (Olympus Corporation, Japan) at high magnification (×400) and counted on five random fields for each section by an investigator unaware to experimental design. Data was analyzed by Image J software and presented as a percent of total number of PI-stained nuclei.
Immunohistochemistry was used to detect the expression of BDNF in the ischemic cortex at 24 h after ischemia. To identify the expression of immune-like BDNF protein, brain sections were incubated for 45 min at 37 °C in a blocking solution (10% normal goat serum, 3% Triton X-100 in PBS) followed by incubation in a monoclonal anti-BDNF (ab203573;1:200, Abcam, UK) overnight. Then, sections were washed in PBS (3 × 5 min) and incubated in FITC-anti-mouse IgG (1:200, Abcam, UK) for 2 h at room temperature. The nuclei were stained by incubating the tissue in PI (1 mg/ml) for 30 s. BDNF protein (green) was detected using a fluorescence microscopy (Olympus Corporation, Japan) at high magnification (×400) and counted on five random fields for each section by an investigator blinded to experimental design. Data was analyzed by Image J software and expressed as a percent of total number of PI-stained nuclei.
Western Blot Assay
Western blot assay was performed according to our previous study [19]. Briefly, 24 h after the cerebral ischemia the brain samples (right hemisphere) were collected, and protein components were extracted. Tissue lysis was performed in the RIPA lysis buffer, and general protease inhibitor cocktail was used to inhibit proteolytic protein degradation during the process.
Protein concentrations were measured using a BCA Protein Assay kit (The Thermo Scientific Pierce BCA Protein Assay Kit, Therfmofisher Scientific, US) and separated using sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) gels, and then transferred to Immobilon NC membranes (Millipore, Boston, MA, USA). After blocking with 5% non-fat milk in Tris-buffered saline and Tween 20 (pH 7.6) (TBST), the membranes were incubated with primary antibodies against Bcl-2, Bax, active caspase-3 and GAPDH at 4 °C overnight. Then, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:5000) at room temperature for 2 h. The antibody incubations were followed by TBST washing three times for 5 min each. Then chromogenic substrate was added to the membranes. The protein bands were detected using a Bio-Rad imaging system (Bio-Rad, Hercules, CA, USA) and quantified using the Quantity One software package (West Berkeley, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization.
Statistical Analysis
For statically analysis of neurological function, brain edema, BBB permeability, TUNEL positive cells, apoptosis-related proteins and BDNF protein the one-way ANOVA and Bonferroni and/or Dunnett’s posthoc method were used. The Kruskal–Wallis and Dunn’s posthoc tests were used to analyze the infarct size (Sigma Stat 4.0; Jandel Scientific, Erkmiceh, Germany). Results are presented as mean ± SEM. Value of P < 0.05 was considered statistically significant. The power of 80% was applied to the analysis of the data.
Results
Cerebral Circulation Measurement in MCA Area
Measurement of local cerebral blood flow (LCBF) by Laser Doppler Flowmetry (LDF) in all the animal groups exhibited that CBF after MCA blockage reduced below 20% of the baseline and conserved during 45 min of MCAO (Fig. 1). No significant differences were observed among groups concerning LCBF during MCAO and re-establishing brain circulation (Fig. 1, P > 0.05).
Irisin Reduces Infarct Size and Improves Neurological Outcomes in a Dose-Dependent Manner
Blockage of the MCA for 45 min and 23 h reperfusion in control group (MCAO + PBS) led to brain damage in MCA territory (157 ± 10 mm3). Central administration of irisin (MCAO + IR) at doses of 0.5 (80 ± 13 mm3), 2.5 (69 ± 10 mm3), 7.5 (44 ± 7 mm3) and 15 µg/kg, (41 ± 12 mm3) extensively reduced infarct size compared with the control (PBS) group (P < 0.001, Fig. 2a, b). Whereas, irisin at dose of 0.1 µg/kg, ICV did not significantly change the infarct size (126 ± 10 mm3) compared to the control group; P > 0.05, Fig. 2a, b
Infarct size (a and d), TTC staining image (c) and neurological deficit scores (c) in control (PBS) and irisin (IR) treated groups at doses 0.1 (IR-0.1), 0.5 (IR-0.5), 2.5 (IR-2.5), 7.5 (IR-7.5) and 15 µg/kg ICV (IR-15). PBS and or irisin were administered into ICV at the beginning of MCAO (0 h) or 1 h and or 3 h after MCAO, neurological outcome and infarct size were measured 24 h after ischemia. Red color is intact area and white color is lesion area. Values are as mean ± SEM (n = 7, each). *P < 0.001, compared to the PBS group
Twenty-four hours after ischemia, neurological deficit score was 10 ± 0.51 in the control group. Central administration of irisin (IR) at doses 7.5 (7 ± 0.45) and 15 (7.5 ± 0.42) µg/kg, ICV, noticeably reduced score of neurological deficit (P < 0.004, Fig. 2c). Other doses of irisin did not significantly change the neurological outcome (P > 0.05, Fig. 2c).
