Abstract
The presence of a brain renin angiotensin system (RAS) is well documented. An overactive brain RAS contributes to the development and progression of cardiovascular and renal disorders among other conditions. In hypertension, an augmented brain RAS leads to an increase in sympathetic nervous system activity. In addition, impaired baroreceptor reflex function, increased vasopressin activity and neuroinflammation are important contributors as well. The relevance of angiotensins in central and peripheral systems, such as neurons and vascular smooth muscle cells, in cardiovascular disease pathogenesis is fairly understood. However, the role of astrocytes is less well studied. Astrocytes are a major contributor to neuroinflammation by increased synthesis and secretion of inflammatory mediators, dysregulated astrogliosis and impaired astrocyte proliferation. Astrocytes may also contribute to impaired neuromodulation. The precise molecular mechanisms by which astrocytes mediate these effects are still not fully understood. Here, we summarize the role of astrocytes in RAS -mediated pathogenesis of hypertension and other cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that the renin angiotensin system (RAS) plays a key role in the pathogenesis of numerous diseases. In hypertension, an overactive brain RAS leads to an increase in sympathetic nervous system activity [1]. Impaired baroreceptor reflex function, increased vasopressin activity and neuroinflammation are important contributors as well [2, 3]. It is no surprise then, that current therapeutic strategies for hypertension, renal and cardiovascular disorders target the RAS [4, 5]. These therapeutic strategies include the use of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers and direct renin inhibitors [4]. Despite several positive outcomes with these treatment measures, many patients remain resistant to treatment. The classical RAS was mainly focused on systemic RAS components and their effects in the periphery. RAS components and signaling pathways involving tissue RAS and brain or central RAS are now emerging. Since the discovery of the brain RAS, numerous research efforts have focused on (i) the distribution of the RAS components in the various brain cell types, and (ii) elucidating the molecular mechanisms involved in brain RAS-mediated pathogenesis [6,7,8,9].
All RAS components are found in the brain, localized in neuronal and non-neuronal cells [10, 11]. There is ongoing debate regarding the presence of the Angiotensin type 1 (AT1R) and Angiotensin type 2 (AT2R) receptors on astrocytes under physiological conditions in vivo [12]. In vitro studies in rat models and human astrocytoma cell lines have confirmed the presence of AT1Rs and AT2Rs in astrocytes [7, 13]. Since this discovery of functional receptors for Angiotensin (Ang) II on astrocytes, it is now accepted that glial cells are actively involved in brain RAS-mediated actions. As such, astrocytes are now emerging as key players in the development and progression of pathological conditions including hypertension. The role of astrocytes in RAS-mediated pathogenesis of hypertension and cardiovascular diseases, and the molecular mechanisms involved are not fully understood. Whether the deleterious effects of the RAS are elicited directly through neuronal AT1Rs, glial AT1Rs or both is not known. It is theorized that in the hypertensive state, glial AT1Rs are directly activated contributing to the disease [12]. These are current areas of active research in our laboratory and others.
The aim of this review is to summarize what is known about the association between brain RAS and astrocytes in the pathogenesis of hypertension and cardiovascular diseases. This review covers the RAS components in the CNS, and the reported peripheral and central actions of the angiotensin peptides that may drive hypertension and other cardiovascular diseases. It also covers the role of astrocytes in the brain and the RAS-mediated central molecular mechanisms that are known to occur in the brain. Finally, we propose further studies that would help to understand the molecular mechanisms involved in hypertension and cardiovascular disease pathogenesis in astrocytes.
Renin Angiotensin System Components
The RAS is described as a hormonal system that regulates arterial pressure, tissue perfusion and extracellular volume [14]. Over the years, the role of the RAS has widened to include functions such as regulation of cell proliferation, apoptosis, fibrosis and proinflammatory activities [15]. Overactivity of this system has been implicated in a number of cardiovascular disorders and the metabolic syndrome [16]. Ang II, the major effector peptide for the RAS is a potent mitogen, a mediator for proinflammatory states, and a vasoactive hormone [15]. Studies have shown that the levels of Ang II in the brain are in the picogram range. This translates to more than a 1000-fold higher levels in the brain compared to circulating Ang II based on the plasma volume in the brain [17]. However, the distribution of AT1Rs differ by brain region. Ang II is the most widely studied ligand of the RAS and is the main focus of this review.
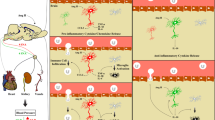
Ang II mediates its effects through two main receptors, the AT1R and the AT2R [18]. Most of the actions of Ang II are elicited by the AT1R [18]. Data continue to emerge for Ang II metabolites and other active RAS peptides (Table 1). As new biologically active RAS peptides and receptors are discovered, the role of the RAS in disease pathogenesis continues to expand. Ang III has similar effects to Ang II and binds to the same receptors (AT1R and AT2R). Other active RAS peptides include Ang IV and its receptor AT4R (IRAP), Ang (1–7) and its receptor Mas, as well as Ang (1–9) which binds to AT2Rs. The enzymes renin, ACE, ACE2, neutral endopeptidase (NEP)/ACE, aminopeptidase A (APA), aminopeptidase N (APN) are involved in the formation of Ang I, Ang II, Ang (1–7) and Ang (1–9), Ang (1–9) to Ang (1–7), Ang III and Ang IV, respectively (see Fig. 1). Recently identified RAS peptides include prorenin and its receptor [19, 20].