The Effect of Irisin on Infarct Size at Different Time Intervals After Brain Ischemia
The infarct size following 45 min MCAO and 23 h reperfusion in the control group was 157 ± 10 mm3. Treatment with irisin (7.5 µg/kg, ICV) at 0 h (44 ± 7 mm3) and 1 h (64 ± 6 mm3) after ischemia significantly diminished the infarct size (P < 0.001, Fig. 2d) whereas; administration of irisin (7.5 µg/kg, ICV) at 3 h (145 ± 19 mm3) after ischemia did not alter infarct size (P > 0.05, Fig. 2d).
The Irisin Protects the Brain Against Edema Formation
In the sham-operated group, percent of brain water content (BWC %) was 78.7 ± 0.4%, at 24 h after surgery. Induction of stroke considerably enhanced BWC to 82.6 ± 0.5% (P < 0.001, Fig. 3a) at 24 h after MCAO. Central administration of irisin (7.5 µg/kg, ICV) notably (81.2 ± 0.3%) reduced the brain edema formation compared to the control (PBS) group (P < 0.001, Fig. 3a). No significant difference was observed (P > 0.05) in the BWC in the non-ischemic hemisphere among groups (P > 0.05, Fig. 3a).
The effects of the optimal dose of irisin (IR-7.5) on brain edema (a) and BBB permeability (b) at 24 h after ischemia. PBS and or irisin were administered ICV at the beginning of MCAO and brain edema and BBB permeability were measured 24 h after ischemia. Values are mean ± SEM (n = 7, each). *P < 0.004 compared to the control (PBS) group. #P < 0.001 compared to the respective sham-operated group
Irisin Did Not Alter MCAO Induced BBB Interruption
The intensity of EB permeation was estimated as an index of BBB disruption following cerebral ischemia. The value of EB in the ischemic region for the control group (11.07 ± 1.05 µg/g tissue) was significantly higher than the sham-operated group (0.63 ± 0.3 µg/g tissue; P < 0.001, Fig. 3b). Central administration of irisin (7.5 µg/kg, ICV) at beginning of MCAO did not alter (8.49 ± 1.35) the EB leakage in the ischemic region when compared with the control group at 24 h after MCAO (P > 0.05, Fig. 3b).
Irisin Reduces MCAO Induced Apoptosis in the Cortex of Cerebral Ischemia
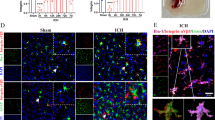
After 45 min MCAO and 23 h of reperfusion, the number of apoptotic cells in the control (MCAO + PBS) group was 58.5 ± 1.2%. It was significantly higher than sham-operated group (10.7 ± 0.84%). Central administration of irisin (7.5 µg/kg, ICV) at beginning of MCAO significantly (26.6 ± 1.42) reduced number of apoptotic cells in the cortex compared with control (PBS) group (P < 0.001, Fig. 4a, b).
TUNEL staining image (a), and quantitative analysis of percent of TUNEL positive cells (b) in sham-operated and control (MCAO + PBS) and irisin (MCAO + IR-7.5) groups. TUNEL positive cells were presented as a percentage of the total number of PI-stained nuclei (×400 fluorescent microscope). The percentage of apoptotic cells was showed as mean ± SEM (n = 6, each). The arrows (white) indicated TUNEL-positive cells. #P < 0.001 compared to respective sham-operated group. *P < 0.001 compared to the control (PBS) group
Irisin Increases Expression of BDNF Protein in Brain Cortex After Ischemia
In immunohistochemical assessment, 24 h after MCAO establishment, we demonstrated that irisin (7.5 µg/kg, ICV) significantly enhanced the expression of BDNF protein in the cortical area of the penumbral zone in the ischemic hemisphere compared with the control (PBS) group (P < 0.001, Fig. 5a, b).
Immune-like reactive BDNF cells (Green) image (a) and quantitative analysis of percent of BDNF expression (b) in sham-operated and control (MCAO + PBS) and irisin (MCAO + IR-7.5) treated groups. The arrows (white) indicated BDNF protein expression. BDNF protein expression was presented as a percentage of the total number of PI-stained nuclei (×400 fluorescent microscope). The BDNF positive reaction was presented as mean ± SEM (n = 6, each). #P < 0.001 compared to respective sham-operated group, *P < 0.001 compared to the control (PBS) group
Irisin Downregulates Bax and Caspase-3 Pro-apoptotic Proteins and Upregulates Bcl-2 Anti-apoptotic Protein
Western blot analysis demonstrated significant elevation of the pro-apoptotic proteins Bax and caspase-3 in the ischemic tissue of the brain; while treatment with irisin significantly downregulated the expression of these proteins (P < 0.001, Fig. 6a–e). Moreover, Bcl-2 protein was downregulated in the ischemic tissue. In the group treated with irisin, expression of Bcl-2 was significantly upregulated (P < 0.001, Fig. 6a–e). In line with these changes, a significant decline in the Bcl-2/Bax relation was observed in the ischemic tissue compared with the sham group (P < 0.001, Fig. 6a–e). This change was reversed in the irisin-treated group and the difference between control group and group treated with irisin was statistically significant (P < 0.001, Fig. 6a–e).
The photograph exhibits the levels of caspase 3, Bax and Bcl-2 in the sham-operated and control (MCAO + PBS) and irisin (MCAO + IR-7.5) groups as detected by western blotting (a). The quantitative analysis shows Bcl-2/GAPDH (b), Bax/GAPDH (c), Bcl-2/Bax (d) and caspase-3/GAPDH (e) ratio at 24 h after MCAO in the sham-operated and control (PBS) and irisin treatment groups (n = 5, each). #P < 0.001 from respective sham-operated group, *P < 0.001 compared to the control (PBS) group
Discussion
Major new finding of the current research is that recombinant irisin has neuroprotective effect against ischemic injury in a dose-dependent manner. This protective effect is maintained up to an hour after ischemia. In the optimum dose, irisin has a protective effect against cerebral edema formation; but it did not alter BBB permeability. According to the findings of the current study, the neuroprotective activity of irisin is mediated by reducing apoptosis and increasing the expression of BDNF in the brain tissue adjacent to the ischemic area in mouse model of MCAO. It is seem that apoptosis reduction in irisin treated group is through inhibiting the expression of Bax and caspase-3 and enhancing the expression of Bcl-2 protein.
We found that central administration of irisin in optimum dose reduces brain damage by 72% compared with the control group. Interestingly, this therapeutic response of irisin was retained up to an hours after ischemia, which it was associated with improvement in neurological function. This result is important because the therapeutic efficacy in clinical setting is estimated via the neurological performance than damage size.
It appears that irisin has potent neuroprotective activity in acute phase of ischemia in the MCAO model of stroke. Data from the present study is in agreement with a recent study by Li et al. that demonstrated peripheral administration of recombinant irisin reduces brain damage in MCAO mice model [11]. Furthermore, Wang et al. reported that irisin strongly reduced infarct size and recovered cardiac performance in a Langendorff perfused heart model [6]. Their study is in line with the findings of the present study in terms of irisin protective effects against ischemic insult.
In the other experiment, we found that treatment with irisin reduced brain edema by 30% relative to control group. This is similar to a study of Li et al. which indicated that intravenous injection of irisin diminished brain water content by about 25% in an experimental model of stroke [11]. Another result of present study showed that cerebrovascular permeability increased extensively at 24 h after cerebral damage, but treatment with irisin did not protect BBB disruption. The question may be raised that how did irisin reduce brain edema following cerebral ischemia? We suggest that irisin may improve brain edema through direct adjusting of the cell volume of neurons and or astrocytes. Further studies are required to confirm this hypothesis.