Schematic representation of the Renin Angiotensin System highlighting the components that are known to be present in astrocytes. ACE angiotensin converting enzyme, AT1R angiotensin type I receptor, AT2R angiotensin type 2 receptor, AT4R angiotensin type 4 receptor, IRAP insulin-regulated aminopeptidase. Receptors are denoted by (*)
Our laboratory and others have studied the components of the RAS in rat primary astrocyte cultures. We have used primary astrocyte cultures as a brain model system to investigate various intracellular signaling pathways by which, the active neuropeptides Ang II and Ang III elicit neuroinflammatory and other cardiovascular actions [21,22,23,24]. Other laboratories have confirmed the presence of angiotensin receptor subtypes in astrocytes in vitro [25] using different techniques [6]. However, as previously mentioned, there is conflicting evidence for the presence of the angiotensin receptor subtypes in non-neuronal cell types in vivo [12, 26]. In addition, the precise pathway for Ang II synthesis in the brain is not fully characterized. Whether Ang II is synthesized intracellularly within all brain cell types or its synthesis is predominantly in neurons is yet to be fully established [12].
Peripheral and Central Actions of Angiotensin Peptides
Mechanisms by which Ang II contributes to the pathogenesis of cardiovascular diseases in the periphery include: (i) overactive vascular inflammatory responses in arterial walls resulting in endothelial dysfunction; (ii) stimulation of reactive oxygen species (ROS) production; and (iii) stimulation of hypertrophic growth and accumulation of extracellular matrix [15]. Degradation products of Ang II such as Ang III and Ang IV also stimulate proinflammatory states, extracellular matrix accumulation, and profibrotic states [15]. Moreover, Ang III mediates kidney functions, sodium and water retention, vasoconstriction, thirst, sodium appetite, and vasopressin release [27].
Overactive brain RAS activity can induce or maintain the hypertensive state. Characteristics of brain RAS-mediated hypertension include increased sympathetic vasomotor tone, impaired baroreceptor reflex, and overexpression of angiotensinogen and renin [3]. The molecular mechanisms suggested were enhanced noradrenaline modulation and increased tyrosine hydroxylase activity [3]. Veerasingham and Raizada have extensively reviewed the contribution of brain RAS dysfunction to hypertension with an emphasis on the molecular mechanisms in neurons [3]. Another contribution to brain RAS-mediated hypertension is dysfunctions involving glial cells, in particular astrocytes. Accumulating evidence shows that astrocytes mediate inflammatory and proliferative activities and, are the main source of angiotensinogen [28] in the brain. The central effects of RAS have not been fully investigated, especially in relation to the role of astrocytes. It was originally thought that the brain RAS-mediated actions occurred mostly in neurons with other cell types providing a supporting role. Mounting evidence now suggests a role for astrocytes in pathophysiological conditions [23, 24, 29,30,31]. The interplay between neurons and astrocytes, and the RAS-mediated paracrine and autocrine signaling mechanisms in the brain require further investigation.
Role of Astrocytes in the Brain
Glial cells or neuroglia are non-neuronal brain cells that form a scaffold to support neurons. They are also referred to as the “glue” of the CNS [32]. Astrocytes or astroglia are the most abundant glial cells of the CNS [30]. Neuroglia includes astrocytes, microglia, oligodendrocytes and progenitor cells also known as radial glia cells [32, 33]. Dysregulation of astroglial functions as well as dysfunction of astroglial properties may be linked to several neurological diseases such as epilepsy, amyotrophic lateral sclerosis, ischaemic stroke, and hepatic encephalopathy [31]. The TGF-β signaling pathway in astroglia was shown to sustain brain inflammation in mice [34]. This signaling mechanism demonstrated a connection between the CNS and the immune system in neuroinflammation and autoimmunity in the brain [34].
Astrocytes have multiple subtypes performing different functions [30]. They secrete growth promoting and growth inhibiting substances that support the growth and development of neurons, maintain synaptic homeostasis, form the tripartite synapse, maintain the blood brain barrier, provide metabolic support for neurons and neuroprotection from oxidative stress [30]. They are also a source of extracellular matrix and pro- and anti-inflammatory cytokines [23, 24, 35, 36]. This suggests both neuroinflammatory and neuroprotective roles for astrocytes. The heterogeneity of the astrocyte subtypes may differ by region, and the properties of the astrocytes within and between regions may also vary [31]. Indeed, this diversity of astrocyte subtypes and functions influences mechanisms of neuromodulation effects involving astrocytes [37].
In response to CNS injury, astrocytes migrate to the site of injury by a process called reactive astrogliosis; this serves to protect both injured and uninjured tissue [37, 38]. Reactive astrogliosis is therefore a suitable indicator of tissue injury [37]. Regulated reactive astrogliosis serves a neuroprotective role, however, dysregulated reactive astrogliosis may be harmful and can contribute to pathophysiological conditions [37, 39]. The role of reactive astrogliosis in diseases has been extensively reviewed [37, 39]. Briefly, reactive astrogliosis can be triggered by numerous signaling molecules that can have harmful or beneficial effects depending on the signaling cascades involved. Mechanisms for harmful effects include impaired neurotransmission and neuroinflammation that may be associated with disruption of the blood brain barrier. The following signaling pathways were suggested as regulators of cell proliferation and inflammatory states associated with reactive astrogliosis: Signal transducer and activator of transcription 3 (STAT3), NFkB, suppressor of cytokine signaling 3, nuclear factor-like 2, cyclic adenosine monophosphate, and oligodendrocyte transcription factor [37, 39].
RAS-Mediated Central Effects and Molecular Mechanisms in Astrocytes
The signaling pathways of the angiotensins differ by tissue, affinity of ligand for the receptor, structural modifications of the receptor, duration of exposure to ligand, and concentration of ligand [18]. Moreover, for astrocytes, the signaling pathways differ by brain region. Numerous signaling pathways have been identified in astrocytes linking these cells to neurological disorders. These pathways mediate astrocyte proliferation, hypertrophy, ROS generation, inflammation, and astrogliosis. Indeed, astrocytes have a major role in neuroinflammation and maintenance of the blood brain barrier. Under physiological conditions, the brain and peripheral RAS act independently due to the blood brain barrier that prevents transfer of RAS peptides [17]. In pathological states, blood brain barrier breakdown increases permeability of circulating peptides [40]. This allows circulating peptides such as Ang II to access brain regions, and in particular, the central cardiovascular centers [40]. The binding of Ang II to its receptors in these regions leads to a cascade of events that may result in increased sympathetic outflow and increased synthesis of inflammatory mediators [12]. Local production of Ang II in the brain is not fully understood. The various components are distributed among different brain cell types [12]. Locally produced Ang II may also cause sympathoexcitation and increased synthesis of inflammatory mediators [40].
Ang II regulates numerous astroglial functions. Ang III is believed to have similar effects to Ang II; however, data for Ang III central actions are sparse. Ang II induces astrocyte proliferation [23, 41,42,43], modulates the synthesis and secretion of various proteins involved in the biochemical and regulatory functions of astrocytes [44], activates numerous intracellular signaling pathways linked to various functions such as inflammatory status, cellular growth and proliferation [23], and neurotransmission. Ang II modulates transcriptional regulation of ACE2 mRNA in astrocytes [23]. Moreover, an altered balance of ACE/ACE2 supporting the generation of Ang II instead of Ang (1–7) was observed in SHRs astrocytes [36]. These findings suggest that in SHRs the Ang II counterregulatory peptide Ang (1–7) is produced to a lower extent. This impairment of the counterregulatory axis, creates an environment in the brain that is susceptible to the deleterious effects of Ang II. Physiological levels of Ang II and its metabolites are required for maintenance of the blood brain barrier [45, 46]. Moreover, Ang II inhibits the inflammatory response of astroglial cells by inhibiting nitric oxide production after exposure to the bacterial endotoxin LPS [47], suggesting a role for Ang II in reactive astrogliosis [48]. Ang II stimulation of glucose uptake and the glucose transporter in cultured astrocytes was also suggested to be associated with reactive astrogliosis and recovery from brain injury [48]. Additionally, Ang II stimulated secretion of plasminogen activator inhibitor 1 and human related-tissue inhibitor of metalloprotease in astrocyte cultures [44].
Upregulated expression of AT1Rs in mice astrocytes was linked to exaggerated sympathetic nervous system activity in myocardial infarction (MI)-induced heart failure. The AT1R upregulation was more pronounced in astrocytes compared to neurons suggesting a role for astrocytes in MI-induced heart failure [49, 50].
Mechanisms in Astrocytes Contributing to Cardiovascular Disease Pathogenesis
To date, the role of astrocytes in cardiovascular disease pathogenesis includes upregulated angiotensinogen and renin levels [3], dysregulated astrogliosis [37, 39], neuroinflammation [2], generation of ROS and proinflammatory cytokines [23, 36], disruption of the blood brain barrier [37], and neuron-astrocyte interaction that favors sympathoexcitation [51]. The precise mechanisms by which these processes contribute to cardiovascular disease pathogenesis are not fully understood. The signaling pathways and physiological responses mediated by the angiotensin peptides in astrocytes can be divided into four main categories as shown below.
Crosstalk with Other Receptors
There is accumulating evidence of crosstalk between the AT1R and other receptors. Crosstalk has been observed for the RAS and the endocannabinoid system, particularly the cannabinoid receptor type 1 (CB1R) [52]. Altered Ang II- and Arachidonyl-2′-chloroethylamide (ACEA)-induced mitogen activated protein kinase (MAPK) activation was observed in astrocytes of hypertensive rat models, suggesting that aberrant or reduced CB1R functions could contribute to the pathophysiology of hypertension [53]. In renal tubules, activation of the dopamine D4 receptor (D4R) was shown to decrease AT1R expression [54]. Furthermore, Ang II mediated hypertension in mutant mice deficient in the D4 receptor [55]. Based on these observations in renal tubules, our laboratory is currently investigating the interaction between the AT1R and the dopamine D4 receptor in astrocytes in relation to hypertension. Transactivation was observed for AT1Rs and the membrane bound tyrosine kinases platelet derived growth factor (PDGF) receptor and epidermal growth factor (EGF) receptor in astrocytes [56]. Crosstalk with other receptors provides a mechanism by which RAS may regulate other signaling pathways which may exacerbate RAS actions directly or indirectly. In the case of the CB1R or D4R which may be counterregulatory to AT1R, homologous regulation by Ang II dampens their inhibitory effects on RAS actions. Some studies have shown anti-inflammatory actions of the CB1R which may counteract the proinflammatory actions of Ang II in cardiovascular disorders [52]. In addition, the D4R is known to regulate blood pressure. Dysregulation of these receptors via crosstalk with AT1R is a potential mechanism by which RAS-mediated cardiovascular disease actions are exacerbated. Moreover, the AT1R crosstalks with tyrosine kinase receptors to induce astrocyte proliferation as discussed below.