In the final experiment, the molecular mechanisms implicated in the neuroprotection of irisin in MCAO model of mice were investigated. According to our findings, treatment with irisin reduced apoptotic cell death in the ischemic cortex at 24 h after establishing stroke. In line with these findings, several investigators reported that irisin suppressed apoptosis and/or activated cell survival signaling pathways in the alveolar epithelial cells [20], the pancreatic β cells [21], the vein endothelial cells [22, 23], heart ischemia [6], and in vivo and in vitro model of brain ischemia [11, 12]. According to the results of the current study, upregulation of Bcl-2 protein by administration of irisin is an important negative regulatory mechanism of apoptotic cell death. Moreover, downregulation of Bax, as a positive regulator of apoptotic cell death, and caspase-3, as an effector protease involved in apoptotic proteolysis, contribute to the tissue preservation against apoptosis after brain ischemic-reperfusion injury. Considering the important role of Bax and Bcl-2 gene products in the regulation of the intrinsic apoptotic pathways, it appears that these pathways play an important role in the beneficial effects of irisin peptide. A previous study has demonstrated that circulatory irisin mediates the exercise-induced neuroprotection against brain ischemia by suppressing oxidative stress, inflammatory response, and Akt and ERK1/2 phosphorylation [11]. Moreover, irisin has been reported to increase the neuronal cell proliferation through the STAT3 signaling pathway [9]. Since STAT3 is known to induce the gene expression of anti-apoptotic Bcl-2 family [24]. It is reasonable to attribute the anti-apoptotic effects of irisin after brain I/R injury, at least in part, to the changes in STAT3 signaling. In addition, it has been reported that Akt activation is associated with the upregulation of ERK1/2 and Bcl-2 and downregulation of Bax and subsequent prevention of apoptotic cell death. Findings of the current study hint at a role for these signaling pathways in the neuroprotection of irisin in MCAO model. This warrants further studies to consider the role of these molecular signaling pathways in the physiology of brain protection against I/R injury in response to extrinsic (e.g. irisin therapy) or intrinsic (e.g. in athletes).
Immunohistochemical assay demonstrated that central injection of irisin led to a significant increase in the expression of BDNF in the ischemic brain area. In agreement with this finding, Wrann et al. reported that FNDC5, precursor of irisin, increased BDNF gene expression in cortical cell cultures and hippocampus of mice after intravenous injection [25]. Their study elucidated a positive relationship between FNDC5 and BDNF gene expression. Another study revealed that irisin beneficial effect on mood in COPD patients is probably via increasing expression of BDNF in the brain [26]. BDNF is a neurotrophic factor that plays a key role in regulating neurogenesis, survival of neurons and microglia, stimulating neuroplasticity and differentiation of cells in the central nervous system [27]. Additionally, irisin may directly participate in regulation of the hippocampus neurogenesis and cell proliferation in mouse [9]. Therefore, we suggest that irisin may act via enhancing BDNF expression or directly lead to neuronal survival following cerebral ischemia and decrease infarct size. In this approach, we suggest evaluating the effects of irisin with and without BDNF blocker in future studies. Other mechanisms may participate with the protective effect of irisin against stroke damage, which has not been investigated in this study. Recent studies reported that irisin suppresses production of pro-inflammatory cytokines of TNF-α, IL-6 and ROS formation following cerebral ischemia [11, 12]. It is well identified that excessive formation of free radicals and pro-inflammatory cytokines plays an important role in stroke pathophysiology [28]. Therefore, we suggest that part of the neuroprotective effects of irisin could be due to the inhibition of the production of pro-inflammatory cytokines and free radicals.
In conclusion, findings of current study show that irisin exerts dose dependent neuroprotective effects in stroke-induced brain damage. This effect is via suppressing apoptosis and increasing the BDNF expression in the ischemic area. Clinical application of the irisin to treat stroke and brain ischemia warrants further investigations.
References
Novelle MG, Contreras C, Romero-Picó A et al (2013) Irisin, two years later. Int J Endocrinol 2013:746281. https://doi.org/10.1155/2013/746281
Roca-Rivada A, Castelao C, Senin LL et al (2013) FNDC5/Irisin is not only a myokine but also an adipokine. PLoS ONE 8:e60563. https://doi.org/10.1371/journal.pone.0060563
Jodeiri Farshbaf M, Ghaedi K, Megraw TL et al (2016) Does PGC1α/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? NeuroMol Med 18:1–15. https://doi.org/10.1007/s12017-015-8370-x (Epub 2015 Nov 26).