Cellular Growth and Proliferation
The MAPKs are a family of protein kinases that are involved in pathways that control cell differentiation, cell proliferation, and cell death [57]. This family of proteins includes the extracellular signal regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) and p38 MAPKs. Ang II is a potent mitogen that induces proliferation in rat astrocytes by multiple signaling cascades. In our laboratory, we have shown that Ang II activates the ERK1/2 MAPK pathway through non-receptor tyrosine kinases Src and Pyk2 [41], and by transactivation of membrane bound receptor tyrosine kinases, PDGF and EGF receptors to stimulate astrocyte growth [56]. Ang II stimulated astrocyte proliferation via JNK signaling with upstream Src but not PKC signaling [43]. Astrocytes were also shown to elicit neuroprotective effects via MKK4/JNK/c-Jun/AP-1 [58], and stimulate p38 MAPKs [59]. Ang III also stimulated astrocyte proliferation by JNK and ERK1/2 MAPKs [21, 42]. Further, the Ang III effects were direct and did not result from its conversion from Ang II.
The AT4Rs are found in both astrocyte and neuronal primary cells [60]. They are more prevalent however, in sensory and cognitive areas compared to the AT1Rs that are prevalent in cardiovascular and osmoregulatory areas [61]. Ang IV increased ERK1/2 MAPKs in astrocytes without intracellular calcium activation. There were also notable increases in growth rate and apoptosis when Ang IV was administered with Ang II [62].
Astrocyte proliferation is a potential mechanism by which Ang II may contribute to cardiovascular disease pathogenesis. The effects may be twofold, astrocytes are a source of RAS components and can lead to increased Ang II production augmenting the central Ang II effects. Secondly, astrocytes are a source of proinflammatory mediators and an increase in astrocyte numbers may lead to stimulation of proinflammatory effects. It is now accepted that neuroinflammation is an important contributor to cardiovascular disease pathogenesis. Additionally, accumulation of astrocytes may contribute to dysregulated astrogliosis and an overall augmentation of deleterious RAS-mediated effects in these cells.
Neuroinflammation
Inflammation can be described as the local reaction of tissue or microcirculation to injury or insult [63]. The inflammatory response is a cascade of events that serves to restore damaged tissue with subsequent scar tissue formation. The inflammatory cascade can be classified into three general steps regardless of the injury. However, the severity and extent of the response will vary. The three main aspects of the inflammatory response include: (i) changes in vascular permeability, (ii) phagocytosis and transmigration of immune cells, and (iii) growth and reconstitution of tissue [63, 64].
Astrocytes are important regulators of neuroinflammation, and may either suppress or exacerbate the immune response [65]. Neuroinflammation is an inflammatory response originating in the CNS. It is characterized by accumulation of microglia and astrocytes, and upregulation of cytokines and immune system components [66]. Neuroinflammation in brain regions controlling sympathetic outflow is associated with resistant hypertension [67]. RAS peptides have also been implicated in the inflammatory process. Both Ang II and prorenin upregulate ROS and proinflammatory cytokines (IL-1β; IL-6; TNF-α), while downregulating the anti-inflammatory cytokine IL-10 [68]. The mechanisms by which Ang II is believed to mediate the inflammatory process in peripheral tissues include: (i) upregulation of prostaglandins and VEGF with altered pressor action and cytoskeletal proteins, (ii) increased proinflammatory mediators, (iii) activation of immune cells, and (iv) increased cellular growth, extracellular matrix and angiogenesis [64, 69]. Ang III was also shown to induce STAT3 leading to an increase in IL-6 mRNA expression in astrocytes [24].
Within the brain, Ang II’s chronic stimulation of sympathetic outflow is a hallmark feature of neurogenic hypertension [67, 70]. In our laboratory, we showed that Ang II induced the production of IL-6 and ROS in rat cerebellum and brainstem astrocytes. This was mediated via the NF-kB/ROS pathway. However, no significant differences were observed in astrocytes isolated from Wistar compared to SHRs in Ang II-induced ROS levels [36]. We have also shown that activation of the JAK/STAT pathway by Ang II induced growth and production of IL-6 in rat astrocytes [23]. Leukocyte infiltration, a characteristic of the inflammatory response is modulated by Ang II in astrocytes. NF-kB was shown to play a role in the regulation of these Ang II effects [71].
It is postulated that there are feed forward and feedback mechanisms in neurogenic hypertension driven by RAS. Ang II and prorenin have direct effects on inflammatory states and ROS production in brain regions (feed forward). In addition, Ang II mediates systemic cytokine signaling across circumventricular organs [67]. Ang II also increases blood brain barrier permeability which may cause infiltration of peripheral immune cells into the brain tissue [67]. This highlights a mechanism by which Ang II may be involved in the pathogenesis of diseases.