Kuloglu T, Aydin S, Eren MN et al (2014) Irisin: A potentially candidate marker for myocardial infarction. Peptides 55:85–91. https://doi.org/10.1016/j.peptides.2014.02.008
Aydin S, Aydin S, Kobat MA et al (2014) Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides 56:141–145. https://doi.org/10.1016/j.peptides.2014.04.002
Wang H, Zhao YT, Zhang S et al (2017). Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol 232(12):3775–3785. https://doi.org/10.1002/jcp.25857
Jiang M, Wan F, Wang F, Wu Q (2015) Irisin relaxes mouse mesenteric arteries through endothelium-dependent and endothelium-independent mechanisms. Biochem Biophys Res Commun 468(4):832–836. https://doi.org/10.1016/j.bbrc.2015.11.040
Dun SL, Lyu R-M, Chen Y-H et al (2013) Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 240:155–162. https://doi.org/10.1016/j.neuroscience.2013.02.050
Moon H-S, Dincer F, Mantzoros CS (2013) Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 62:1131–1136. https://doi.org/10.1016/j.metabol.2013.04.007
Hashemi M-S, Ghaedi K, Salamian A et al (2013) Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231:296–304. https://doi.org/10.1016/j.neuroscience.2012.11.041
Li D-J, Li Y-H, Yuan H-B et al (2017) The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 68:31–42. https://doi.org/10.1016/j.metabol.2016.12.003
Peng J, Deng X, Huang W et al (2017) Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol Immunol 91:185–194. https://doi.org/10.1016/j.molimm.2017.09.014
Wu H, Guo P, Jin Z et al (2018) Serum levels of irisin predict short-term outcomes in ischemic stroke. Cytokine. https://doi.org/10.1016/j.cyto.2018.02.017
Icli A, Cumhur Cure CE M3, et al (2016) Novel myokine: irisin may be an independent predictor for subclinic atherosclerosis in Behçet’s disease. J Investig Med 64(4):875–881. https://doi.org/10.1136/jim-2015-000044 (Epub 2016 Mar 3).
Panagiotou G, Mu L, Na B et al (2014) Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism 63(10):1265–1271. https://doi.org/10.1016/j.metabol.06.001 (Epub 2014 Jun 9)
Akhoundzadeh K, Vakili A, Shadnoush M, Sadeghzadeh J (2018) Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci 43(1):32–40
Hadadha M, Vakili A, Bandegi AR (2015) Effect of the inhibition of hydrogen sulfide synthesis on ischemic injury and oxidative stress biomarkers in a transient model of focal cerebral ischemia in rats. J Stroke Cerebrovasc Dis 24:2676–2684
Behrouzifar S, Vakili A, Bandegi AR et al (2018) Neuroprotective nature of adipokine resistin in the early stages of focal cerebral ischemia in a stroke mouse model. Neurochem Int 114:99–107
Javedan G, Shidfar F, Davoodi SH et al (2016) Conjugated linoleic acid rat pretreatment reduces renal damage in ischemia/reperfusion injury: unraveling antiapoptotic mechanisms and regulation of phosphorylated mammalian target of rapamycin. Mol Nutr Food Res 60:2665–2677. https://doi.org/10.1002/mnfr.201600112
Shao L, Meng D, Yang F et al (2017) Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun 487:194–200. https://doi.org/10.1016/j.bbrc.2017.04.020
Liu S, Du F, Li X et al (2017) Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 12:e0175498. https://doi.org/10.1371/journal.pone.0175498
Song H, Wu F, Zhang Y et al (2014) Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS ONE 9:e110273. https://doi.org/10.1371/journal.pone.0110273
Lu J, Xiang G, Liu M et al (2015) Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 243:438–448. https://doi.org/10.1016/j.atherosclerosis.2015.10.020
Bhattacharya S, Ray RM, Johnson LR (2005) STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J 392:335–344. https://doi.org/10.1042/BJ20050465
Wrann CD, White JP, Salogiannnis J et al (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Papp C, Pak K, Erdei T et al (2017) Alteration of the irisin-brain-derived neurotrophic factor axis contributes to disturbance of mood in COPD patients. Int J Chronic Obstr Pulm Dis 12:2023–2033. https://doi.org/10.2147/COPD.S135701
Begni V, Riva MA, Cattaneo A (2016) Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin Sci 131(2):123–138. https://doi.org/10.1042/CS20160009
Vakili A, Mojarrad S, Akhavan MM, Rashidy-Pour A (2011) Pentoxifylline attenuates TNF-α protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res 1377:119–125. https://doi.org/10.1016/j.brainres.2011.01.001
Acknowledgements
This work was financially supported by a research grant from Vice Chancellor for Research of the Semnan University of Medical Sciences (Grant Number: 917).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Asadi, Y., Gorjipour, F., Behrouzifar, S. et al. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem Res 43, 1549–1560 (2018). https://doi.org/10.1007/s11064-018-2569-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2569-9