In human astrocytes, Ang II and Ang III increased prostaglandin levels and enhanced Ca2+ activation of phospholipase C (PLC) [72]. Other active RAS peptides also have either a regulatory or a counterregulatory role in mediating inflammatory states. Ang (1–7) stimulated bradykinin production, prostaglandin synthesis and vasodilation [72]. The main downstream signaling pathway identified thus far for Ang (1–7)/Mas is activation of NO incorporating bradykinin receptors and the cGMP/PKG pathway [73]. In a mouse model of Alzheimer’s disease, AT4R was shown to mediate anti-inflammatory effects [74]. The ACE2/Mas axis exhibits anti-inflammatory effects by stimulating bradykinin release, reducing Ang II levels and increasing Ang (1–7) and NADPH oxidase 4 expression [75, 76]. Bradykinin B2 subtype receptors are found on astrocytes [77]. Both Ang (1–7) and Ang (1–9) stimulate bradykinin release in the periphery [76]. Bradykinin facilitates vasorelaxation and vasodilation [78]. Other studies have reported proinflammatory effects of bradykinin in astrocytes, resulting from increased IL-6 expression via a NFkB pathway [79]. The role of brain bradykinin on cardiovascular effects is not clear; however, enhanced pressor response to bradykinin was observed in SHRs compared to Wistar Kyoto rats [80]. The bradykinin B1 receptor subtype which is also found in the CNS did not mediate the pressor effects observed in SHRs [80]. Determining the role of brain angiotensins on the bradykinin-mediated pressor effects may identify other astrocyte-mediated pathophysiological effects in cardiovascular diseases.
Neuroinflammation is well established as a significant contributor to cardiovascular disease pathogenesis. RAS-mediated generation of inflammatory mediators are outlined above. There are numerous mechanisms by which neuroinflammation may induce or maintain hypertension and other cardiovascular disorders. A number of mechanisms are discussed elsewhere [2]. Astrocytes are an important source of pro- and anti-inflammatory cytokines, they maintain the blood brain barrier, they provide metabolic support to neurons and they maintain synaptic homeostasis. As such, astrocyte dysfunction can lead to a host of neuroinflammatory and deleterious actions including breakdown of blood brain barrier giving access to circulating peptides, increased sympathoexcitation, neuronal death or impaired neuronal functions, and increased ROS generation. RAS-induced proliferation of astrocytes can magnify these effects in addition to generating more Ang II, sustaining these effects.
Neurotransmission
A number of roles have been suggested for astrocytes in neurotransmission [81]. In addition to forming a scaffold to guide neuronal development and facilitate synapse formation [82], astrocytes are also involved in glutamate clearance and energy supply to neurons [82]. Prostaglandins stimulate glutamate release by astrocytes in a calcium-dependent manner [83]. Accumulation of glutamate in the synaptic cleft can result in overstimulation of neurotransmission [81]. Ang II inhibits astrocyte glutamate transporter 1; this stimulates sustained neuronal activity and SNS outflow [51]. A suggested mechanism was Ang II induced ROS generation [51]. Similarly, impaired calcium signaling possibly resulting in neurotransmitter excitation in astrocytes was implicated in alcoholism [84]. Ang II at physiological concentrations stimulates calcium flux in astroglia cells [85]. An exaggerated RAS may result in aberrant calcium signaling, this may be another mechanism via which Ang II, and astrocytes are involved in disease pathogenesis. Roles for astrocytic calcium mobilization and molecular mechanisms involved was reviewed [86]. It was concluded that more effective tools for measuring calcium in astrocytes may help to advance our understanding of calcium signaling and astrocyte functions. Angiotensins are known to stimulate calcium signaling in astrocytes [87]; however, the signaling pathways differ by brain region and species.
Other RAS-Mediated Mechanisms in Astrocytes
In astrocytes, Ang II induced the expression of c-fos and c-myc via the ERK1/2 MAPK signaling pathway. Ang II also induced cjun expression however, inhibition of the ERK1/2 pathway did not affect its expression [88]. Additionally, Ang II activated CREB in rat astrocyte cultures [62]. Others have shown that Ang II differentially activates PLC in neonatal rat astrocytes [89].
Another mechanism by which Ang II may modulate astrocyte functions is by aberrant gene regulation. Recent studies have implicated microRNAs in RAS-mediated cardiovascular inflammation [90]. MicroRNAs regulate gene expression [90], and members of micro RNA subfamilies are regulated by Ang II via the AT1R [91, 92]. High levels of the microRNA family miR-181 were observed in astrocytes compared to neurons, and have been implicated in neuroinflammation involving astrocytes [38]. RAS-mediated neuroinflammation and the role of microRNAs in astrocytes warrants exploration.
Perspectives
The role of RAS in cardiovascular diseases and hypertension has been established for over four decades. Brain RAS has been highlighted as a significant contributor to the development and maintenance of the hypertensive condition. Astrocytes have now emerged as major players in RAS-mediated central effects. Astrocytes express the major receptors for the active RAS peptides Ang II and Ang III. Activation of these receptors in brain regions with cardiovascular functions modulate cell growth and proliferation, neuroinflammation and neurotransmission. Moreover, these receptors interact with other receptors such as the CB1R and the D4R to elicit other central effects. It is not fully established whether the AT1Rs become activated in both neurons and astrocytes to cause these effects. In vitro studies have shown increased levels of proinflammatory cytokines and ROS after Ang II stimulation in astrocyte enriched cultures. These inflammatory mediators not only induce neuroinflammation but also trigger excitotoxicity. Moreover, a response to traumatic brain injury includes activation of the RAS to restore homeostasis. However, this is a double-edged sword as this response can lead to proinflammatory states and increased oxidative stress. Since astrocytes are involved in RAS-mediated neuroinflammatory and oxidative responses, their activation may drive deleterious outcomes to brain injury. In support of this, inhibition of AT1Rs improved neurorestoration, inhibited astrogliosois and decreased proinflammatory response during brain traumatic injury [93]. This suggests a role for astrocytes in traumatic brain injury. The neuroinflammatory role of astrocytes spans multiple areas and remain an active area of investigation. In addition to astrocytes, other glial cells are major contributors to RAS-mediated central effects. Studies in individual cell types have provided insight into the contributions of each. However, there still exists a great need to investigate the interactions between the cell types and whether this interaction may be dysregulated in hypertension and other cardiovascular diseases. When these interactions are known, they can then be targeted for therapeutic intervention.
Future Directions
It is evident that astrocytes play a vital role in the CNS and in RAS-mediated central effects. Their role in neuroprotection and neuroinflammation is of particular interest because neuroinflammation is a major contributor to pathological conditions. Finding therapeutic strategies that could swing the pendulum to enhance their neuroprotective capabilities would be a desirable advancement in the field. This however, requires more studies elucidating the molecular mechanisms involved in the neuroprotective actions of astrocytes. Our laboratory has carried out in vitro studies on molecular mechanisms in RAS-mediated central effects in primary astrocytes. Investigating these molecular mechanisms in neuron-astrocyte cocultures, will help us to determine whether RAS-mediated neuroinflammatory actions are occurring directly in astrocytes or due to paracrine signaling from neurons, or both. Neuron-astrocyte interactions have been shown to be integral in RAS-mediated central effects. Investigations carried out in single cell cultures provide baseline responses but do not account for the multiple interactions between cell types that do exist in vivo. Moreover, it is postulated that cells grown in culture may exhibit certain pathophysiological characteristics not observed in normal conditions in vivo. Examining these RAS-mediated mechanisms in vivo, particularly the actions of astrocytes and how they interact with other brain cells, would be a welcomed step forward in developing therapeutic strategies.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- ACEA:
-
Arachidonyl-2′-chloroethylamide
- Ang:
-
Angiotensin
- APA:
-
Aminopeptidase A
- APN:
-
Aminopeptidase N
- AP-1:
-
Activator protein 1
- AT1R:
-
Angiotensin type 1 receptor
- AT2R:
-
Angiotensin type 2 receptor
- CB1R:
-
Cannabinoid receptor type 1
- cGMP:
-
Cyclic guanosine monophosphate
- CNS:
-
Central nervous system
- CREB:
-
Cyclic adenosine 3, 5-monophosphate response element-binding protein
- EGFR:
-
Epidermal growth factor receptor
- ERK1/2:
-
Extracellular signal-regulated kinase
- IL:
-
Interleukin
- IRAP:
-
Insulin-regulated aminopeptidase receptor
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- MAPK:
-
Mitogen-activated protein kinase
- MKK4:
-
Mitogen-activated protein kinase kinase 4
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NEP:
-
Neutral endopeptidase
- NFkB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PDGF:
-
Platelet-derived growth factor
- PLC:
-
Phospholipase C
- PKC:
-
Protein kinase C
- PKG:
-
Protein kinase G
- RAS:
-
Renin angiotensin system
- ROS:
-
Reactive oxygen species
- SHR:
-
Spontaneously hypertensive rat
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- VEGF:
-
Vascular endothelial growth factor
References
Tsuda K (2012) Renin-angiotensin system and sympathetic neurotransmitter release in the central nervous system of hypertension. Int J Hypertens 2012:11
Haspula D, Clark MA (2018) Neuroinflammation and sympathetic overactivity: mechanisms and implications in hypertension. Auton Neurosci 210:10–17
Veerasingham SJ, Raizada MK (2003) Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139(2):191–202
Shearer F, Lang CC, Struthers AD (2013) Renin-angiotensin-aldosterone system inhibitors in heart failure. Clin Pharmacol Ther 94(4):459–467
Cravedi P, Remuzzi G, Ruggenenti P (2011) Targeting the renin angiotensin system in dialysis patients. Semin Dial 24(3):290–297
McKinley MJ et al (2003) The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35:901–918
Raizada MK et al (1987) Distinct angiotensin II receptor in primary cultures of glial cells from rat brain. Proc Natl Acad Sci USA 84(13):4655–4659
Colin S et al (1991) Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci USA 88(17):7567–7571
Sumners C et al (1994) Peptide receptors in astroglia: focus on angiotensin II and atrial natriuretic peptide. Glia 11(2):110–116
Bickerton RK, Buckley JP (1961) Evidence for a central mechanism in angiotensin induced hypertension. Proc Soc Experim Biol Med 106(4):834–836
Ganten D et al (1971) Angiotensin-forming enzyme in brain tissue. Science 173(3991):64
de Kloet AD et al (2015) Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol 309(5):R444–R458
Tallant EA et al (1991) Human astrocytes contain two distinct angiotensin receptor subtypes. Hypertension 18(1):32–39
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13(8 Suppl B):9–20
Ruiz-Ortega M et al (2001) Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension 38(6):1382–1387
Jiang F et al (2014) Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol 11(7):413–426
Phillips MI (1987) Functions of angiotensin in the central nervous system. Annu Rev Physiol 49:413–435
Mehta PK, Griendling KK (2006) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol 292:C82–C97
Shan Z et al (2008) Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol 93(5):701–708
Sealey JE (1995) Evidence for cardiovascular effects of prorenin. J Hum Hypertens 9(6):381–384
Clark MA, Nguyen C, Tran H (2012) Angiotensin III induces c-Jun N-terminal kinase leading to proliferation of rat astrocytes. Neurochem Res 37(7):1475–1481
Clark MA, Nguyen C, Tran H (2013) Distinct molecular effects of angiotensin II and angiotensin III in rat astrocytes. Int J Hypertens 2013:782861
Kandalam U, Palanisamy M, Clark MA (2012) Angiotensin II induces cell growth and IL-6 mRNA expression through the JAK2-STAT3 pathway in rat cerebellar astrocytes. JAK-STAT 1(2):83–89
Kandalam U et al (2014) Angiotensin III induces signal transducer and activator of transcription 3 and interleukin-6 mRNA levels in cultured rat astrocytes. J Renin-Angiotensin-Aldosterone Syst 16(4):758–767
Tallant EA, Diz DI, Ferrario CM (1996) Identification of AT1 receptors on cultured astrocytes. Adv Exp Med Biol 396:121–129
O’Callaghan EL et al (2011) Regulation of angiotensinogen by angiotensin II in mouse primary astrocyte cultures. J Neurochem 119(1):18–26
Yugandhar VG, Clark MA (2013) Angiotensin III: a physiological relevant peptide of the renin angiotensin system. Peptides 46:26–32
Stornetta RL et al (1988) Astrocytes synthesize angiotensinogen in brain. Science 242(4884):1444–1446
Gowrisankar YV, Clark MA (2016) Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem 138(1):74–85
Blackburn D et al (2009) Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia 57(12):1251–1264
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7(3):194–206
Purves D, Augustine GJ, Fitzpatrick D et al (2001) Neuroglial cells. In: Purves D, Augustine GJ, Fitzpatrick D (eds) Neuroscience. Sinauer Associates, Sunderland
Jäkel S, Dimou L (2017) Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci 11:24
Lanz TV et al (2010) Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120(8):2782–2794
Wiese S, Karus M, Faissner A (2012) Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 3:120
Gowrisankar YV, Clark MA (2016) Angiotensin II induces interleukin-6 expression in astrocytes: role of reactive oxygen species and NF-kappaB. Mol Cell Endocrinol 437:130–141
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Hutchison ER et al (2013) Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 61(7):1018–1028
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647
Biancardi VC et al (2014) Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension 63:572–579
Clark MA, Gonzalez N (2007) Src and Pyk2 mediate angiotensin II effects in cultured rat astrocytes. Regul Pept 143(1):47–55
Clark MA, Tran H, Nguyen C (2011) Angiotensin III stimulates ERK1/2 mitogen-activated protein kinases and astrocyte growth in cultured rat astrocytes. Neuropeptides 45(5):329–335
Clark MA, Guillaume G, Pierre-Louis HC (2008) Angiotensin II induces proliferation of cultured rat astrocytes through c-Jun N-terminal kinase. Brain Res Bull 75(1):101–106
Olson JA et al (1991) Angiotensin II induces secretion of plasminogen activator inhibitor 1 and a tissue metalloprotease inhibitor-related protein from rat brain astrocytes. Proc Natl Acad Sci USA 88(5):1928–1932
Antonella Muscella FA, Marsigliante S, Levi G (2000) Angiotensin II modulates the Activity of Na+, K+- ATPase in cultured rat astrocytes via the AT! receptor and protein kinase C-δ activation. J Neurochem 74(3):1325–1331
Wosik K et al (2007) Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci 27(34):9032–9042
Chandler LJ et al (1995) Angiotensin II decreases inducible nitric oxide synthase expression in rat astroglial cultures. Am J Physiol 268(3 Pt 1):C700–C707
Tang W et al (1995) Angiotensin II increases glucose uptake and glucose transporter-1 mRNA levels in astroglia. Am J Physiol 268(3 Pt 1):E384-90
Isegawa K et al (2014) Angiotensin II type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol 307(10):H1448
Isegawa K et al (2013) Brain angiotensin II type 1 receptors in astrocytes, not neurons, are upregulated in mice with myocardial infarction-induced heart failure. J Card Fail 19(10):S142
Stern J et al (2016) Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 68:1483–1493
Haspula D, Clark MA (2016) Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J Neurochem 139(4):523–536
Haspula D, Clark MA (2017) MAPK activation patterns of AT1R and CB1R in SHR versus Wistar astrocytes: evidence of CB1R hypofunction and crosstalk between AT1R and CB1R. Cell Signal 40(Supplement C):81–90
Chen K et al (2015) Activation of D(4) dopamine receptor decreases AT(1) angiotensin II receptor expression in rat renal proximal tubule cells. Hypertension 65(1):153–160
Bek MJ et al (2006) Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 47(2):288–295
Clark MA, Gonzalez N (2007) Angiotensin II stimulates rat astrocyte mitogen-activated protein kinase activity and growth through EGF and PDGF receptor transactivation. Regul Pept 144(1–3):115–122
Pearson G et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–183
Mahesh VB, Dhandapani KM, Brann DW (2006) Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol 246(1–2):1–9
Alanazi AZ, Patel P, Clark MA (2014) p38 mitogen-activated protein kinase is stimulated by both angiotensin II and angiotensin III in cultured rat astrocytes. J Recept Signal Transduct 34(3):205–211
Greenland K, Wyse B, Sernia C (1996) Identification and characterization of angiotensin IV binding sites in rat neurone and astrocyte cell cultures. J Neuroendocrinol 8(9):687–693
Wyse B, Greenland K, Sernia C (1995) Specific binding sites for (3–8)angiotensin in C6 glioma cells. Brain Res 681(1–2):41–46
Holownia A, Braszko JJ (2007) The effect of angiotensin II and IV on ERK1/2 and CREB signalling in cultured rat astroglial cells. Naunyn Schmiedebergs Arch Pharmacol 376(3):157–163
Spector WG, Willoughby DA (1963) The inflammatory response. Bacteriol Rev 27:117–154
Suzuki Y, Ruiz-Ortega M, Egido J (2000) Angiotensin II: a double-edged sword in inflammation. J Nephrol 13(Suppl 3):S101–S110
Colombo E, Farina C, Astrocytes: key regulators of neuroinflammation. Trends Immunol 37(9): 608–620
Morales I et al (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8:112
Winklewski PJ et al (2015) Brain inflammation and hypertension: the chicken or the egg? J Neuroinflamm 12:85
Winklewski PJ et al (2015) Brain inflammation and hypertension: the chicken or the egg? J Neuroinflamm 12(1):85
Suzuki Y et al (2003) Inflammation and angiotensin II. Int J Biochem Cell Biol 35(6):881–900
Cardinale JP et al (2012) Angiotensin II–induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59(1):113–121
Fuchtbauer L et al (2011) Angiotensin II Type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun 25(5):897–904
Jaiswal N et al (1991) Subtype 2 angiotensin receptors mediate prostaglandin synthesis in human astrocytes. Hypertension 17(6 Pt 2):1115
Xu P, Sriramula S, Lazartigues E (2011) ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol 300(4):R804–R817
Royea J et al (2017) Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J Neurosci 37:5562–5573
Lo J et al (2013) Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar–Kyoto rats and spontaneously hypertensive rats. Exp Physiol 98(1):109–122
Tom B et al (2001) Bradykinin potentiation by angiotensin-(1–7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension 38(1):95–99
Cholewinski AJ et al (1991) Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem 57(4):1456–1458
Kuga T et al (1997) Bradykinin-induced vasodilation of human coronary arteries in vivo: role of nitric oxide and angiotensin-converting enzyme. J Am Coll Cardiol 30(1):108–112
Schwaninger M et al (1999) Bradykinin induces interleukin-6 expression in astrocytes through activation of nuclear factor-κB. J Neurochem 73(4):1461–1466
Cloutier F et al (2004) Correlation between brain bradykinin receptor binding sites and cardiovascular function in young and adult spontaneously hypertensive rats. Br J Pharmacol 142(2):285–296
Murphy-Royal C et al (2017) Astroglial glutamate transporters in the brain: regulating neurotransmitter homeostasis and synaptic transmission. J Neurosci Res 95(11):2140–2151
Auld DS, Robitaille R (2003) Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron 40(2):389–400
Bezzi P et al (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391(6664):281–285
Wu S, Wu Z, Wang D (2015) Alcohol abrogates intracellular Ca2+ elevation by angiotensin II and ATP in cultured rat astrocytes. Figshare, London, pp 101–104
Wang D et al (1996) Angiotensin II regulation of intracellular calcium in astroglia cultured from rat hypothalamus and brainstem. J Neurochem 67(3):996–1004
Agulhon C et al (2008) What is the role of astrocyte calcium in neurophysiology? Neuron 59(6):932–946
Gebke E et al (1998) Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience 85(2):509–520
Delaney J et al (2008) Regulation of c-fos, c-jun and c-myc gene expression by angiotensin II in primary cultured rat astrocytes: role of ERK1/2 MAP kinases. Neurochem Res 33(3):545–550
Tallant EA, Higson JT (1997) Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia 19(4):333–342
Pacurari M, Tchounwou PB (2015) Role of MicroRNAs in Renin-Angiotensin-Aldosterone System-mediated cardiovascular inflammation and remodeling. Int J Inflamm 2015:7
Jeppesen PL et al (2011) Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br J Pharmacol 164(2):394–404
Eskildsen TV et al (2013) Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14(6):11190–11207
Villapol S et al (2015) Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain 138(11):3299–3315
Gallagher PE et al (2006) Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290(2):C420-6
Machado RDP, Santos RAS, Andrade SP (1999) Opposing actions of angiotensins on angiogenesis. Life Sci 66(1):67–76
Flores-Muñoz M et al (2012) Adenoviral delivery of angiotensin-(1–7) or angiotensin-(1–9) inhibits cardiomyocyte hypertrophy via the mas or angiotensin type 2 receptor. PLoS ONE 7(9):e45564
Flores-Muñoz M et al (2011) Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol 589(4):939–951
Liu M, Shi P, Sumners C (2016) Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J Neurochem 136(1):163–171
Simões e Silva AC, Teixeira MM (2016) ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107(Supplement C): 154–162
Jiang T et al (2012) Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br J Pharmacol 167(7):1520–1532
Young CN, Davisson RL (2015) Angiotensin-II, the brain, and hypertension: an update. Hypertension 66(5):920–926
Dupont AG et al (2009) IRAP and AT1 receptor mediated effects of angiotensin IV. J Intern Med 265(3):401–403
Wright JW et al (1996) Angiotensin III and IV activation of the brain AT1 receptor subtype in cardiovascular function. Peptides 17(8):1365–1371
Oliveira MA et al (1999) Synergistic effect of angiotensin-(1–7) on bradykinin arteriolar dilation in vivo. Peptides 20(10):1195–1201
Diz DI et al (2008) Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol 51(6):542–548
Acknowledgements
This work was supported by a President’s Faculty Research and Development Grant (#335435) from Nova Southeastern University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
O’Connor, A.T., Clark, M.A. Astrocytes and the Renin Angiotensin System: Relevance in Disease Pathogenesis. Neurochem Res 43, 1297–1307 (2018). https://doi.org/10.1007/s11064-018-2557-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2557-0